How to Cite | Publication History | PlumX Article Matrix
Sunil Chandra Pradhan1* and Ajaya Kumar Patra2
1Department of Zoology, Utkal University, VaniVihar, Bhubaneswar, India.
2Kandarpur Degree College, Kandarpur, Cuttack, India.
Corresponding Author E-mail: e-mail:sunilch_pradhan@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2867
ABSTRACT:
The work was assessed the field performance of Polythene cover on morphology, fruit yield and post maturation changes on growing tomato (Lycopersicon esculentum). Data revealed that use of polythene gave adverse effect on average height in treated condition but favourable for matured ripen fruits (41.5%). Significant correlation observed in the post maturation (0.731, P≤0.01) and fruits ripening time (0.605, P≤0.01). The fruit yield was found to be significantly increased by 61.23%. Polythene induced ripening had shown the variation in the amount of lycopene (429.3mg/100gm), protein (1.92%), ascorbic acid (23.68 mg/ 100gm) and the sugar (5.02%). Polythene covering was significant and positively correlated with lycopene (0.788, P<0.01), lipid(0.853, P<0.01),ascorbic acid(0.515 P<0.01) but negatively correlated with protein(-0.221P<0.05).Maturity at harvest is a very important attribute to tomatoes quality that found to be regulated by the use of polythene. Analysis of variance also showed statistically significant result in the lipid (F-value=14.688801,p≤0.01) ,sugar(F value= 25.52718,p≤0.01) and ascorbic acid (F-value=9.44343,p≤0.01).
KEYWORDS: Biochemical features, Lycopersicon esculentum, Polyethylene, Post mature changes.
Download this article as:| Copy the following to cite this article: Pradhan S. C, Patra A. K. Effect of Polyethylene on Fruit Yield and Morpho-Biochemical Features During Ripening of a Local Tomato Cultivar Grown in a Tropical Environmental Condition, India. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Pradhan S. C, Patra A. K. Effect of Polyethylene on Fruit Yield and Morpho-Biochemical Features During Ripening of a Local Tomato Cultivar Grown in a Tropical Environmental Condition, India. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/371J6uX |
Introduction
The role of plants in maintaining human health is well documented. Fruits and vegetables possess protective effect against different degenerative diseases due to the presence of various phytochemicals, carotenoids, vitamins and minerals(Srivastava and Kulshreshtha,2013).The common garden tomatoes are botanically classified as a fruit but many people considered as a vegetable. The department of Agriculture,USA has defined tomato as a vegetable belongs to Solanaceous family(Opadotun et al., 2016). L. esculentum is one of the highly valuable and constitutes among the major vegetable crops around the world.It is a daily used food item due less sugar, low acidic PH ,good seasoning and delicious in taste(Beckles 2012).β-carotene, polyphenols and vitamin C of tomato are thought to be potent antioxidants that has been rich in phytochemicals(Tyssandier 2004).Many factors such as cultivation method, growth parameters, temperature, light, soil type, mineral nutrients and ripening conditions have been affecting ripening and harvesting timing and also the quality of vegetation.
Vegetable production increases manifolds in recent years but the per capita consumption of vegetables in India is only about210 gm /day/person which is far lower than the minimum dietary need of 300 gm/day/person( Singh and Chaubey, 2013). It is indispensable to adopt different methodologies to enhance production. Even though the humid tropical weather favours the cultivation of a wide range of vegetables, a number of constraints including a shortage of water, land, labour etc. limit the cultivation. As per the plasticulture society, there is extensive use of polythene, plastic plate, coverings, seedbeds etc. to augment the yields (Lamont et al., 2000). These are widely used as a popular model to study plant biology under stresses, microbial infections and diseases(Wang et al., 2010; Fischer et al., 2011; Page et al., 2010).
The present study has set up to investigate the possible correlation between polyethene cover and the morphological-chemical changes, fruit yields, assess the maturity and ripening process in a tropical area like Bhubaneswar.
Materials and Methods
The experimental item Lycopersicon esculentum was chosen because of its easy cultivation, medium in size, short life span, easy to handle, less cost, small space requirement and widely consumed items in the locality. The experiment was conducted in spring-summer of 2012-2013 in tropical environmental conditions at Bhubaneswar, Khurda with average fall of rain 1449.1 mm and temperature varied between 19.7°C (winter) to 32.5°C(summer). Physico-Chemical properties revealed the soil as lateritic, slightly acidic pH (5.4)with less amount of phosphorous, calcium but the medium amount of potassium and nitrogen.
The desired healthy seeds were procured from University of Agriculture, Odisha and treated with 10% fungicide. A 25feet square area was taken for seed bade. The soil was tilted loosen, mix with manure (cow dung) and levelled. The four sides of the nursery were covered with net. After 2-3 days seeds were germinated and grew up a little seedling on 25-30 days. The good and healthy seedling had been selected and transplanted from nursery to the main field, keeping a distance of two feet among lines and plants. Black Polyethylene (30×15 length and 0.3-micron thickness) was washed and treated with5% fungicides. The young plants, flowers, small fruits and half ripen fruits were covered with this polyethylene.
Preparation of Samples
Maturity was assessed by observing colour as proposed by Nielsen(2003). The fresh tomatoes were cleaned and divided into two parts. One part was blended into a paste for determination of moisture while the other part was used for biochemical analysis.
Moisture Content
A known weight of the sample(5g) was taken on a pre-weighed aluminium dish (M1). The dish containing sample(M2) was placed in the oven at 100oc for two hours, the dish was a move to desiccators and allowed to cool. The dish containing a dried sample was weighed (M3)( AOAC 1975).
Lipid Content
Lipid was extracted through Soxhletˋs Apparatus (AOAC 2004). Test sample (four gm) was taken in a thimble that put in Soxhletˋs extractor. Extraction process carried on for 4 hours. The thimble was kept in a desiccator and weighed.
Protein Content
Protein was estimated by the Kjeldahl method (Imtiaz et al., 2015). The test sample was taken into Kjeldahl flask and digested with H2SO4. The digested sample was made alkaline and titrated with Hydrogen Chloride solution. The protein content was obtained multiplying the nitrogen value (6.25).
Total Sugar Content
An aliquot from the filtrate was taken and ten ml of hydrochloric acid added and the inversion was carried out. The contents neutralized with sodium hydroxide using phenolphthalein as an indicator and titrated ( Ranganna 1986).
Lycopene Content (mg/100gm)
Tomato fruits were finely ground. One gm of the puree was centrifuged and the supernatant collected. The absorbance of supernatant (hexane layer) containing lycopene was taken at a wavelength of 503 nm( Fish et al., 2002).
Ascorbic Acid Content (mg/100gm)
It was determined as per Ranganna(1979) by taking a homogenized sample and titrating with indophenol solution until a faint pink colour develops.
Statistical Analysis
The statistical analysis was performed with MSTAT-Statistical package and ANOVA was used to assess the level of significance (MSTAT 1988; Rajablariani et al.,2012).
Result and Discussions
Morphological Features
During the course of the experiment both normal and polyethene covered treated plants, flowers and fruits were measured, analyzed and recorded (Table 1). The average height of young plant ranged from 18.5-23.4 cm in normal or control and 16.8-20.5cm in treated condition. The frequency of fallen young tomato flowers found to be varied 9% to 15.5 % in normal and 19.5% to 39.0%under polythene cover. The frequency of fallen young tomato fruits during experimental periods found to be varied 7.8% to 14.3 % in normal and 17.5% to 33.0% under polythene cover and the highest overall per cent (34%) of fallen fruits were observed at 14 days of observation. The per cent of fallen flowers, fruits and increased in size under polyethylene cover fruits were found to have a linear relationship with duration of treatment. Ashrafuzzaman et al., (2011) also expressed a similar view in their experiment. Post mature changes in the increase of fruit size (Figure 1) by 9.4% to 18.7% in normal and 28.2% to 41.5 % under polythene covered whereas fruit yield under polythene cover increased by 61.23%.Diametric analysis of tomato fruits found to have average transverse diameter 2.7cm and weight 65gram in normal and average transverse diameter 3.8cm and weight became 89.5 gram in treated condition. Duration of ripening fruit(Figure 2) also found to be reduced in treated condition by 55.4 per cent. The initial ripening from the day of maturation took 4days and full ripening in 6.5dayswhereas untreated condition took 5 to 10.5 days.Use of Polyethylene cover found to have significant correlation(Table 2)with fallen flowers(0.211, P≤0.05), post maturation time(0.731, P≤0.01), transverse diameter(0.539, P≤0.01) , fruits ripening time(0.605, P≤0.01)and fruit yield( 0.571 , P≤0.01) of tomato plants.
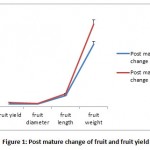 |
Figure 1: Post mature change of fruit and fruit yield |
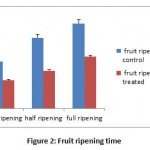 |
Figure 2: Fruit ripening time |
Table 1: Morphological features of Tomato plant.
| Percent of fallen tomato flowers in control and treated | |||||||||
| Observation
In days |
total plant for observation
|
Total no. of flowers for observation | % of fallen flowers | ||||||
| normal | Polythene covered | normal | Polythene covered | normal | Polythene covered | normal | Polythene covered | ||
| 7 | 7 | 20 | 20 | 100 | 100 | 9 | 19 | ||
| 14 | 14 | 20 | 20 | 100 | 100 | 15 | 39 | ||
| Percent of fallen young tomato fruits in control and treated | |||||||||
| Observation
In days |
Total plant for observation
|
Total fruits for observation | % of fallen fruits | ||||||
| normal | Polythene covered | normal | Polythene covered | normal | Polythene covered | normal l | Polythene covered | ||
| 7 | 7 | 20 | 20 | 100 | 100 | 7 | 17 | ||
| 14 | 14 | 20 | 20 | 100 | 100 | 14 | 33 | ||
| Per cent of post mature changes of tomato fruits in control and treated | |||||||||
| Observation
In days |
Total plant for observation
|
Total fruits for observation | % of increase fruits size | ||||||
| normal | Polythene covered | normal | Polythene covered | normal l | Polythene covered | normal | Polythene covered | ||
| 7 | 7 | 20 | 20 | 100 | 100 | 10 | 28 | ||
| 14 | 14 | 20 | 20 | 100 | 100 | 18 | 41 | ||
*normal=Without polythene cover(control), Polythene covered=Experimental
Table 2: Correlation coefficients (r) between polythene and morphological features
| parameters | Correlation coefficient(r) | Significance |
| Polythene & fallen flowers | 0.211 | * |
| Polythene & fallen young fruits | 0.103 | * |
| Polythene & post mature changes of fruits | 0.731 | ** |
| Polythene & transverse diameters | 0.539 | ** |
| Polythene & fruits ripening time | 0.605 | ** |
| Polythene & fruits yield | 0.571 | ** |
*, ** Significantly correlated at 0.05 and 0.01 respectively
Click here to View figureThe test result showed that Polyethylene had a more adverse effect on young plants but favourable for matured and ripen a condition. This may be due to the effects that light absorption and reflection from polyethene cover had on the plant’s phytochrome regulatory system. Experimental work of Orzolek et al. (2000) documented a polythene impact on maturing conditions. Other possibilities of adverse effects are reduced supply of carbohydrate at early development, competitive inhibitors that may be indigenous or exogenous from other sources. The microclimatic conducive parameters may be developed by the polyethene might have provided a suitable condition for producing higher growth and development. The rise of output related with favourable microclimate condition, light induces parameters, pathogen management. The similar role of respiratory substrate and inhibitors in the vegetation also analyzed by Ba-RG and Burg(1967).
Biochemical Features
The biochemical analysis(Table3), correlation coefficient(Table4) and ANOVA(Table5) were performed and statistically analyzed. The major component of the fruit was moisture(Table 3) whose value was ranged from 88.15% to 93.81 %. The moisture content was found to be decreased with maturation and ripening process. The amount of moisture content corresponds with the values reported by the study of Gupta et al .,(2006) and De Souza et al., (2008). A negative correlation was observed between polythene & moisture (r= 0.097). Lipid content fluctuated from 0.81to 1.17 %. In the present investigation, the total lipid level showed a gradual increase with the maturation process. The positive correlation was observed between polythene &lipid (r=.853at p≤0.01). Significant variations(Table5) also observed in the analysis of variance (F-value=14.688801,p≤0.01).Protein content was found to be varied from 1.92 to 2.57%. The decreased values were obtained with the maturation process. It has been observed a reduced protein amount with increase maturation that may be due to a rise in the secretion of volatile substances. A significant negative correlation observed with polythene cover(r= -.221*at p≤0.05). The amount of sugar content ranged from 1.8 to 5.02%. The highest value was 5.02% in 14 days of treatment with positive correlation (0.287, P≤0.01).ANOVA analysis showed significant variations (F value= 25.52718, p≤0.01). The results also agreed with Petro-Turza(1987)but the result of another work found to be noted from 3.44 to 0.54 per cent that may be due to a different cultivar Melkamu et al.,( 2008).Lycopene content fluctuated from 147.6 (mg/100gm) to 429.3 (mg/100gm). The systems of cultivation seem to have a significant influence on the lycopene due to high correlation coefficient(0.788 at p≤0.01) and variance value(F-value=4.53815, p≤0.05).The full maturation and redness of Lycopersicon due to increased synthesis of lycopene pigment. The work of Radzevičius et al.,(2009)also confirmed that the pigmentation directly related with ripening and maturation process. Gonzalez-Cebrino et al.,(2012) reported that lycopene contents changed widely among all investigated cultivars during ripening, increasing significantly from6.57 mg kg-1 (III ripeness stage) to 132.64 mg kg-1 (VI ripeness stage).The ascorbic acid varied from 12.5(mg/100gm) to23.68 (mg/100gm). This value indicates that ripening has a considerable correlation (r=.515**at p≤0.01) as well as in the variance analysis (F-value= 9.443143, p≤0.01). Such type of observation has been reported by investigator Oliveiraet al.,( 2013).The response of polyethene cover can be assessed in two developing stages. The initial phase leads to a developmental pause and abscission but the later stage corresponds with enhances growth and maturation which was marked with fast tissue growth and increase ribosomal as well as enzymatic activities. Polyethene induced maturity and ripening may cause more glycogenolytic metabolic activities. Reduced growth in the initial phase due to wanting of the respiratory substrate. Polyethene may also affect the indigenous secretion of ethylene, the rise of temperature and induced visible light spectrum promoting reduction of time and causes early maturation without affecting texture and quality.
Table 3: Biochemical features of Tomato
| PARAMETERS | Days | |||
| 7 | 14 | |||
| Biochemical constituents | CONTROL | TREATED | CONTROL | TREATED |
| Moisture(%) | 93.81±0.22 | 92.45±0.03 | 92.28±0.02 | 88.15±0.08 |
| Total lipid(%) | 0.81 | 0.92 | 0.88 | 1.17 |
| Total protein(%) | 2.57±0.02 | 2.36±0.05 | 2.20±0.02 | 1.92±0.02 |
| Total sugar(%) | 1.8 | 2.1e | 3.17 | 5.02d |
| Lycopene(mg/100gm) | 147.6 | 218.5 | 264.8 | 429.3 |
| Ascorbic acid(mg/100gm) | 12.5 | 17.4 | 18.5 | 23.68 |
Table 4: Correlation coefficients (r) between polythene and biochemical features
| Parameters | Correlation coefficient® | Significance |
| Polythene & Moisture | -0.097 | ns |
| Polythene & Total lipid | 0.853 | ** |
| Polythene & Total protein | -0.221 | * |
| Polythene & Total sugar | 0.287 | * |
| Polythene & Lycopene | 0.788 | ** |
| Polythene & Ascorbic acid | 0.515 | ** |
*, ** Significantly correlated at 0.05 and 0.01 respectively
Table 5: Analysis of variance (ANOVA )on biochemical feature
| Parameter | Unit | SS | MS | ANOVA
F-Value |
P-Value |
| Moisture | percent | 368.521
|
74.091 | 1.371094NS
|
P≥0.05
|
| Lipid | % | 3.834
|
0.703 | 14.688801**
|
p≤0.01 |
| Protein | % | 217.3771
|
29.70643
|
0.897974 NS
|
P≥0.05
|
| Sugar | % | 6520.935
|
1508.93573 | 25.52718**
|
p≤0.01 |
| Lycopene | mg /100mg | 7.577352
|
1.64332 | 4.53815* | p≤0.05 |
| Ascorbic acid | mg /100mg | 31073.8
|
7010.9153 | 9.443143**
|
p≤0.01 |
Conclusion
The ripening and biochemical composition of fresh tomato depend on cultivars, maturity, light, season, temperature, climate, irrigation, soil fertility, cultural practice etc. besides these factors, the impact of polythene has been well marked. This study has commercial importance as ripening and harvesting at a proper time always give benefit to the farmer and consumers.
Acknowledgment
The authors are extremely grateful to head, Utkal University for providing necessary facilities to the conduct of this experiment.
Funding Source
This research was not supported by any grant or financial supports.
Conflict of Interest
Authors declare no conflicts of interest.
References
- O.A.C. Association of Official Analytical Chemists, Official Methods of Analysis 12th Edition. 1975; Washington. D.C.
- O.A.C. American Association of Agricultural Chemists: Approved Methods of AOAC, 2004; St Paul, Minnesota.
- Ashrafuzzaman M., Hamid A., Ismail M. R. and Sahidullah S. Effect of Plastic Mulch on growth and Yield of Chilli( annuum L.). Braz Arch of Biol and Technol.2011;54(2) : 321- 330.
- Ba-RG P. and Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967; 42: 144-152.
- Beckles M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum Lycopersicum L.) fruit. Postharv. Biol. Technol. 2012; 63:129-140.
- De Souza A.S., Soraia V.B., Magalhães N.F., Ricardo H.V. and Azevedo A.D. Spray- dried tomato powder: Reconstitution properties and colour. Braz Arch of Biol and Technol.2008; 51(4): 807-814.
- Fischer , Kulandaivelul C. , Allal F. and Stephan,W. Adaptation to drought in two wild tomato species: the evolution of the Asr gene family. New Phytol .2011;190: 1032- 1044.
- Fish W.W., Perkins-Veazie P. and Collins J.K. A quantitative assay for lycopene that utilizes reduced volumes of organicsolvents. J of Food comp and Ana.2002; 15:309-317.
- G onzález-Cebrino , Lozano M., Fernández-León A.M. and Ayuso M.C. Effects of ripening stage and cultivar on quality parameters and carotenoids content of six tomato cultivars grown under organic conditions. Acta hort. 2012; (936):71-77.
- Gupta S., Ghuman B.S. and Sandhu K.S. Preparation of tomato powder on small scale J of Food Sci and Technol.2006; 43(1): 31-33.
- Imtiaz K., Komal H., Rasheed A.r, Ashraf K., Muhammad S., Abid F., Ijaz A.and Mukhtar A.Proximate chemical composition of brinjal, Solanum melongena L (Solanales: Solanaceae), genotypes and its correlation with the natural enemies in Peshawar.J of Ent and Zool Studies .2015; 3(5): 07-11.
- Lamont W.,J. Orzolek M.D., Otjen L.and Simpson T.Production of potatoes using plasticulture. Proc. Natl. Agr. Plastics Congr. 2000;29:599–601.
- Melkamu M., Seyoum T., Woldetsadik K. Effects of pre- and post harvest treat- ments on changes in sugar content of tomato. Afr J of Biotechnol.2008; 7(8):1139-1144.
- MSTAT C. Michigan statistics, Microcomputer statisticalprogram, Michigan State university, USA, 1988.
- S. Food analysis laboratory manual. Kluwer Academic/Plenum Publishers,NY.2003; pp 45-49.
- Oliveira B, Moura C. F. H. , Gomes-Filho E. and Marco C. A. The Impact of OrganicFarming on Quality of Tomatoes Is Associated to Increased Oxidative Stress during Fruit Development: PLoS ONE.2013; 8(2):e5635:1-6.
- Orzolek D.New Concepts in Plasticulture for Tomatoes and Peppers,Th Pennsylvania StateUniversity, University Park, PA. 2000; 863-115.
- Opadotun O.O. Adekeye S.A. Ojukwu E.O. and Adewumi A.A.Comparative Analysis of Nutritional Values of Tomatoes Subjected to Different Drying Conditions. Int J of Basic and ApplSci.2016;5(1):6-9.
- Page , Gouble B., Valto B., Bouchet J., Kretzchmar A., Causse M., Renard C. and Faurobert, M. Protective proteins are differentially expressed in tomato genotypes differing for their tolerance to low-temperature storage. Planta. 2010 ; 232: 483-500.
- Petro-Turza M.Flavor of tomato and tomato products. Food Rev Int .1987;2(3):309-351.
- R adzevicius A., Karklelienė R., Viskelis P., Bobinas C., Bobinaitė R., and Sakalauskie S. Tomato (Lycopersicon esculentum Mill.) fruit quality and physiologica parameters at different ripening stages of Lithuanian cultivars. Agro res,2009; 7(2):712-718.
- Rajablariani R., Hassankhan F. and Rafezi R. Effect of colored plastic mulches on yield of tomato and weed biomass. Int J of Env Sci and Dev.2012:3(6) 590-593.
- Ranganna S. Mannul of Analysis of Fruit and Vegetable Products. Tata Mc Grow Hill Publishing Co. Ltd., 1979; New Delhi..
- Ranganna S. Hand Book of Analysis and Quality Control for Fruit and Vegetable Products, 2nd Ed. Tata McGraw Hill. 1986; New Delhi.
- Srivastava S. and Kulshreshtha K. Nutritional Content and Significance of Tomato Powder. Annal of Ari Zone. 2013;52(2); 121-124.
- Singh B. and Chaubey T. Vegetable research in India-An overview. Prog Hort. 2013; 45:9- 35.
- Tyssandier V. Feillet-Coudray C. Caris-Veyrat C. and Guilland G. Effect of Tomato Product Consumption on the Plasma Status of Antioxidant Microconstituents and on the Plasma Total Antioxidant Capacity in Healthy Subjects. J Am Coll N .2004;23(2):148-56.
- Wang M., Jiang P. and YU K. Effects of exogenous epibrassinolide on photosynthetic characteristics in tomato (Lycopersicon esculentum Mill) seedlings under weak light stress. J Agr Food Chem.2010;58: 3642-3645.

This work is licensed under a Creative Commons Attribution 4.0 International License.





