How to Cite | Publication History | PlumX Article Matrix
Department of Biological Sciences, College of Sciences, Northern Border University, Arar, Saudi Arabia.
Corresponding Author E-mail : alshrari@live.com
DOI : http://dx.doi.org/10.13005/bbra/2861
ABSTRACT:
The Human Papillomavirus (HPVs), especially the high-risk HPVs, are firmly connected with cervical cancer. This research aims to evaluate the knowledge and attitudes toward cervical cancer and HPVs for scanning and prevention. A cross-sectional survey was performed among 434 Health College students in the Northern region of Saudi Arabia to obtain this information. In the present study, 402 students were encompassed in the final analysis. The results revealed that the estimated necessary knowledge about HPV and cervical cancer was 31.07%. The medical knowledge concerning the treatment of HPVs and cervical cancer was 30.98%. The pharmaceutical knowledge was 29.35%, wherein the knowledge about the HPV vaccine was only 8%. The most common barrier preventing the students from receiving the HPV vaccine was inadequate available information (22.13%). The present study showed an insufficient degree of understanding concerning HPV and cervical cancer among students of the Health College in the Northern region of Saudi Arabia. It is recommended that there is a requisite for educational involvement and awareness fights to increase HPV and Cervical Cancer essential knowledge awareness. It is also suggested that vaccines should be granted for mass scale practice and should be incorporated in the national immunization drive of the country for dropping the problem of cervical cancer.
KEYWORDS: Awareness; Cross-Sectional Study; Cervical Cancer; Human Papillomavirus (HPV); Northern Border University
Download this article as:| Copy the following to cite this article: Ahmed S. A. Knowledge and Awareness toward Human Papillomavirus (HPV) and Cervical Cancer among Health College Students in the Northern Region of Saudi Arabia. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Ahmed S. A. Knowledge and Awareness toward Human Papillomavirus (HPV) and Cervical Cancer among Health College Students in the Northern Region of Saudi Arabia. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/3gKGa7T |
Introduction
The human papillomavirus (HPVs) infection is implicated in the development of cervical cancer. Continuing infection by the high-risk HPV (specifically, type 16) can initiate cancer of the vulva, cervix, vagina, anus, oropharynx, and penis (Crosbie et al. 2013; Bernard et al. 2010). The connection between cervical cancer and HPVs infection is well recognized (Sundstrom et al. 2019; Chen et al. 2019; Almazrou et al. 2020; Jradi and Bawazir, 2019). In 2018, a total of 570,000 incidences of HPVs infection-related cancer appeared, whereas 311,000 mortalities were acknowledged. It has also been recognized that cervical cancer ranks the second most common form of cancer in females of 15-44 years group (https://gco.iarc.fr/today). Cervical cancer is the ninth most frequently diagnosed cancer among the Saudi women of 15-44 years group (Jradi and Bawazir, 2019; https://gco.iarc.fr/today; Bruni et al. 2019). In 2018, 316 Saudi women were diagnosed with cervical cancer, wherein 158 resulted in death (World Health Organization, 2017). According to literature (Turki et al. 2013), the prevalence of the HPV infection is escalating in a worrying proportion in Saudi Arabia. Three preventive vaccines (Table 1) for young girls (9-13 years), and young adults (13-126 years) have been introduced (Sundstrom et al. 2019; Markowitz et al. 2007; Rashid et al. 2016; Harper et al. 2004; Villa et al. 2005). It also noted that the available vaccines could protect more than 2/3rd of the population against HPV-16 and HPV-18 genotype infections (Alsbeih, 2014; Gosadi, 2019; Hussain et al. 2016; Doorbar, 2006). An increase in the degree of perception and the elementary familiarity of the disease, along with its available vaccines, helps to control and prevent the disease. Similarly, the outcome of the present study of the subject matter can help control the prevalence of cervical cancer caused by HPV in Saudi Arabia. Accordingly, the author aimed to perform the titled survey in the Northern Region of Saudi Arabia.
Table 1: Types of HPV vaccines
| Name | Valency | Specific HPV | Cervical cancer cases caused by specific HPV | Genital warts caused by specific HPV |
| Cervarix® | Bivalent
(2 types) |
16, 18 | 71% | No |
| Gardasil® | Quadrivalent (4 types) | 6, 11,
16, 18 |
71% | 90 % |
| Gardasil 9® | Nonavalent (9 types) | 6, 11, 16, 18, 31, 33, 45, 52, 58 | 90 % | 90 % |
Methodology
This study was undertaken from September 2016 to November 2017 at the Northern Border University (NBU, Saudi Arabia), which is located in the Northern Region of Saudi Arabia. Four health colleges, namely, The College of Medicine, The College of Nursing, The College of Pharmacy, and the College of Applied Medical Sciences, located in Arar and Rafha cities were included in the present study. The affiliated students of the university were from the urban areas as well as the rural areas, but the quasi-totalities were belonging to urban areas. This cross-sectional study was performed by a pre-tested questionnaire (Table 2), which was shared with the students after their consent. A total of 434 students participated in this study, wherein 258 girl students and 144 boy students (18-26 years) contributed to the study. The collected records from the survey were analyzed anonymously by allocating arbitrary codes.
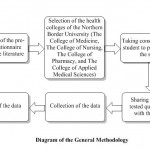 |
Diagram 1: Diagram of the General Methodology |
Results
Of the 402 students interviewed, the number of girls was 258 and 144 boys (64.18% and 35.82 % respectively). The results are summarized as the number of answers given by the respondents for each question (Table 2). A total of 8040 answers were presented by the interviewed students (20 x 402 = 8040), wherein 2625 (32.64%) answered as ‘‘Don’t know’’. Accordingly, it can be assumed that every third participant student was unaware of HPV and cervical cancer. Moreover, 46.26 % (186 students) of the total number of students never heard about cervical cancer. Human papillomavirus (HPV) as a correct answer for the possible causes of cervical cancer is known by 133 students (33.08 %), and 24.62% of the students answered that HIV causes cervical cancer. The knowledge about prevention and vaccine for cervical cancer showed similar values among the students.
Table 2: The pre-test questionnaire and the obtained results (N = 402)
| S. No. | Question | Options | Number of response (Percentage) |
| 1 | Have you ever heard about cervical cancer? | Yes | 216 (53.73) |
| No | 186 (46.26) | ||
| 2 | Cervical cancer is known to be caused by a high risk of….? | Human Immunodeficiency Virus (HIV) | 99 (24.62) |
| Methicillin-Resistant Staphylococcus aureus (MRSA) | 31 (7.71) | ||
| Hepatitis B virus (HBV) | 27 (6.71) | ||
| Neisseria meningitidis (NM) | 18 (4.47) | ||
| Human papillomavirus (HPV) | 133 (33.08) | ||
| Candida Albicans (CA) | 22 (5.47) | ||
| None of this | 72 (17.91) | ||
| 3 | Cervical cancer is preventable. | True | 167 (41.54) |
| False | 68 (16.92) | ||
| Don’t know | 167 (41.54) | ||
| 4 | Is there any vaccine available for cervical cancer? | Yes | 107 (26.62) |
| No | 113 (28.11) | ||
| Don’t know | 182 (45.27) | ||
| 5 | What do you think HPV causes? | Respiratory tract infections (RTIs) | 24 (5.97) |
| A skin rash | 29 (7.21) | ||
| A sexually transmitted disease | 180 (44.78) | ||
| A liver cancer | 22 (5.47) | ||
| None of these don’t know | 18 (4.48) | ||
| Don’t know | 129 (32.09) | ||
| 6 | Is there Any Vaccine available for HPV? | Yes | 156 (38.81) |
| No | 69 (17.16) | ||
| Don’t know | 177 (44.03) | ||
| 7 | Antibiotics can cure HPV | Yes | 114 (28.36) |
| No | 127 (31.59) | ||
| Don’t know | 161 (40.05) | ||
| 8 | HPV is transmitted | Yes | 216 (53.73) |
| No | 56 (13.93) | ||
| Don’t know | 130 (32.34) | ||
| 9 | If it transmitted, HPV is mainly transmitted through | Not transmitted | 38 (9.45) |
| Aerosols of respiratory droplets | 14 (3.48) | ||
| Contaminated surfaces | 11 (2.74) | ||
| Sexual contact | 144 (35.82) | ||
| Mosquitoes | 9 (2.24) | ||
| All of these | 72 (17.91) | ||
| None of these | 5 (1.24) | ||
| Don’t know | 109 (27.11) | ||
| 10 | HPV can infect both males and females | True | 192 (47.76) |
| False | 101 (25.12) | ||
| Don’t know | 109 (27.11) | ||
| 11 |
If there is a vaccine for HPV, is it available in Saudi Arabia? |
Yes | 75 (18.66) |
| No | 95 (23.63) | ||
| Don’t know | 232 (57.71) | ||
| 12 | The Vaccines treat HPV infection and HPV-associated disease | True | 125 (31.09) |
| False | 73 (18.16) | ||
| Don’t know | 04 (50.75) | ||
| 13 | Which age group HPV vaccine should be given? | 0-5 y | 23 (5.72) |
| 5-9 y | 27 (6.72) | ||
| 9-20 y | 49 (12.19) | ||
| 20-30 y | 85 (21.14) | ||
| 30 & above | 64 (15.92) | ||
| Don’t know | 154 (38.31) | ||
| 14 | Who can get the HPV vaccine? | Male | 13 (3.23) |
| Female | 129 (32.09) | ||
| Both | 160 (39.8) | ||
| Don’t know | 100 (24.88) | ||
| 15 | Do Individuals need to be screened for HPV before getting vaccinated? | Yes | 166 (41.29) |
| No | 57 (14.18) | ||
| Don’t know | 179 (44.53) | ||
|
16 |
Available cervical cancer screening test? | Pap test and liquid-based cytology (LBC) | 60 (14.93) |
| Visual inspection with Acetic Acid (VIA) | 25 (6.22) | ||
| HPV testing for high-risk HPV types | 49 (12.19) | ||
| All of these | 85 (21.14) | ||
| None of these | 17 (4.23) | ||
| Don’t know | 66 (41.29) | ||
|
17 |
Can it (vaccine) be given to a woman already having an HPV infection? | Yes | 97 (24.13) |
| No | 120 (29.85) | ||
| Don’t know | 185 (46.02) | ||
|
18 |
How many doses of the HPV vaccine are required? | Zero | 11 (2.74) |
| One | 56 (13.93) | ||
| Two | 35 (8.71) | ||
| Three | 37 (9.20) | ||
| Four & above | 22 (5.47) | ||
| Don’t know | 241 (59.95) | ||
|
19 |
What do you think will be the most important obstacle preventing yourself to receive/advice HPV vaccination? | High cost | 54 (13.43) |
| Worry about the efficacy of the vaccine | 45 (11.19) | ||
| Availability of vaccine | 65 (16.17) | ||
| Worry about complications | 75 (18.66) | ||
| Inadequate information | 89 (22.14) | ||
| Above the age | 74 (18.41) | ||
|
20 |
What are your sources of knowledge and information on HPV-associated disease and prevention? | Medical school teachings | 153 (38.06) |
| Newspaper | 9 (2.24) | ||
| Internet | 81 (20.15) | ||
| Friends | 27 (6.72) | ||
| Books | 27 (6.72) | ||
| Television | 7 (1.74) | ||
| Don’t hear about it | 98 (24.38) |
Furthermore, only 167 students (41.54%) were sure that cervical cancer is preventable, whereas 235 students (58.45%) did not know or supposed that cervical cancer is not avoidable. The majority of the participants (61.19%) were unaware of the available vaccine for HPV, while 38.80% were aware that this type of cancer is preventable by vaccine. It has also been observed that only 75 students (18.66%) knew that the vaccine for HPV is available in Saudi Arabia. Question 12 was related to the effect of available vaccines against HPV and associated illness. The data collected from the questionnaire showed that 31.09% of students knew that vaccines could treat HPV infection and HPV-associated disease, whereas 68.90% did not know about it.
According to the World Health Organization (WHO), Merck’s Gardasil received approval from the US Food and Drug Administration (FDA) in 2006. Shortly afterward, it was provisionally recommended for girls and women aged nine to 26 years. In the present study, only 49 students (12.19%) were able to reveal the correct age group for which the HPV vaccines have been approved, whereas 38.30% did not know the ideal age for such vaccines. About 39.8% of respondents answered that both males and females could get the HPV vaccines, 3.23% supposed that only a male can get vaccines, and 32.09% revealed that only a female could get these vaccines. It is well known that ‘‘HPV vaccination combined with regular screening is the best strategy to avoid developing cervical cancer (World Health Organization, 2017). However, no health checkup is needed before getting the vaccine. The percentage of students who were aware of it was 14.18%, whereas others did not know the answer (44.53%) or gave the wrong answer (41.29%).
The cervical cancer screening test is recommended in women aged > 30 years every five years, including a combined check of cytology and HPV testing (co-testing). The standard cervical cytology test is recommended alone every three years (Saslow et al. 2012;
Moyer and U.S. Preventive Services Task Force, 2012; Domgue et al. 2019). One query focused on the awareness of the interviewed students about the cervical cancer screening test. The results were varied from don’t’ know (41.29%) to Pap test (14.93%) and HPV test (12.19%). Once infected by the HPV virus, will it be possible getting the vaccine? The majority of participants (46.02) were not aware of it, 24.13% responded that it is possible, whereas 29.85% did not believe in HPV vaccine efficacy in HPV post-infected women. The WHO recommends that two doses of an HPV vaccine be given to 9-14 years old girls as a priority (World Health Organization, 2017). It was surprising that only 8.71% of students gave the correct answer, whereas others either provided incorrect answers or did not know about vaccine doses. The most critical obstacle preventing the participants from receiving/advising HPV vaccination was inadequate information (22.14%). This was followed by the worry about the complications (18.66%), above the age (18.41), vaccine availability (16.17%), high cost, and the concern about the efficacy of the vaccine. It has also been identified that 38.06% of students consider medical school teaching as the primary source of information about HPV associated diseases and prevention. This was followed by the internet (20.15), books & friends (6.72%), newspaper (2.24), and television (1.74%). A large part of the students (24.38%) did not hear about it.
Discussion
Cervical cancer is the ninth most frequently diagnosed cancer among Saudi women of the 15-44 age group (Jradi and Bawazir, 2019; Bruni et al. 2019). About 316 Saudi women had cervical cancer in 2018, wherein 158 died of this disease (World Health Organization, 2017). Cervical cancer is preventable as well as curable if detected early (Rashid et al. 2016; Alsbeih, 2014). Mostly advanced-stage cases are reported in Saudi Arabia due to inaccessible screening tests (Alsbeih, 2014; Dosoky et al. 1995; Manji, 2000). The age-standardized incidence rate of cervical cancer cases attributable to HPV (per 100,0000 women) in Saudi Arabia is relatively low as compared to other countries (2.5 in Saudi Arabia, 1.9 for Yemen, 6.4 for UAE, 8.4 for UK and 75.3 in Swaziland) (Bruni et al. 2019). In 2013, the WHO had reported that 6.51 million Saudi women older than 15 years are at risk of developing cervical cancer (World Health Organization, 2017), and HPV-16 and HPV-18 cause 70% of the worldwide cervical cancers. The HPV-16 (30%), HPV-18 (8.0%), and HPV-45 (5%) are the most prevalent genotypes reported in Saudi Arabia (Turki et al. 2013).
A combination of knowledge, awareness, educational programs, HPV screening, and HPV vaccination can reduce and control cervical cancer cases (Rashid et al. 2016). Accordingly, the present study aimed to get information about the level of awareness and knowledge toward cervical cancer, HPV, and attitude to HPV vaccination among health college students. We observed a persistent answer ”don’t know” and the present cross-sectional study and about half of the interviewed students did not hear at all about cervical cancer.
Globally, the awareness level among students about cervical cancer and HPV is weak. The knowledge regarding the prevention and vaccine is also found to be very poor. This is alarming because the concept of the protection against this type of cancer is primarily built upon the procurement of necessary and basic understanding and awareness toward HPV and cervical cancer. In the present study, 61.19% of the interviewed students assumed that there is no available vaccine for HPVs; 18.66% knew that there is an available vaccine against HPV in Saudi Arabia; 39.8% answered that both sexes could get the vaccine. This is the first report undertaken to understand the awareness about cervical cancer and HPV in the Northern Region of Saudi Arabia. However, previous studies have been conducted in other regions of the country like Riyadh (Almazrou et al. 2020; Jradi and Bawazir, 2019). A lack of awareness about HPV, cervical cancer, and the HPV vaccine among the studied women groups in Riyadh has been reported (Jradi and Bawazir, 2019). This study further elaborated that the health care providers (other than physicians) (30%) were not aware of the preventive measures, wherein 63.3% did not exercise any screening procedure for cervical cancer because of the lack of screening facilities. This study corroborates our finding, and the reason for the reduced level of the HPV, cervical cancer, and the HPV vaccine are well harmonized (Jradi and Bawazir, 2019). Among other reasons, misconception, non-availability of the educational programs at the academic and community level, and the socio-cultural factors (modesty and decency) may explain the reduced level of awareness among participants about the HPV, cervical cancer and its vaccine. The lack of information about HPV linked to the lack of knowledge of the vaccines is one of the numerous barriers that prevent vaccination recommendation.
Conclusion
This study exposed a reduced level of knowledge about HPV and cervical cancer among students of the Health Colleges in the Northern Border University. It is imperative to improve awareness about this subject among the participant students. Further, it is believed that there exists no national HPV immunization program in Saudi Arabia. However, such programs are running successfully in many countries, for example, in the USA (National Breast and Cervical Cancer Early Detection Program, NBCCEDP), in UK (Cervical screening), in Australia (The National Cervical Screening Program). It is suggested that the National Transformation Program to ensure the realization of Kingdom’s Vision 2030 in the domain of Public Health System and Health Disasters Management will be the adequate framework for incorporating The ‘‘Saudi Arabian Cervical Screening Program: SACSP’’.
Acknowledgment
The author, thanks to all the students of the Northern Border University, who participated in this study.
Conflict of Interest
The author declares that there is no conflict of interest.
Funding Source
There is no funding source.
References
- Crosbie E. J., Einstein M. H., Franceschi S., Kitchener H. C. Human papillomavirus and cervical cancer. Lancet. 2013; 382(9895): 889‐899.
- Bernard H. U., Burk R. D., Chen Z., van Doorslaer K., zur Hausen H., de Villiers E. M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010; 401(1): 70‐79.
- Sundstrom B., Smith E., Delay C., Luque J. S., Davila C., Feder B., Paddock V., Poudrier J., Pierce J. Y., Brandt H. M. A reproductive justice approach to understanding women’s experiences with HPV and cervical cancer prevention. Soc. Sci. Med. 2019; 232: 289-297.
- Chen J., Deng Y., Ao L., Song Y., Xu Y., Wang C. C., Choy K. W., Tony Chung K. H., Du Q., Sui Y., Yang T., Yang J., Li H., Zou C., Tang, T. The high-risk HPV oncogene E7 upregulates miR-182 expression through the TGF-β/Smad pathway in cervical cancer. Cancer lett. 2019; 460: 75-85.
- Almazrou S., Saddik B., Jradi H. Knowledge, attitudes, and practices of Saudi physicians regarding cervical cancer and the human papilloma virus vaccine. J. Infec. Public Health. 2020; 13(4): 584-590.
- Jradi H., Bawazir, A. Knowledge, attitudes, and practices among Saudi women regarding cervical cancer, human papillomavirus (HPV) and corresponding vaccine. Vaccine. 2019; 37(3): 530-537.
- https://gco.iarc.fr/today (Accessed on March 1, 2020)
- Bruni L., Albero G., Serrano B., Mena M., Gómez D., Muñoz J., Bosch F. X., de Sanjosé S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 17 June 2019. 2019 [https://www.hpvcentre.net/statistics/reports/XWX.pdf; Accessed on March 1, 2020].
- World Health Organization. Questions and answers about HPV vaccination: Information for parents and caregiver. 2017: 1-17. (http://www.euro.who.int/__data/assets/pdf_file/0009/356841/Q-and-A_HPV_Parents_EN.pdf; Accessed on March 1, 2020)
- Turki R., Sait K., Anfinan N., Sohrab S., Abuzenadah, A. Prevalence of Human Papillomavirus in Women from Saudi Arabia. Asian Pac. J. Cancer Prev. 2013; 14(5): 3177-3181.
- Markowitz L. E., Dunne E. F., Saraiya M., Lawson H. W., Chesson H., Unger E. R., Centers for Disease Control and Prevention (CDC), & Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommend. Reports. 2007; 56: 1-24.
- Rashid S., Labani S., Das B. C. Knowledge, Awareness and Attitude on HPV, HPV Vaccine and Cervical Cancer among the College Students in India. PLoS One. 2016; 11(11): e0166713.
- Harper D. M., Franco E. L., Wheeler C., Ferris D. G., Jenkins D., Schuind A., Zahaf T., Innis B., Naud P., De Carvalho N. S., Roteli-Martins C. M., Teixeira J., Blatter M. M., Korn A. P., Quint W., Dubin G., GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004; 364(9447): 1757-1765.
- Villa L. L., Costa R. L., Petta C. A., Andrade R. P., Ault K. A., Giuliano A. R., Wheeler C. M., Koutsky L. A., Malm C., Lehtinen M., Skjeldestad F. E., Olsson S. E., Steinwall M., Brown D. R., Kurman R. J., Ronnett B. M., Stoler M. H., Ferenczy A., Harper D. M., Tamms G. M., Barr E. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005; 6(5): 271-278.
- Alsbeih G. HPV Infection in Cervical and Other Cancers in Saudi Arabia: Implication for Prevention and Vaccination. Front. Oncol. 2014; 4: 65.
- Gosadi I. M. National screening programs in Saudi Arabia: Overview, outcomes, and effectiveness. J. Infect. Public Health. 2019; 12(5): 608-614.
- Hussain A., Alkhenizan A., McWalter P., Qazi N., Alshmassi A., Farooqi S. Abdulkarim A. Attitudes and perceptions towards HPV vaccination among young women in Saudi Arabia. J. Family Community Med. 2016; 23(3): 145-150.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. (Lond.). 2006; 110(5): 525-541.
- Saslow D, Solomon D., Lawson H. W., Killackey M., Kulasingam S. L., Cain J., Garcia F. A., Moriarty A. T., Waxman A. G., Wilbur D. C., Wentzensen N., Downs L. S., Jr Spitzer M., Moscicki A. B., Franco E. L., Stoler M. H., Schiffman M., Castle P. E., Myers E. R., ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012; 62(3): 147-172.
- Moyer V. A., U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012; 156(12): 880-891.
- Domgue J. F., Cunningham S. A., Yu R. K., Shete, S. Prevalence and determinants of cervical cancer screening with a combination of cytology and human papillomavirus testing. Ann. Epidemiol. 2019; 36: 40-47.
- Dosoky M., Ismail N., Dagastani M. Preinvasive cervical carcinoma in Saudi Arabia. Lancet. 1995; 345(8950): 650.
- Manji M. Cervical cancer screening program in Saudi Arabia: action is overdue. Ann. Saudi Med. 2000; 20(5-6): 355-357.

This work is licensed under a Creative Commons Attribution 4.0 International License.






