How to Cite | Publication History | PlumX Article Matrix
Taoufik Hakim1, Souad Lekchiri1, Mohamed El Amine Afilal2, Mostafa Ellouali1, Hafida Zahir1 and Hassan Latrache1*
1Bioprocess and Biointerfaces Laboratory, Faculty of Sciences and Techniques, Sultan Moulay Slimane University, Beni Mellal, Morocco.
2Biochemistry and Biotechnology Laboratory, Mohamed First University, Oujda, Morocco.
Corresponding Author E-mail: latracheh@yahoo.fr
DOI : http://dx.doi.org/10.13005/bbra/2856
ABSTRACT: The choice of the best support for microbial adhesion can improve the start-up speed and efficiency of dairy wastewater treatment by biofilm bioreactors. In this study, three substrates were tested: PP (polypropylene), PET (Polyethylene terephthalate), and PVC (polyvinyl chloride). By using the contact angle method, the surface physicochemical characteristics of the bacterium, inert substrates, and substrates after dairy wastewater (DWW) conditioning film were measured to understand its impact on adhesion as well as the most suitable material to optimize bacterial adhesion. DWW conditioning film affects the physicochemical characteristics of plastic supports and improves the initial adhesion of bacteria to substrates. Results of initial adhesion tests for untreated and treated supports showed differences in how bacterial cells adhered to substrates. Before treatment, PVC and then PP showed a significant adhesion capacity, double that of PET. After modifying by DWW, initial bacterial adhesion increased by 106 (105 to 1011 CFU/cm2) and PVC demonstrated the highest adhesion capacity, followed by PP and finally PET. Therefore, before the modification of the supports by DWW, PP and PVC are in the same rank for the initial bacterial adhesion and after the modification, PVC seems to be the best for initial bacterial adhesion.
KEYWORDS: Conditioning film; Dairy wastewater; Initial adhesion; Physicochemical properties
Download this article as:| Copy the following to cite this article: Hakim T, Lekchiri S, Afilal M. E, Ellouali M, Zahir H, Latrache H. Modifying Supports Surfaces by Dairy Wastewater Conditioning Film and Relationship with Initial Bacterial Adhesion. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Hakim T, Lekchiri S, Afilal M. E, Ellouali M, Zahir H, Latrache H. Modifying Supports Surfaces by Dairy Wastewater Conditioning Film and Relationship with Initial Bacterial Adhesion. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/2GOoax7 |
Introduction
The dairy industry represents an economic and nutritional interest for the sums it generates and dairy products are very important for human nutrition. But this industry generates a lot of wastewater. The quantity of wastewater varies between 0.2 and 10 L for one liter of treated milk1. Dairy wastewater (DWW) is highly loaded with organic matter2.
Treatment of this wastewater is becoming an absolute necessity for environmental protection. There are several methods of treating DWW to reduce its organic matter loading: electrochemical3, membrane filtration4, reverse osmosis5,coagulation-flocculation 6, land treatment7, and biological treatments such as anaerobic biofilm reactor8. Among the anaerobic bioreactors, it is worth citing the anaerobic digester, which not only produces little sludge but also generates bio-methane used as an energy source. After anaerobic digestion begins, biogas detection only takes place from the second week of start-up 9 due to the time required to accumulate a sufficient amount of biomass to treat the organic matter. Often, when the content is renewed in a bioreactor, some of the biomass is lost through the effluent, which delays the bioprocess 10. The use of supports inside the reactors allows the microorganisms to bind to support surfaces, form a biofilm, and increase the surface area of contact between the microorganisms and the organic material. Supports, also, can help in maintaining the biomass that may be lost at a reactor outlet and increasing the start-up speed of the bioreactor after the renewal of its contents.
Many different studies have concluded that physicochemical characteristics, roughness, pH, ionic strength, and porosity play an important role in the initial adhesion of bacteria to substrates 10–16. One aspect of microorganisms’ adhesion to bio carriers that has received little attention is the conditioning film phenomenon: the deposit of nutrients on material surfaces when immersed in a liquid medium17. Substances in organic materials such as sugars and proteins can adsorb to surfaces, forming a conditioning film and affecting physicochemical characteristics, roughness, surface charge, and wettability. The conditioning film, in turn, affects the adhesion of bacteria to the surface17.
To our knowledge, no work has highlighted the effect of DWW conditioning film or, more generally, any organic material on the supports in the bioreactor for wastewater treatment. In this work, our objective is to compare 3 plastic supports before and after treatment with DWW, demonstrate how the DWW conditioning film modifies both the bio carriers’ physicochemical characteristics and the bacterium adhesion to substrate surfaces treated and untreated with DWW.
Materials and Methods
Bacterial strain and the preparation of a bacterial suspension
The bacterial strain used as a biological model is an optional anaerobic Gram-positive bacterium from a laboratory digester. This bacterium is added to the consortium of microorganisms in the anaerobic digester to increase the quantity of biogas 18. This strain is grown in a liquid Luria Bertani medium (LBL) at 37°C for 24 hours. The bacteria are then collected by centrifugation (5000 g for 15 min), washed twice with a solution of KNO3 at 0.1 M of ionic strength. Finally, the bacterial suspension was prepared with a solution of KNO3 (0.1M).
Plastic Supports
Three ordinary plastic carrier materials were selected for their low cost, durability, and availability and because they are commonly used as mobile carrier materials in anaerobic digesters: polyethylene terephthalate (PET:(C10H8O4)n), polypropylene (PP:(C3H6)n), and polyvinyl chloride (PVC:(C2H3Cl)n). Each plastic material was cut into 1.5 cm2 square coupons for contact angle measurement experiments, and into 1 cm2 square for adhesion tests. The plastic supports were immersed in ethanol for 15 min to disinfect and remove dirt from their surface and subjected to a sonication bath in sterile distilled water for 10 min. The coupons were rinsed several times with sterile distilled water. Then they were dried in a sterile area before being stored in a sterile condition for later use.
Contact angle measurement
The Bacteria
The method for measuring contact angles on bacterial layers has been described by Busscher et al 19. Briefly, the prepared bacterial suspension is deposited on a cellulose acetate filter (0.45 μm) using a filtration ramp, producing a bacterial mat whose thickness probably represents 50 to 100 cells. This film is placed on a glass support and allowed to evaporate. The contact angle is then measured. Water (w), formamide (f), and diiodomethane (d) were used as reference solvents. A drop is formed at the end of a syringe to be deposited on the sample surface. A sequence of digital images is immediately acquired (Windrop) using a CCD camera placed on a goniometer (GBX Instruments, France). Three measurements are made for each sample and for each solvent. The experiment is repeated three times. The free surface energies are determined: the Lifshitz-Van der Waals γLW, electron acceptor γ+, and electron donor γ– using the equation (1) of Van Oss et al20. In this approach the contact angles (θ) can be expressed as in Equation (1).
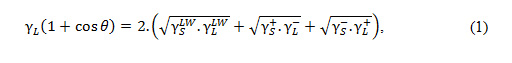
And the quantitative hydrophobicity can be estimatedby using Equation (2).
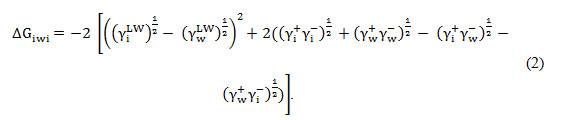
The supports with and without conditioning film
The effect of DWW conditioning film on supports’ physicochemical characteristics was studied by comparing the physicochemical characteristics of these plastic materials before and after their treatment with DWW. Treatment consisted of immersing the materials in DWW for 3 hours at 37°C and then drying in a sterile area.
For untreated supports, the contact angle was measured after cleaning, disinfecting, and drying. For treated supports, the contact angle was measured after treatment with DWW. The basic principle is the same as for bacteria. The contact angles of the supports were measured using the sessile drop technique of the three probe liquids of different polarity and with known surface energy.
Initial Adhesion Tests
Ten ml of bacterial suspension containing approximately 108 CFU/ml was incubated in a petri dish containing PET, PP, PVC coupons (cleaned and disinfected according to the protocol described above) untreated and treated by sterile DWW for 3 hours at 37°C. After incubation, the coupons were then rinsed three times with sterile distilled water to remove non-adherent bacteria. The plastic coupons were immersed in test tubes containing sterile physiological water (NaCl: 9 g/l). The bacterial cells were detached from the inert supports using a sonication bath (ultrasonic) for 5 min21. The adhered bacteria were harvested by the sonication method and CFUs were counted using the serial dilution technique of the bacterial suspension (dilution up to 10-3 in the case of untreated supports and 10-9 for supports treated with DWW).
Results and Discussion
Surface Energy Cmponents of the Bacterium
Qualitative hydrophobicity θw is a measure of the contact angle between a bacterial surface and a drop of water and quantitative hydrophobicity ΔGiwi is the free energy of interaction between any substance (i) and water (w). The contact angle measurements of the bacterium were taken and then used to determine the surface energy components (Table 1).
Table 1: Contact angle and surface energy components of the bacterium
| Contact angle (°) | Surface energy (mJ/ m2) | |||||||
| Surface | θd | θf | θw | γLW | γ+ | γ– | γAB | ΔGiwi |
| Bacterium | 43.5 | 38.6 | 33.8 | 37.5 | 0.59 | 50.62 | 10.93 | 31.1 |
| Std. dev. | 1.5 | 2 | 1.4 | 0.8 | 0.1 | 4.5 | ||
Bacterium43.538.633.8 37.50.5950.6210.9331.1Std. dev.1.521.4 0.80.14.5d = diiodomethane, f = formamide and w = waterγLW surface energy of Lifshitz-Van der Waals, γ+ electron acceptor, γ– electron donor, the γAB Lewis acid–base surface tension and ΔGiwi: the free energy of interaction between two entities of that material when immersed in waterStd. dev.= Standard deviationIn view of the results obtained, our bacterium has a hydrophilic character qualitatively (θw = 33.8°<65°)22. A quantitative approach affirms this result, finding that the strain tested has a positive free surface energy (ΔGiwi = 31.1 mj/m2>0)20. Moreover, this strain has a strong electron donor character (γ–= 50.62 mj/m 2), whereas the electron acceptor properties are very low (γ + = 0.59 mj/m2). In light of these results, Latrache et al 23have shown that the hydrophobicity measured by the contact angle is directly correlated with the high N / C ratio and inversely correlated with that of O / C ratio and have also shown that the hydrophilicity of Escherichia coli is related to the presence of polysaccharides, while hydrophobicity is related to the presence of proteins. Also, the carboxyl and phosphate groups contribute to the negative charges present at Escherichia coli cell surface24.
Plastic supports’ physicochemical charactersThe contact angle measurements for the different plastic supports were taken before and after the supports were treated with DWW and then used to determine the surface energy components (Figure 1).All the supports (U) are hydrophobic and have agreat qualitative hydrophobicity (θw(PETU) = 80.9°; θw (PPU) = 77.8°; θw (PVCU) = 73.3°) (Figure 1.a). Like the qualitative approach (θw), the quantitative hydrophobicity (∆Giwi) of the three supports havea hydrophobic character (∆Giwi (PETU)= -63.2mJ/m²; ∆Giwi (PPU)= -42.3mJ/m²; ∆Giwi (PVCU)=-48.5mJ/m²) (Figure 1.b). The electron donor γ– character for the three untreated supports have a feeble valor ( γ–(PETU)= 4.9mJ/m²; γ–(PPU)= 4.1 mJ/m²; γ–(PVCU)= 5.3mJ/m²) (Figure 1.c) and the valor of electron acceptor character is very feeble ( γ+(PETU)= 0 mJ/m²; γ+(PPU)= 0.25mJ/m²; γ+(PVCU)= 1 mJ/m²) (Figure 1.d).
 |
Figure 1: Physicochemical characteristics of supports (PET, PP, and PVC) untreated (U) and treated (T) with OMWW. (a) Qualitative hydrophobicity; (b) Quantitative hydrophobicity; (c) Electron donor property; (d) Electron acceptor property; U Untreated; T Treated with DWW. |
After treatment with DWW , the support surfaces show a change in their qualitative hydrophobicity θw (θw (PETT) = 78.97°; θw (PPT) = 100.84°, θw (PVCT) = 93.90°) (Figure 1.a) and quantitative hydrophobicity ∆Giwi (∆Giwi (PETT)=-39.08 mj/m²; ∆Giwi (PPT)=-79.03 mj/m²; ∆Giwi (PVCT)=-79.03 mj/m²) (Figure 1.b). The DWW conditioning film increased the valor of the electron donor γ– character for PET ( γ–(PETT)= 13.28 mJ/m²) and decreased the valor for PP and PCV (γ–(PPT)= 0.60 mJ/m²; γ–(PVCT)= 2.26 mJ/m²) (Figure 1.c), while the valor of electron acceptor character remained very feeble with a slight increase for PET and small decrease por PP and PVC ( γ+(PETT)= 0.25 mJ/m²; γ+(PPT)= 0.55 mJ/m²; γ+(PVCT)= 0.06 mJ/m²) (Figure 1.d).Initial adhesion tests of a bacterium to supports treated and untreated with DWWThis section presents results regarding the adhesion power of the selected bacterial strain to several supports that differ by their physicochemical characteristics and by whether they were conditioned in DWW. The bacterial strain’s ability to attach to untreated supports compared to those treated with DWW is presented in Figure 2.
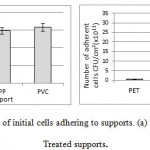 |
Figure 2: Number of initial cells adhering to supports. (a) Untreated supports; (b) Treated supports. |
In Figure 2.a, results of the adhesion tests show a marked difference among the untreated support materials (PET, PP, and PVC) in their ability to promote initial bacteria adhesion. PET is the substrate with least colonization by the studied bacterial strain, whereas PVC contains the most of adherent bacteria, followed by PP; both PVC and PP had over 2 times more colonization than PET [PVC:1.58 105 CFU/cm2; PP:1.48 105 CFU/cm2; PET: 0.72 105 CFU/cm²]. The adhesion on these untreated substrates decreases in this order PVC>PP>PET. Treatment with DWW increases bacteria adhesion across all supports by a factor of 106 (from 105 UFC/cm2 for untreated supports to 1011 UFC/cm2for treated supports) (Figure 2.b). Notably, after treatment with DWW, PET is the substrate with least colonization by the studied bacterial strain, whereas PVC contains the higher colonization (56 times more than PET and 4 times more than PP), followed by PP (13.8 times more than PET) [PET: 0.43 1011 CFU/cm2; PP: 5.95 1011 CFU/cm2; PVC: 24.25 1011 CFU/cm²]. The adhesion on these treated substrates decreases in this order PVC>PP>PET.
Microbial adhesion to a surface is quite complex because it involves electrostatic, Van der Waals and acid-base components. Our study consisted in determining the physicochemical characteristics of the bacterium and the supports treated and untreated with DWW.
Various studies have shown that a conditioning film can be formed by several organic substances such as proteins, polysaccharides, lipids, nucleic acids, and exopolysaccharides 25,26. Conditioning film formation is a multi-step phenomenon; as an example, on stainless steel in a marine environment, proteins adsorb first followed by carbohydrates27,28.
It should be noted that DWW has a complex composition. Some authors have mentioned that DWW resembles milk but is diluted, the organic and mineralogical composition differs according to the industrial process ( milk, cheese, yogurt and butter) used and that detergents and other products used in the production process is found in trace amounts in the effluent at the outlet of the dairy plant 29. It is known that milk is a complex biological fluid consisting of several components including lactose, proteins, fats and calcium phosphate. According to Mittelman 30, the adsorption of milk and its components on the substrate surface occurs within 5 to 10 s.
Because of this, other authors put into perspective the role of the medium, especially the conditioning film, on bacterial adhesion 17,25.
Treatment with DWW does alter the natural character of the three supports, with remarkable changes in the ∆Giwi and θw values (Figure 1.a and b). The ΔGiwidecreased for the PET support after DWW treatment and increased greatly for the PP and PVC supports (Figure 1.b). The electron donor character value increased greatly for the PET (γ–(PETU)= 4.9 mJ/m² to γ–(PETT)= 13.28 mJ/m²) following DWW treatment and decreased greatly for PP (γ–(PPU)= 4.1 mJ/m² to γ–(PPT)= 0.60 mJ/m²; and PVC (γ–(PVCU)= 5.3 mJ/m² to γ–(PVCT)= 2.26 mJ/m²) (Figure 1.c).
The results show that the hydrophobicity, qualitatively and quantitatively, increased after treatment with DWW for PVC and PP supports and remainedalmost stable qualitatively and increased quantitatively for PET.These results are consistent with those of Hamadi et al 31 who had worked on stainless steel and found that steel coated with milk is more hydrophobic than uncoated steel.The electron donor character for PP and PVC are stronger for untreated supports than for treated supports with DWW, these results are the same to those found for stainless steel 21. For PET it is the opposite.
The contact angle method gave very detailed results in terms of hydrophobicity and electron donor/acceptor character for the three supports (PET, PP, PVC) before and after modification with DWW (Figure 2). From a qualitative and quantitative point of view, we found that all the untreated polymer materials have a clearly hydrophobic character. Moreover, all these materials have a low electron donor/acceptor character. Many different studies have shown the same tendency in the surface physicochemical characteristics for these untreated polymers 32–37.
In this study, adhesion tests of the bacterial strain were performed on polyethylene terephthalate (PET), polypropylene (PP), and polyvinyl chloride (PVC). Different results were observed between supports that were modified with DWW and those that were untreated. Microorganism adhesion to surfaces is, as with any inert colloidal particle, largely governed by physicochemical interactions. The sum of these interactions—including electrostatic, Lifshitz-Van der Waals, and acid-Lewis base interactions—can be attractive or repulsive. These interactions depend on the physicochemical properties of microorganisms’ surface, substrate surface, and suspension medium characteristics. These physicochemical properties include hydrophobicity, electrostatic charge, and electron donor/electron acceptor character. All the factors likely to modify the physicochemical surface properties of one of the elements involved in the adhesion phenomenon can thus favor or limit microorganisms’ fixation 35.
In addition, basic chemistry states that one hydrophilic entity naturally attracts another hydrophilic entity 38 and vice versa. Previous research38–40 have reported that hydrophobicity cannot systematically explain the results of microbial adhesion to a support and that acid-base interactions play a very important role in the adhesion phenomenon 13,41,42. According to these assertions, the adhesion of the studied bacterium on the surfaces of supports modified with DWW may be due in part to the acid-base interactions between the strong bacterium’s electron-donating character and the weak supports’ electron-accepting character, which may also explain the adhesive power of this bacterium on modified and untreated supports with DWW.
Hydrophobicity and electron acceptor/donor characteristics were used here to explain these results. Electrostatic forces were not taken into account because the tests were carried out in a liquid with a high ionic strength43. It is well known that bacteria are usually charged negatively in a liquid medium 44. To avoid charge interference between the bacteria cells and the DWW, we used high ionic strength of cell suspension.
As mentioned in different studies, polysaccharides can have a hydrophobic or hydrophilic character depending on the state of freedom in solution as well as their three-dimensional conformation, which can influence the physicochemical parameters of the supports treated with DWW 36,45. Hamadi et al 21have shown that physicochemical parameters including hydrophobicity and electron acceptor/donor character of a stainless steel surface can be modified by fatty acid and proteins after conditioning by milk.
In our case, the modification of the three supports’ physicochemical characteristics (hydrophobicity and electron donor/acceptor character) is due to DWW properties (carbohydrate, fat content and proteins). The concentration and type of molecules adsorbed on the surface of a material are conditioned by the nature of this material (∆Giwi, hydrophobicity, electron donor/acceptor character, electrostatic charges, etc.) 46,47. This may explain the differences we found concerning hydrophobicity and electron donor/acceptor character between the modified supports.
The more the hydrophobicity decreases (θw (PETU) = 80.9°; θw (PPU) = 77. 8°; θw (PVCU) = 76.3 °) (Figure 2.a)., the more the bacterial adhesion increases for untreated supports [PET: 0.72 105 CFU/cm²; PP:1.48 105 CFU/cm2; PVC:1.58 105 CFU/cm2] (Figure.1a). The work of Pringle and Fletcher 48 who found a relationship between the contact angle to water (varies from 0° to 110°) and the adhesion of different bacteria on four different surfaces. Also, Absolom et al 49 showed a linear relationship between the contact angle to water of different varieties of polymers (ranging from 58° to 110°) and bacterial adhesion.
In our case, the thermodynamic theory (physicochemical proprieties) cannot explain the high number of adhered cells on the treated supports. It must be other factors like specific biological interaction (that include ligand-receptor bond) involved in the bacterial adhesion 50.
The components of DWW probably adsorb differently on the 3 supports due to difference of plastic chemical composition (more on PVC than PP and less on PET). Thus, DWW conditioning film increase the bacterial adhesion differently, PVC contains the higher colonization (56 times more than PET and 4 times more than PP) followed by PP (13.8 times more than PET).
Conclusions
The bacterium has a very pronounced hydrophilic character both qualitatively and quantitatively and a strong donor electron character.
Untreated supports with DWW have a hydrophobic character and a very weak electron acceptor/donor character.
After treatment, PET support retained its hydrophobic character with change compared to untreated supports. PVC and PP treated supports have an increase in hydrophobicity and a decrease in the electron donor character.
Bacterial adhesion to untreated supports is affected by hydrophobicity. In fact, the more the hydrophobicity increases, the more the bacterial adhesion increases, and the amount of cell adherence is double for PVC and PP comparted to PET for untreated supports. After treatment with DWW, the conditioning film of DWW significantly enhanced the bacterial adhesion for all three supports (from 105 UFC/cm² to 1011CFU/cm²).
In conclusion, the choice of support material impacts bacterial adhesion, especially after taking into account the DWW conditioning film, which promotes a high level of bacterial adhesion.
Acknowledgments
We deeply thank Dr. Abdeslam Jaafari for his technical support to produce this work.
Conflict of Interest
The authors declare no conflict of interest.
References
- Balannec B., Vourch M., Rabiller-Baudry M., Chaufer B. Comparative study of different nanofiltration and reverse osmosis membranes for dairy effluent treatment by dead-end filtration. Sep Purif Technol. 2005;42(2):195–200.
CrossRef - Lhanafi S., Aba-AakiZ R., Et-Taleb S., Elhaouti R., Abbaz M., Ez-Zahery M.Caractérisation des effluents laitiers en vue de leur valorisation: Cas de lactosérum. J Mater Environ Sci. 2014;5:2489–94.
- Mohan S. V., Mohanakrishna G., Velvizhi G., Babu V. L., Sarma P.N. Bio-catalyzed electrochemical treatment of real field dairy wastewater with simultaneous power generation. Biochem Eng J. 2010;51(1–2):32–9.
CrossRef - Bortoluzzi A. C., Faitão J. A., Di Luccio M., Dallago R. M., Steffens J., Zabot G. L. Dairy wastewater treatment using integrated membrane systems. J Environ Chem Eng. 2017;5(5):4819–27.
CrossRef - Vourch M., Balannec B., Chaufer B., Dorange G. Treatment of dairy industry wastewater by reverse osmosis for water reuse. Desalination. 2008;219(1–3):190–202.
CrossRef - Mateus G. A. P., Formentini-Schmitt D. M., Nishi L., Fagundes-Klen M. R., Gomes R. G., Bergamasco R. Coagulation/flocculation with Moringa oleifera and membrane filtration for dairy wastewater treatment. Water, Air, Soil Pollut. 2017;228(9):342.
CrossRef - Healy M. G., Rodgers M., Mulqueen J. Treatment of dairy wastewater using constructed wetlands and intermittent sand filters. Bioresour Technol. 2007;98(12):2268–81.
CrossRef - Karadag D., Köroʇlu O. E., Ozkaya B., Cakmakci M. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem. 2015;50(2):262–71.
CrossRef - Weiland P. Biogas production: Current state and perspectives. Appl Microbiol Biotechnol. 2010;85(4):849–60.
CrossRef - Muñoz Sierra J. D., Oosterkamp M. J., Wang W., Spanjers H., van Lier J. B. Comparative performance of upflow anaerobic sludge blanket reactor and anaerobic membrane bioreactor treating phenolic wastewater: Overcoming high salinity. Chem Eng J. 2019;366(December 2018):480–90.
CrossRef - Mao Y., Quan X., Zhao H., Zhang Y., Chen S., Liu T. Accelerated startup of moving bed biofilm process with novel electrophilic suspended biofilm carriers. Chem Eng J. 2017;315:364–72.
CrossRef - daSilva A. N., Macêdo W. V., Sakamoto I. K., Pereyra D. de L. A. D., Mendes C. O., Maintinguer S. I.Biohydrogen production from dairy industry wastewater in an anaerobic fluidized-bed reactor. Biomass and Bioenergy. 2019;120(November 2018):257–64.
CrossRef - Hamadi F., Latrache H., Mabrrouki M., Elghmari A., Outzourhit A., Ellouali M. Effect of pH on distribution and adhesion of Staphylococcus aureus to glass. J Adhes Sci Technol. 2005;19(1):73–85.
CrossRef - Tang B., Yu C., Bin L., Zhao Y., Feng X., Huang S. Essential factors of an integrated moving bed biofilm reactor-membrane bioreactor: Adhesion characteristics and microbial community of the biofilm. Bioresour Technol. 2016;211:574–83.
CrossRef - Liu Y., Yang S. F., Tay J. H., Liu Q. S., Qin L., Li Y. Cell hydrophobicity is a triggering force of biogranulation. Enzyme Microb Technol. 2004;34(5):371–9.
CrossRef - Oh Y. J., Lee N. R., Jo W., Jung W. K., Lim J. S. Effects of substrates on biofilm formation observed by atomic force microscopy. Ultramicroscopy. 2009;109(8):874–80.
CrossRef - Kanematsu H., Barry D. M., (ed): Biofilm and Materials Science. Biofilm and Materials Science. Springer International Publishing Switzerland. 2015; 17–23.
CrossRef - Afilal M. E., Belkhadi N., Daoudi H., Elasri O. Methanic fermentation of different organic substrates. J Mater Environ Sci. 2013;4(1):11–6.
- Busscher H. J., Weerkamp A. H., van Der Mei H. C., van Pelt A. W., de Jong H. P., Arends J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl Environ Microbiol. 1984;48(5):980–3.
CrossRef - van Oss C. J., Chaudhury M. K., Good R. J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem Rev. 1988;88(6):927–41.
CrossRef - Hamadi F., Latrache H., Asserne F., Elabed S., Zahir H., Saad I. K. Quantitative Adhesion of Staphylococcus aureus on Stainless Steel Coated with Milk. Food Nutr Sci. 2013;04(03):299–304.
CrossRef - Vogler E. A. Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interface Sci. 1998;74(1–3):69–117.
CrossRef - Latrache H., El Ghmari A., Karroua M., Hakkou A., Ait Mousse H., El Bouadili A. Relations between hydrophobicity tested by three methods and surface chemical composition of Escherichia coli. New Microbiol. 2002;
CrossRef - Hamadi F., Latrache H., Elghmari A. Determination of Escherichia coli Negative Charge Concentration From XPS Data and Its Variation with pH. J Surf Anal. 2005;12(3):293–302.
CrossRef - Taylor G. T, Zheng D., Lee M., Troy P. J., Gyananath G., Sharma S. K. Influence of surface properties on accumulation of conditioning films and marine bacteria on substrata exposed to oligotrophic waters. Biofouling. 1997;11(1):31–57.
CrossRef - Zaidi B. R., Bard R. F., Tosteson T. R. Microbial specificity of metallic surfaces exposed to ambient seawater. Appl Environ Microbiol. 1984;48(3):519–24.
CrossRef - Compère C., Bellon-Fontaine M. N., Bertrand P., Costa D., Marcus P., Poleunis C. Kinetics of conditioning layer formation on stainless steel immersed in seawater. Biofouling. 2001;17(2):129–45.
- Poleunis C., Compère C., Bertrand P. Time-of-flight secondary ion mass spectrometry: Characterisation of stainless steel surfaces immersed in natural seawater. J Microbiol Methods. 2002;48(2–3):195–205.
- Ozturk I., Eroglu V., Ubay G., Demir I. Hybrid upflow anaerobic sludge blanket reactor (HUASBR) treatment of dairy effluents. Water Sci Technol. 1993;28(2):77–85.
CrossRef - Mittelman M. W. Structure and Functional Characteristics of Bacterial Biofilms in Fluid Processing Operations. J Dairy Sci. 1998;81(10):2760–4.
CrossRef - Hamadi F., Asserne F., Elabed S., Bensouda S., Mabrouki M., Latrache H. Adhesion of Staphylococcus aureus on stainless steel treated with three types of milk. Food Control. 2014;38(1):104–8.
CrossRef - Nguyen V., Karunakaran E., Collins G., Biggs C.A. Physicochemical analysis of initial adhesion and biofilm formation of Methanosarcina barkeri on polymer support material. Colloids Surfaces B Biointerfaces. 2016;143:518–25.
CrossRef - Habouzit F., Gévaudan G., Hamelin J., Steyer J. P., Bernet N. Influence of support material properties on the potential selection of Archaea during initial adhesion of a methanogenic consortium. Bioresour Technol. 2011;102(5):4054–60.
CrossRef - Assaidi A., Ellouali M., Latrache H., Mabrouki M., Timinouni M., Zahir H. Adhesion of Legionella pneumophila on glass and plumbing materials commonly used in domestic water systems. Int J Environ Health Res. 2018;28(2):125–33.
CrossRef - van Oss C. J. (ed): Interfacial Forces in Aqueous Media, 2ndedn.New York:CRC press.2006; 217-220.
CrossRef - van Oss C. J, Chaudhury M. K., Good R. J. The Mechanism of Phase Separation of Polymers in Organic Media—Apolar and Polar Systems. Sep Sci Technol. 1989;24(1–2):15–30.
CrossRef - van Oss C. J., Good R. J., Busscher H. J. Estimation of the polar surface tension parameters of glycerol and formamide, for use in contact angle measurements on polar solids. J Dispers Sci Technol. 1990; 11(1):75-81.
CrossRef - McEldowney S., Fletcher M. Variability of the influence of physicochemical factors affecting bacterial adhesion to polystyrene substrata. Appl Environ Microbiol. 1986;52(3):460–5.
CrossRef - Pratt-Terpstra I. H., Weerkamp A. H., Busscher H. J. On a relation between interfacial free energy-dependent and noninterfacial free energy-dependent adherence of oral streptococci to solid substrata. Curr Microbiol. 1988 Nov;16(6):311–3.
CrossRef - Sjollema J., Van der Mei H. C., Uyen H. M. W., Busscher H.J. The influence of collector and bacterial cell surface properties on the deposition of oral streptococci in a parallel plate flow cell. J Adhes Sci Technol. 1990;4(1):765–77.
CrossRef - Hamadi F., Latrache H., Mallouki B., Mliji E., El Ghmari A., Mabrouki M. Adhesion of Escherichia coli to glass under different pH. J Pure Appl Microbiol. 2008;2(2):295–302.
CrossRef - Henriques M., Azeredo J., Oliveira R. Adhesion of Candida albicans and Candida dubliniensis to acrylic and hydroxyapatite. Colloids Surfaces B Biointerfaces. 2004; 33(3-4):235-241.
CrossRef - Bellon-Fontaine M. N., Rault J., van Oss C. J. Microbial adhesion to solvents: A novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surfaces B Biointerfaces. 1996;7(1–2):47–53.
CrossRef - van Loosdrecht M. C. M., Lyklema J., Norde W., Zehnder A. J.B. Bacterial adhesion: a physicochemical approach. Microb Ecol. 1989;17(1):1–15.
CrossRef - van Oss C. J. Interfacial Forces in Aqueous Media- 2nd ed. Taylor Fr Gr. 2006;V36.
CrossRef - Rosmaninho R., Santos O., Nylander T., Paulsson M., Beuf M., Benezech T.Modified stainless steel surfaces targeted to reduce fouling – Evaluation of fouling by milk components. J Food Eng. 2007; 80(4):1176-1187.
CrossRef - Rubio C., Costa D., Bellon-Fontaine M. N., Relkin P., Pradier C. M., Marcus P. Characterization of bovine serum albumin adsorption on chromium and AISI 304 stainless steel, consequences for the Pseudomonas fragi K1 adhesion. Colloids Surfaces B Biointerfaces. 2002;24(3–4):193–205.
CrossRef - Pringle J. H., Fletcher M. Influence of substratum wettability on attachment of freshwater bacteria to solid surfaces. Appl Environ Microbiol. 1983;45(3):811–7.
CrossRef - Absolom D. R., Lamberti F. V., Policova Z., Zingg W., van Oss C. J., Neumann A. W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983;46(1):90–7.
CrossRef - Vitte J., Benoliel A. M., Pierres A., Bongrand P. Is there a predictable relationship between surface physical-chemical properties and cell behaviour at interface. Eur Cells Mater. 2004;7(SUPPL.1):19.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





