How to Cite | Publication History | PlumX Article Matrix
Jaciara S. de Araujo1 , Juliene da C. Rocha1
, Juliene da C. Rocha1
![]() , Marcos A. O. Filho1
, Marcos A. O. Filho1
![]() , Vitor T. Ribeiro1
, Vitor T. Ribeiro1 , Luan T. C. de P. Vasconcelos1
, Luan T. C. de P. Vasconcelos1
![]() , Nathalia K. de Araujo2
, Nathalia K. de Araujo2 , Eduardo L. de B. Neto1
, Eduardo L. de B. Neto1 and Everaldo S. dos Santos1*
and Everaldo S. dos Santos1*
![]()
1Biochemical Engineering Laboratory, Chemical Engineering Department, Centre of Technology, Federal University of Rio Grande do Norte, 59072-970 Natal, Brazil
2Pharmacy Deparment, Federal University of Rio Grande do Norte, Av. General Gustavo Cordeiro de Farias S/N, Petrópolis, 59012-570, Natal – RN, Brazil.
Corresponding Author E-mail : everaldo@eq.ufrn.br
DOI : http://dx.doi.org/10.13005/bbra/2850
ABSTRACT: Rhamnolipids are biosurfactants synthesized by different species of microorganisms. In this study, the influence of carbon/nitrogen ratio (C/N) and percentage of inoculum on rhamnolipid production by Pseudomonas aeruginosa AP029-GLVIIA using glucose as substrate was evaluated. The critical micellar concentration (CMC) and surface tension were analyzed for the highest biosurfactant concentration, which presented values of 49.63 mg/L and 29.5 mN/m, respectively. Emulsification rates were determined for different solvents and showed the bioproduct's ability to form stable emulsions for up to 90 days. The efficiency of the biosurfactant in removing petroleum present in the sand was 16.8% and the antimicrobial activity of the rhamnolipid against fungal species was determined, showing its potential to inhibit fungi of the species Candida tropicalis and Candida albicans.
KEYWORDS: Antifungal Activity; Bioremediation; Emulsification; Pseudomonas aeruginosa; Rhamnolipid
Download this article as:| Copy the following to cite this article: De Araujo J. S, Rocha J. da C, Filho M. A. O, Ribeiro V. T, Vasconcelos L. T. C. de P, De Araujo N. K, Neto E. L. de B, Dos Santos E. S. Production of Rhamnolipids by Pseudomonas aeruginosa AP029-GLVIIA and Application on Bioremediation and as a Fungicide. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: De Araujo J. S, Rocha J. da C, Filho M. A. O, Ribeiro V. T, Vasconcelos L. T. C. de P, De Araujo N. K, Neto E. L. de B, Dos Santos E. S. Production of Rhamnolipids by Pseudomonas aeruginosa AP029-GLVIIA and Application on Bioremediation and as a Fungicide. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/31wlx9k |
Introduction
Surfactants are an important class of chemical compounds synthesized in large part by petroleum derivatives (Gudiña et al., 2015a; Padilha et al., 2015) They are formed by hydrophobic and hydrophilic portions, which are distributed at the interface between liquid phases causing the decrease of surface and interfacial tensions ( Akbari et al., 2018; Ehinmitola et al., 2018; Gudiña et al., 2016; Grüninger et al., 2019; Mondal et al., 2015; Mondal et al., 2016, Mondal et al., 2017a). The surfactant production is expected to increase to 24 million tons and be worth approximately $ 120 million by 2020 (Jiang et al., 2020).
Currently, the studies about biosurfactants have been expanded due to the high environmental impact caused by some chemical surfactants. In addition to having similar properties to chemical surfactants, these amphiphilic bioproducts have advantages such as biodegradability, low toxicity and stability under extreme conditions of pH, temperature and salinity (França et al., 2015). Surfactants have potential to be applied in numerous products or fields, such as, detergents, paints, paper products, pharmaceuticals, cosmetics, petroleum, food, and water treatment (Costa et al., 2010; Mondal et al., 2017b).
Biosurfactants can be produced by different strains of microorganisms (bacteria, filamentous fungi and yeasts) using renewable raw materials with low cost as a substrate (Abdel-Mawgoud et al., 2010; Araújo et al., 2013). These molecules are classified into five major groups: lipopeptides, glycolipids, fatty acids, phospholipids and polymeric biosurfactants (Geetha et al., 2018).
Among the glycolipids there are the rhamnolipids, composed of rhamnose molecules and one or two units of β-hydroxydecanoic acid, which are present mainly in four isoforms (Mulligan, 2005). The production of these molecules occurs predominantly by Pseudomonas aeruginosa and the z is classified as mono and di-rhamnolipids according to the amount of rhamnose present in the structure. In addition, the proportion of these two forms can be influenced by nutritional and environmental conditions of microbial growth (Oluwaseun et al., 2017; Varjani and Upasani, 2017). Mono-rhamnolipids congeners show increase emulsification and antimicrobial properties in comparison to di-rhamnolipids (Sood et al., 2020). Other species of Pseudomonas have also been reported as producing rhamnolipids, such as P. chlororaphis, P. plantarii, P. putida and P. fluorescens (Randhawa and Rahman, 2014). The main characteristics of these biosurfactants are related to their ability to reduce the surface tension of water to between 28 and 30 mN/m, reduce interfacial tension between water and hydrocarbons, and have a critical micelle concentration (CMC) between 10 and 200 mg/L (França et al., 2015; Gudiña et al., 2015a).
Although can be applied in different areas, the production of biosurfactant on a large scale is not yet totally feasible, since the cost with the production and recovery of this product is relatively high (Souza et al., 2018). However, an alternative to reduce production costs would be the use of low-cost raw materials such as frying oils, sugarcane and beet molasses and cassava wastewater (Banat et al., 2014). But, even with some limitations it is estimated that in 2023 approximately 524 tons of biosurfactant will be traded, which will be responsible for a turnover of US$ 2.7 billion (Felipe and Dias, 2017).
In this context, the objective of this study was to evaluate the production of rhamnolipids by Pseudomonas aeruginosa AP029-GLVIIA by varying the carbon/nitrogen ratio (C/N), based on a simple and affordable source of carbon and energy (glucose), and the percentage of inoculum. Thus, the produced rhamnolipids were characterized in terms of CMC, emulsification index, bioremediation and antifungal activity against the species Candida albicans and Candida tropicalis.
Material and Methods
Chemical
The main chemicals used during this study were corn oil (Cargil Co. – SP, Brazil), D-glucose (Synth Co. – SP, Brazil), hexadecane (Sigma Co., USA), iron sulfate II heptahydrate (Synth Co. – SP, Brazil), kerosene (Líder Co. – RN, Brazil), magnesium sulfate heptahydrate (Synth Co. – SP, Brazil), monobasic potassium phosphate (Synth Co. – SP, Brazil), motor oil (Petronas Co.-MG, Brazil), peptone (BD Co. – SP, Brazil), sodium chloride (Cinética Co. – PR, Brazil), sodium nitrate (Cinética Co. – PR, Brazil), sodium phosphate dibasic heptahydrate (Synth Co. – SP, Brazil), soybean oil (Bunge Co. – SP, Brazil), and yeast extract (BD Co. – SP, Brazil) they were all of analytical grade.
Microorganism and Maintenance
Pseudomonas aeruginosa AP029-GLVIIA was isolated from an oil well in the city of Mossoró (Rio Grande do Norte, Brazil) and deposited in the culture collection of the Department of Antibiotics in the Federal University of Pernambuco (UFPE – Brazil). The microorganism was maintained in petri dish with PCA (Plate Count Agar) at 5 °C (Araújo et al., 2017).
Inoculum and Culture Medium
For inoculum the microorganism was transferred from the petri dish to 250 mL conical flasks containing 100 mL of medium consisting of 3.0 g/L yeast extract, 5.0 g/L sodium chloride and 5.0 g/L peptone at pH 7.0 then after 24 hours of cultivation at 38 °C and 200 rpm, aliquots were transferred to the production medium (Peng et al., 2012). The production medium (pH 6.5) consisted of a saline solution of MgSO4.7H2O (1.0 g/L), Na2HPO4.7H2O (1.1 g/L), KH2PO4 (1.5 g/L), NaNO3 (2.0 g/L), FeSO4.7H2O (0.1 g/L) and glucose. The influence of glucose on the production of rhamnolipids was evaluated by varying its concentration for 10.0, 18.0 and 26.0 g/L.
Five runs were performed in order to evaluate different culture conditions. The percentage of inoculum was 3.0, 10.0 and 17.0% (v/v) and the C/N ratio was 5, 9 and 13. All experiments were assayed in duplicate at 38 °C and 200 rpm for 72 hours using 100 mL of solution (production medium and inoculum) in 250 mL flasks. The pH of the crude broth was measured by potentiometer mPA 210 (Tecnopon, Brazil) and adjusted to 8.0. Then the medium containing the rhamnolipids was centrifuged (centrifuge 5804 R, Eppendorf, USA) at 1370 x g for 10 minutes and the supernatant obtained was used for further analysis.
Analytical Methods
Determination of Biomass
The biomass quantification was performed by the dry mass method as described by Bezerra et al. (2012). The crude broth was centrifuged (centrifuge 5415 D, Eppendorf, Germany) at 15700 x g for 15 minutes. Each point was measured in triplicate and the cell concentration (g/L) was estimated according to Equation 1:
Cbiomass = ((mass of the tube with biomass-Empty tube mass) / 2) x 1000 (1)
Determination of Glucose
Glucose quantification was evaluated by the 3,5 dinitro-salicylic acid (DNS) method according to Miller (1959). The analyses were performed in triplicate.
Avaliation of Total Proteins
Measurement of total proteins was performed according to Bradford (1976). The assays were performed in duplicate.
Recovery and Quantification of the Rhamnolipids
The recovery of the rhamnolipids was performed first by acid precipitation of the supernatant I obtained from the centrifugation as commented in topic 2.2. The cell-free broth was acidified to pH 2.0 using HCl (6M) and stored at 4 °C overnight. The sample was then centrifuged at 1370 x g for 10 minutes. The supernatant from that centrifugation was discarded and 5 mL of distilled water and petroleum ether in the ratio of 1: 1 (v/v) were added to the precipitate. This procedure was repeated three times and at each repetition the emulsion formed by the ether and the rhamnolipids were removed and stored. Finally, the organic phase obtained from the last step was taken to the rotary evaporator V-850 (Büchi, Switzerland). Ten mL of distilled water was added to the obtained concentrate and stored (Peng et al., 2012). The quantification of the rhamnolipids was performed by the thioglycolic colorimetric method according to Oliveira et al. (2013).
Properties of the Biosurfactant
Critical Micellar Concentration (CMC)
Different dilutions of a 100 mg/L crude rhamnolipids mixture (1.65, 4.96, 6.20, 9.92, 24.82, 33.08, 49.63 and 100 mg/mL) were performed to determine the CMC. The surface tension for each defined concentration was measured using the Phoenix 150 SEO tensiometer and the CMC values were obtained in triplicate (Araújo et al., 2017).
Emulsification Index
The emulsification index was determined by the method of Cooper and Goldenberg (1987). In this case 2.0 mL of supernatant I was added to a tube containing 2.0 mL of the working solvent: hexadecane, toluene, kerosene, soybean oil, corn oil and motor oil. After 24 hours, the emulsification index (E) was measured according to Equation 2, described by Wei et al. (2005). These measurements were repeated every 15 days until completing 90 days and were performed in triplicate.
E (%) = (height of the emulsion/ total height)x 100 (2)
Assessment of Potential for Bioremediation
The evaluation for oil recovery was carried out using sand from a beach (Praia do Meio) of Natal (RN) – Brazil, containing 10% (w/w) of oil in Erlenmeyers of 250 mL. The mixture was allowed to stand for 24 hours and, subsequently, 40 mL of rhamnolipids (1.0 g/L) were added to each flask. Samples were incubated at 40 °C and 100 rpm for 24 h. Then the water/oil mixture was centrifuged at 5000 rpm for 25 minutes in order to quantify the mass of purified oil. The control assay was performed using distilled water under the same conditions and all experiments were performed in triplicate (Gudiña et al., 2015a; Pereira et al., 2013).
Evaluation of Antifungal Activity
The tests to evaluate the antifungal activity of the biosurfactant were carried out following the methodology described by the Clinical and Laboratory Standards Institute (CLSI) with modifications (Cockerill et al., 2012). The antifungal action of purified and unpurified rhamnolipids was evaluated against two yeast strains: Candida albicans ATCC 90028 and Candida tropicalis ATCC 13803. In a 96-well plate, 50.0 μL of the fungal suspension with 105 CFU/mL in Müeller Hinton broth (MH), supplemented with 0.2% glucose, were added to the rhamnolipids (7.425, 3.71, 1.85 , 0.93 and 0.46 μg/mL) and fluconazole (0.58 μg/mL) and then incubated at 35.0 ± 2.0 °C, under agitation of 200 rpm. The optical density at 595 nm was evaluated using a microplate reader (Epoch Biotek, Winooski) at zero time and after 24 hours.
Statistical Analysis
The analysis of the emulsification index and the antifungal activity were performed in triplicate and evaluated by the Tukey test using the software Statistica 7.0 (StatSoft Co, USA) and GraphPad Prism 5.0 (La Jolla California, USA).
Results and Discussion
Production of Rhamnolipids
Rhamnolipid production by Pseudomonas aeruginosa AP029-GLVIIA using glucose as substrate was evaluated by changing the C/N ratio and the percentage of inoculum added to the culture medium. In the five conditions studied, the concentrations of biomass, glucose, rhamnolipid and total proteins were analyzed, as well as pH variation.
According to Table 1, it can be seen that as the C/N ratio increased there was an increase in both biomass formation and rhamnolipid production. Indeed, it is known that these metabolites formation is favored under nitrogen limiting conditions Santos et al. (2002). The highest production of biosurfactant occurred for a ratio C/N of 13 with percentage of inoculum of 3.0%. It should be highlighted that Sousa et al. (2014) found a similar result producing rhamnolipids using glycerol as the carbon source. But, comparing the runs 2, 4 and 5, in which there was an increase in the amount of inoculum, it was observed that the product and the biomass had their values decreased and increased, respectively. The decrease in the amount of biosurfactant produced may be associated with the Quorum Sensing (QS) shown by Pseudomonas aeruginosa. The QS consists of a bacterial communication system capable of coordinating functions as motility and virulence agents, as well as controlling the levels of important compounds for biofilms formation, such as rhamnolipids, lectin A and siderophores (Kariminik et al., 2017). In general, the production of rhamnolipids was favored by using a higher C/N ratio and a lower percentage of inoculum.
During the cultivation, glucose consumption varied from 82.5 to 90.4%, then showing good assimilation of the substrate by the microorganism. In addition, the highest consumption occurred for the first 24 hours of each experiment. The values are of the same magnitude as shown by Bezerra et al. (2012); Sousa et al. (2014) that obtained substrate uptake of 91.9 and 50.8%, respectively, when used cassava wastewater and glycerol as substrate. Different carbon sources have been used for the production of rhamnolipids, for instance, Ramírez et al. (2015) investigated the olive-mill waste and Varjani and Upasani (2016) evaluated crude oil, nonane, decane, dodecane, N-paraffins, kerosene, diesel, xylene, glucose and glycerol, with glucose being the substrate that presented the highest yield in production of rhamnolipids. Additionally, Mondal et al. (2017) used different carbon sources and observed that glucose provided the best result for the biosurfactant synthesis.
According to Table 1, the concentration of total proteins increased in proportion to the increase in the amount of rhamnolipid, probably because these metabolites are capable of increasing the permeability of the cell membrane, and consequently, the concentration of proteins in the medium (Shao et al., 2017). However, the decrease in the protein concentration as shown in the runs 4 and 5 may be associated with the production of proteases but the rhamnolipids production was almost unchanged due to the QS (Bouyahya et al., 2017).
With regard to pH during cultivation it ranged from 5.88 to 8.20 when considering all runs performed, however for the maximum rhamnolipids concentration it ranged between 6.28 and 6.58. Similar results were shown by Varjani and Upasani (2017) that reported that rhamnolipids synthesis by Pseudomonas sp. is favored by pH between 6.0 and 6.5. In addition, the production of total proteins showed an interesting relationship with pH. It can be seen that as protein concentration increases there was an increase in the pH value, as shown in Figure 1, indicating metabolism for proteins formation with ammonium formation that affected pH by increasing it (Santos et al., 2002).
Table 1: Effect of C/N ratio and percentage of inoculum on rhamnolipid production, cell growth, pH and total protein production.
| Run | C/N Ratio | Inoculum
(%) |
Biomass
(g/L) |
Time1
(h) |
Rhamnolipid
(g/L) |
Time2
(h) |
pH3 | Total proteins
(g/L) |
| 1 | 5 | 3 | 1.33 0.02 | 12 | 0.30 0.00 | 4 | 6.52 0.03 | 0.149 0.01 |
| 2 | 9 | 3 | 1.57 0.06 | 24 | 0.78 0.01 | 60 | 6.28 0.05 | 0.248 0.01 |
| 3 | 13 | 3 | 2.50 0.04 | 24 | 0.84 0.06 | 24 | 6.44 0.05 | 0.426 0.00 |
| 4 | 9 | 10 | 2.03 0.20 | 12 | 0.39 0.03 | 12 | 6.58 0.03 | 0.233 0.00 |
| 5 | 9 | 17 | 1.93 0.14 | 10 | 0.40 0.01 | 48 | 6.46 0.00 | 0.123 0.00 |
1,2 Time at which maximum values of biomass and product were reached, respectively.
3pH corresponding to the highest concentration of rhamnolipids obtained in the assay.
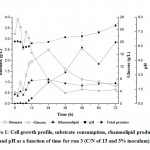 |
Figure 1: Cell growth profile, substrate consumption, rhamnolipid production and pH as a function of time for run 3 (C/N of 13 and 3% inoculum). |
Characterization of Biosurfactant
Critical Micellar Concentration (CMC)
In the present study the CMC of the unpurified (crude) rhamnolipids produced by P. aeruginosa AP029-GLVIIA was evaluated. The CMC determination was performed by measuring the surface tension of the cell free broth corresponding to the point of greatest rhamnolipid concentration (24 hours, run 3). The rhamnolipids produced were able to reduce the surface tension of water from 71.94 ± 1.07 to 29.42 ± 1.41 mN/m with a CMC of 49.63 mg/L. It is emphasized that the CMC depends on the pH, temperature, ionic strength and surfactant structure. But, as the rhamnolipids were synthesized in ionic medium, the influence of pH will be more significant when compared to the other mentioned parameters. An interesting fact concerns the variation in the value of CMC when considering the different isoforms adopted by rhamnolipids (Kłosowska-Chomiczewska et al., 2017). Samadi et al. (2012) observed that for a mixture of rhamnolipids (RLs), CMC was 22 mg/L and surface tension of 26 mN/m. However, when it was applied only mono-rhamnolipids (RL1), the CMC decreased to 15 mg/L while the tension reached 25 mN /m. Finally, for a mixture of di-rhamnolipids (RL2) the CMC reached 30 mg/L and tension of 29.5 mg/L. Gogoi et al., (2016) when studying rhamnolipid production obtained CMC values of 110 and 72 mg/L for crude and purified rhamnolipid, respectively. Regarding the surface tension, the purified rhamnolipid reached 29.5 mN/m. In contrast, Sodium Dodecyl Sulphate (SDS), a chemical surfactant widely used in industry, has CMC values of up to 2890 mg/L and surface tension of 37 mN/m. Thus, when comparing these values with those of the rhamnolipids, it can be seen that the the latter has higher efficiency, since the values obtained are smaller (Bognolo, 1999).
Emulsification index
The formation of the emulsion occurs when a liquid phase is dispersed in the form of droplets in a continuous liquid phase (Desai and Banat, 1997). Emulsification tests were performed with the cell-free supernatant (24 hours, run 3) and they were determined using six organic solvents: hexadecane, toluene, kerosene, soybean oil, corn oil and motor oil. In order to evaluate the stability of the emulsion formed, the indices were measured every 15 days until to complete 90 days.
Figure 2 shows the results of the emulsion formed in the first 24 hours: corn oil (57.47%), toluene (58.62%), soybean oil (59.32%), kerosene (62.07%), hexadecane (66.30%) and motor oil (77.55%). There were oscillations over time, but the emulsification index values remained above 50% for the hydrocarbons, except for the toluene which kept the emulsion for only 24 hours. In relation to the oils only the corn was unable to maintain the emulsion higher than 50% in the last 30 days. In all solvents the emulsion formed at the top of the system, indicating that the rhamnolipids are responsible for forming water-in-oil (W/O) emulsions (Nguyen and Sabatini, 2011)
 |
Figure 2: Emulsification index determined at different times in oils (A) and hydrocarbons (B). |
Table 2 presents a comparison among the present study and of the emulsification indexes of some biosurfactants shown by reports on literature about the solvents herein assayed.
Table 2: Comparison of emulsification indexes of different biosurfactants
| Microorganisms | Carbon Sources | Solvents | Emulsification index | References |
| Pseudomonas aeruginosa AP029/GLVIIA | Glucose | Hexadecane, toluene, kerosene, soybean oil, corn oil and motor oil | 57.47 to 77.55% | This study |
| Pseudomonas aeruginosa LBI | Natural oils | Kerosene and toluene | 70 to 100% | Costa et al. (2006) |
| Pseudomonas aeruginosa AP029/GLVIIA | Cassava | Kerosene | 65% | (Bezerra et al. (2012) |
| Pseudomonas aeruginosa #112 | Corn steep liquor and molasse | Hexadecane | 60% | Gudiña et al. (2016) |
| Pseudomonas
aeruginosa UCP0992 |
Corn steep liquor | Soy, corn and motor oil | 62.5 to 100% | Rufino et al. (2016) |
| Pseudomonas aeruginosa NCIM 5514 | Glucose | Hidrophobic solvents | 17.1 to 82.3% | Varjani and Upasani (2016) |
| Bacillus subtilis ICA56 | Glucose | Motor oil | 79% | França et al. (2015) |
Assessment of Potential for Bioremediation
In this study, a previous test for the recovery of contaminated sand oil was carried out. From the experiment it was possible to determine that the rhamnolipids were able to remove 16.8 ± 1.6% of the petroleum when compared to the control test (sand/ petroleum/distilled water). When studying different surfactins produced by species of Bacillus subtilis, Pereira et al. (2013) achieved oil removal results between 19.0 and 22.0% using a 1.0 g/L rhamnolipid solution. In similar work, Gudiña et al. (2015b) obtained values of 15.0, 26.3 and 25.1% when using 1.0, 2.5 and 5.0 g/L surfactin. On the other hand, Gudiña et al. (2015a) produced rhamnolipids and used them to removal of petroleum showing values of 22.1; 43.7 and 55.0% for the same concentrations of rhamnolipids presented in the present study. Recently, Das and Kumar (2019) demonstrated that biosurfactant was able to recover 46.5% of the crude oil present at a sand pack column.
Evaluation of Antifungal Activity
Analyses of antimicrobial activity were performed using purified and unpurified rhamnolipids, however, only the purified one was able to inhibit the growth of the microorganisms Candida albicans ATCC 90028 and Candida tropicalis ATCC 13803. Figure 3 shows the inhibition of fungi versus the concentrations of rhamnolipids (7.42, 3.71, 1.85, 0.93 and 0.46 μg/mL) and the applied control, fluconazole, (0.58 μg / mL). According to the results, the concentration of rhamnolipid showing higher antifungal activity was 7.42 μg/mL for the two yeasts assayed. In addition, to Candida tropicalis the biosurfactant concentration of 3.71 μg/mL did not show statistical difference when compared with the control (fluconazole) while for yeast Candida albicans all tested concentrations have similar action to fluconazole.
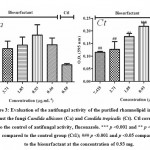 |
Figure 3: Evaluation of the antifungal activity of the purified rhamnolipid incubated against the fungi Candida albicans (Ca) and Candida tropicalis (Ct). |
The present study demonstrates that the rhamnolipids produced by P. aeruginosa AP029-GLVIIA have potential to act as antifungal agents.
Abalos et al. (2001) used rhamnolipids to inhibit the growth of the following microorganisms: Aspergillus niger and Gliocadium virens (16 μg/mL), Chaetomium globosum, Penicillium chrysogenum and Aureobasidium pullulans (32 μg/mL), Botrytis cinerea and Rhizoctonia solani (18 μg/mL). The values presented in parentheses correspond to the Minimum Inhibitory Concentration (MIC).
In addition to antifungal activity, rhamnolipids also have a high potential to inhibit bacterial growth. Tedesco et al. (2016) applied biosurfactants against the bacteria Staphylococcus aureus and Burkholderia cepacia obtaining values MIC of 1.56 and 3.12 μg/mL. Oluwaseun et al. (2017) evaluated the antimicrobial activity of rhamnolipids, produced by Pseudomonas aeruginosa C1501, against various microorganisms (Staphylococcus aureus, Bacillus cereus, Escherichia coli, Saccharomyces cerevisiae, Aspergillus flavus and Aspergillus niger). The results showed that this bioproduct has the capacity to be used at industrial, food and biomedical applications. On the other hand, Ndlovu et al. (2017) studied the antibacterial and antifungal activity of biosurfactant extracts by Bacillus amyloliquefaciens and Pseudomonas aeruginosa against antibiotic resistant (Staphylococcus aureus, Escherichia coli) and fungal pathogens (Candida abicans, Cryptococcus neoformans). The biosurfactant presented antimicrobial action about all microorganisms analyzed.
Recently, Ferreira et al. (2019) investigated the antimicrobial activity of rhamnolipids against Gram-positive and Gram-negative food pathogens (Bacillus cereus, Listeria monocytogenes and Staphylococcus aureus) under different pH. The study suggests that the biosurfactant can be enhanced in acid food.
Conclusion
The Pseudomonas aeruginosa AP029-GLVIIA was able to produce rhamnolipids using glucose as the carbon source. The best condition for rhamnolipids production and biomass formation was using a C/N ratio and inoculum percentage of 13.0 and 3.0%, respectively. The rhamnolipids were able to form stable emulsions in different organic solvents, besides presenting satisfactory responses in relation to surface tension (29.42 ± 1.41 mN/m) and critical micellar concentration (49.63 mg/L). In addition, the tests of oil removal and antifungal activity showed that this kind of biosurfactant has potential for interesting biotechnological applications.
Acknowledgements
The authors thank CAPES and CNPq (Grant: 305251/2017-1) for the financial support for this work.
Compliance with Ethical Standards
Conflict of Interest
Authors declare there is no conflict of interest.
Funding Source
The authors thank CAPES and CNPq (Grant: 305251/2017-1) for the financial support for this work.
References
- Padilha C. E. A., Padilha C. A. A., Souza D. F. S. et al. Prediction of rhamnolipid breakthrough curves on activated carbon and Amberlite XAD-2 using Artificial Neural Network and Group Method Data Handling models . J. Mol. Liq. 2015; 206:293–299.
- Gudiña E. J., Rodrigues A. I., Alves E. et al. Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresour. Technol. 2015; 177:87–93.
- Gudiña E. J., Rodrigues A. I., Freitas V. et al. Valorization of agro-industrial wastes towards the production of rhamnolipids. Bioresour. Technol. 2016; 212:144–50.
- Jiang J., Zu Y., Li X. et al. Recent progress towards industrial rhamnolipids fermentation: process optimization and foam control. Bioresour. Technol. 2020; 298:1–10.
- França I. W. L., Lima A. P., Lemos J. A. M. et al. Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catal. Today. 2015; 255:10–15.
- Akbari S., Abdurahman N. H., Yunus R. M., Fayaz F., Alara O. R. Biosurfactants—a new frontier for social and environmental safety: a mini review. Biotechnology Research and Innovation. 2018; 2: 81-90.
- Costa S. G. V. A. O., Nitschke M., Lépine F. et al. Structure, properties and applications of rhamnolipids produced by Pseudomonas aeruginosa L2-1 from cassava wastewater. Process Biochem. 2010; 45:1511–16.
- Abdel-Mawgoud A. M., Lépine F., Déziel E. Rhamnolipids: Diversity of structures, microbial origins and roles. Applied Microbiology and Biotechnology. 2010; 86:1323–36.
- Araújo L. V., Freire D. M. G., Nitschke M. Biossurfactantes: propriedades anticorrosivas, antibiofilmes e antimicrobianas. Quim. Nova. 2013; 3:848–58.
- Grüninger J., Delavault A., Ochsenreither K. Enzymatic glycolipid surfactant synthesis from renewables. Process Biochemistry. 2019; 87: 45-54.
- Geetha S. J., Banat I. M., Joshi S. J. Biosurfactants: Production and Potential applications in Microbial Enhanced Oil Recovery (MEOR). Biocatal. Agric. Biotechnol. 2018; 14:1–30.
- Mulligan C. N. Environmental applications for biosurfactants. Environ. Pollut. 2005; 133:183–98.
- Oluwaseun A. C., Kola O. J., Mishra P. et al. Characterization and optimization of a rhamnolipid from Pseudomonas aeruginosa C1501 with novel biosurfactant activities. Sustain. Chem. Pharm. 2017; 6:26–36.
- Varjani S. J., Upasani V. N. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour. Technol. 2017; 232:389–97.
- Sood U., Singh D. N., Hira P. et al. Rapid and solitary production of mono-rhamnolipid biosurfactant and biofilm inhibiting pyocyanin by a taxonomic outlier Pseudomonas aeruginosa strain CR1. J. Biotechnol. 2020; 307:98–106.
- Randhawa K. K. S., Rahman P. K. S. M. Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front. Microbiol. 2014; 5:1–7.
- Souza K. S. T., Gudiña E. J., Schwan R. F. et al. Improvement of biosurfactant production by Wickerhamomyces anomalus CCMA 0358 and its potential application in bioremediation. J. Hazard. Mater. 2018; 346:152–58.
- Banat I. M., Satpute S. K., Cameotra S. S. et al. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014; 5:1–18.
- Felipe L. D. O., Dias S. D. C. Surfactantes sintéticos e biossurfactantes : vantagens e desvantagens. Química Nov. na Esc. 2017; 39:228–36.
- Araújo C. K. C., Campos A. O., Padilha C. E. A. et al. Enhancing enzymatic hydrolysis of coconut husk through Pseudomonas aeruginosa AP 029/GLVIIA rhamnolipid preparation. Bioresour. Technol. 2017; 237:20–26.
- Peng X., Yuan X. Z., Zeng G. M. et al. Extraction and purification of laccase by employing a novel rhamnolipid reversed micellar system. Process Biochem. 2012; 47:742–48.
- Bezerra M. S., Holanda V. C. D., Amorim J. A. et at. Produção de biotensoativo utilizando Pseudomonas aeruginosa (P.A.) e resíduo agroindustrial (manipueira) como substrato. Holos 2012; 1:14–27.
- Miller G. L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959; 31:426–28.
- Bradford M. M. A. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976; 72:248–54.
- Oliveira A. C. S. M., Bezerra M. S., Padilha C. E. A. et al. Recovery of Rhamnolipids Produced by Pseudomonas aeruginosa Using Acidic Precipitation, Extraction, and Adsorption on Activated Carbon. Sep. Sci. Technol. 2013; 48:2852–59.
- Cooper D. G., Goldenberg B. G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987; 53:224–29.
- Wei Y. H., Chou C. L., Chang J. S. Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. Biochem. Eng. J. 2005; 27:146–54.
- Pereira J. F. B., Gudiña E. J., Costa R. et al. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 2013; 111:259–68.
- Cockerill F. R., Wikler M. A., Alder J. et al. (Clinical and Laboratory Standards Institute): Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th Wayne, Pennsylvania. 2012; pp 1–32.
- Ehinmitola E. O., Aransiola E. F., Adeagbo O.P. Comparative study of various carbon sources on rhamnolipid production. South African Journal of Chemical Engineering. 2018; 26: 42-48.
- Santos A. S., Sampaio A. P. W., Vasquez G. S. et al. Evaluation of different carbon and nitrogen sources in production of rhamnolipids by a strain of Pseudomonas aeruginosa. Appl. Biochem. Biotechnol. 2002; 98:1025–35.
- Sousa J. R., Correia J. A. C., Melo V. M. M. et al. Cinética e caracterização de ramnolipídeos produzidos por Pseudomonas aeruginosa MSIC02 utilizando glicerol como fonte de carbono. Quim. Nova 2014; 37:431–41.
- Kariminik A., Baseri-Salehi M., Kheirkhah B. Pseudomonas aeruginosa quorum sensing modulates immune responses: An updated review article. Immunol. Lett. 2017; 190:1–6.
- Ramírez I. M., Tsaousi K., Rudden M. et al. Rhamnolipid and surfactin production from olive oil mill waste as sole carbon source. Bioresour. Technol. 2015; 198:231–36.
- Varjani S. J., Upasani V. N. Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: Production, characterization and surface active properties of biosurfactant. Bioresour. Technol. 2016; 221:510–16.
- Mondal M. H., Sarkar A., Maiti T. K. et al. Microbial assisted (Pseudomonas sp.) production of novel bio-surfactant rhamnolipids and its characterisation by different spectral studies. J. Mol. Liq. 2017; 242:873–78.
- Shao B., Liu Z., Zhong H. et al. Effects of rhamnolipids on microorganism characteristics and applications in composting: A review. Microbiol. Res. 2017; 200:33–44.
- Bouyahya A., Dakka N., Et-Touys A. et al. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac. J. Trop. Med. 2017; 10:729–43.
- Kłosowska-Chomiczewska I. E., Mędrzycka K., Hallmann E. et al. Rhamnolipid CMC prediction. Journal of Colloid and Interface Science. 2017; 488:10–19.
- Samadi N., Abadian N., Ahmadkhaniha R. et al. Structural characterization and surface activities of biogenic rhamnolipid surfactants from Pseudomonas aeruginosa isolate MN1 and synergistic effects against methicillin-resistant Staphylococcus aureus. Folia Microbiol. (Praha). 2012; 57:501–08.
- Gogoi D., Bhagowati P., Gogoi P. et al. Structural and physico-chemical characterization of a dirhamnolipid biosurfactant purified from Pseudomonas aeruginosa: application of crude biosurfactant in enhanced oil recovery. RSC Adv. 2016; 6:70669–81.
- Bognolo G. Biosurfactants as emulsifying agents for hydrocarbons. Colloids Surfaces A Physicochem. Eng. Asp. 1999; 152:41–52.
- Desai J. D., Banat I. M. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997; 38:47-64.
- Nguyen T. T., Sabatini D. A. Characterization and emulsification properties of rhamnolipid and sophorolipid biosurfactants and their applications. Int. J. Mol. Sci. 2011; 12:1232–44.
- Costa S. G. V. A. O., Nitschke M., Haddad R. et al. Production of Pseudomonas aeruginosa LBI rhamnolipids following growth on Brazilian native oils. Process Biochem. 2006; 41:483–88.
- Rufino R. D., Neves G., Luna J. M. et al. Conservation of the Biosurfactant Produced by Pseudomonas aeruginosa for Environmental Applications. Chem. Eng. Trans. 2016; 49:535–40.
- Gudiña E. J., Fernandes E. C., Rodrigues A. I. et al. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 2015b; 6:1–7.
- Das A. J., Kumar R. Production of biosurfactant from agro-industrial waste by Bacillus safensis J2 and exploring its oil recovery efficiency and role in restoration of diesel contaminated soil. Environ. Technol. Innov. 2019; 16:1–10.
- Abalos A., Pinazo A., Infante M.R. et al. Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir. 2001; 17:1367–71.
- Tedesco P., Maida I., Esposito F. P. et al. Antimicrobial activity of monoramnholipids produced by bacterial strains isolated from the Ross Sea (Antarctica). Mar. Drugs. 2016; 14:1–14.
- Ndlovu T., Rautenbach M., Vosloo J. A. et al. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express. 2017; 7:1–19.
- Ferreira J. F., Vieira E.A., Nitschke M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Research International. 2019; 116: 737-744.
- Mondal M. H., Malik S., Roy A. et al. Modernization of surfactant chemistry in the age of gemini and nio-surfactants – a review. RSC Advances. 2015; 5(112): 92707-92718.
- Mondal M. H., Roy A., Malik S., et al. Review on chemical bonded geminis with cationic heads: second generation interfactants. Res. Chem. Intermed. 2016; 42(3): 1913-1928.
- Mondal M. H., Sakar A., Maitini T. K. et al. Microbial assisted (pseudomonas sp.) production of novel bio-surfactant rhamnolipids and its characterisation by different spectral studies. J. Mol. Liq. 2017a; 242: 873-878.
- Mondal M. H., Malik S., Garain A., et al. Extraction of natural surfactant saponin from soapnut (Sapindus mukorossi) ans its utilization in the remediation of hexavalent chromium from contaminated water. Tenside Surfact. Det. 2017b; 54(6): 519-529.

This work is licensed under a Creative Commons Attribution 4.0 International License.





