Manuscript accepted on :
Published online on: --
Plagiarism Check: Yes
Reviewed by: PAVITHRA AMRITKUMAR
Second Review by: Aneeza soobadar
Final Approval by: Dr. rer. nat.Elsayed Ahmed
The Phytoremediation of Chromium from Soil Using Cirsium Vulgare and the Health Effects
Ayşe Handan Dökmeci1 and Sevinç Adiloğlu2*
1Department of Emergency and Disasters Management, Tekirdağ Namık Kemal University, 59030 Tekirdağ, Turkey.
2Department of Soil Science and Plant Nutrition, Tekirdağ Namık Kemal University, 59030 Tekirdağ, Turkey.
Corresponding Author E-mail: sadiloglu@hotmail.com
DOI : http://dx.doi.org/10.13005/bbra/2857
ABSTRACT: The spreadingof toxic substances as a result of human activities has become a serious problem for ground, atmosphere, and water ecosystems. Many of these toxic substances are pesticides, heavy metals such as chromium, and they cause serious health problems due to contamination ofsoil and food chain. In this study, the phytoremediation capacity of the medicinal plant Cirsium vulgarein the soil which was contaminated by Cr heavy metal, and the toxic effects of bioaccumulation using phytoremediation method were investigated. For this purpose, 30 mgkg-1 Cr heavy metal contaminant was applied to each pot as Chromium (IV)-oxide. To increase the absorption of this contaminant by the Cirsium vulgare, 0, 3, 6, 8, and 10 mmol/kg doses of EDTA were applied to the pots, respectively. According to the results, with the increasing doses of EDTA, Cr content of the above-ground parts of the plant reached to the highest value in the 6 mmol/kg EDTA chelate dosein 8.23mg/kgchromium for plant; after that, Cr accumulation decreased as a result of the toxic effect occurred inside the plant. These increases were determined as statistically significant(P<0,01). The results have demonstrated that the medicinal Cirsium vulgareplant is effective accumulator for the phytoremediation of the chromium-contaminated soils.
KEYWORDS: Chromium; Cirsium; Hyperaccumulators; Phytoremediation; Toxicity; Vulgare
Download this article as:| Copy the following to cite this article: Dökmeci A. H, Adiloğlu S. The Phytoremediation of Chromium from Soil Using Cirsium Vulgare and the Health Effects. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Dökmeci A. H, Adiloğlu S. The Phytoremediation of Chromium from Soil Using Cirsium Vulgare and the Health Effects. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/34R1a8C |
Introduction
While the most of the physicochemical technologies completely destroy the biological activity in soil and create an ill-conditioned environment for the plant growth, the phytoremediation method protects the biological quality and physical structure of the soil. However, in order to use phytoremediation method more efficiently, the molecular, biochemical, and physiological processes should be understood well, which characterize the accumulation of heavy metals (HMs)in the plant’s structure1-3.
HMs are among the most dangerous metals because they accumulate and remain in the tissues of living organisms, plants, soil, and water via bioconcentration (absorption from the environment) and biomagnification (absorption by food chain). Naturally occurring bioindicators are used to assess the health of the environment and can be also an important tool for detecting the changes in the environment, either positive or negative, and their subsequent effects on human society4.Hyperaccumulator plants can be used as a bioindicator. Hyperaccumulator plants are generally used in contaminated areas to remove metals. The plants which include copper, chromium, cadmium, lead, nickel, cobalt more than 0.1% (1.000 mgkg-1) or 1% (>10.000 mgkg-1) zinc and manganese are defined as metal hyperaccumulator plants 5,6.The soil ground is suitable for vegetation and covered by different plant types except toxic substances. Plants accumulate metals in their bodies through the roots. Phytoremediation is an alternative method for the cleaning of contaminated areas using plants. The studies give us the opportunity to select appropriate plant types to use in phytoremediation. The plants which are suitable for phytoremediation are not only able to accumulate the vast amount of HMsor organic compounds, but also should survive on low mineral soils and produce a high biomass7,8.So, it is important to be able to accumulate heavy metals such as chromium by plants in terms of both human and environmental health. There are not comprehensive studies on bioaccumulation of chromiumusing C. Vulgare.
Medicinal Plant: C. Vulgare
vulgare is a biennial plant that forms a rosette during the first growing season. Its flower and seed are formed the second year. Flowers are bright pink. The maximum annual rate of spread is over 5000 seeds per plant 9,10.It does not reproduce vegetative or have rhizomes. Each seed is topped with a cottony plume which makes them easily dispersed by the wind. The therapeutic use of plants goes back to prehistoric eras. Even in ancient time, people had tried herbal, animal, and mineral products to relieve their pains and they used many natural substances against illnesses for ages11.C.vulgare which is also known as common thistle, cotton thistle, woolly thistle, is a wild and medicinal plant which grows on the meadows, pastures or uncultivated lands. For the medical purpose, it has been used as anti-hemorrhoidal, anti-rheumatic or for injuries externally. C.vulgare has been used for different purposes (wrench, arthralgia, crick, psychological disorders) in traditional Chinese medicine and by NativeAmericans.
Chromium as an Environmental Contaminant
Chromium has a wide area of usage in metallurgy, glass painting, anodized aluminum, organic synthesis, tannery, and wood preservation industries12. Cr (VI) generally exists as chromate (CrO4-2). Cr (VI) is easy to accumulate and transport comparing to Cr (III), and its removal from water is more difficult. While EPA classifies Cr (VI) as carcinogenic, which is taken by inhalation, it classifies Cr (III) as the unknown cause of cancer. The US EPA determines Cr (VI) as one of the 17 most dangerous chemicals13.Cr (VI) can be made less toxic form by transforming it into Cr (III). Chromium can be found everywhere in environment including air, water, and soil. Cr content which is found naturally in soil varies between 10 and 50 mgkg-1 depending on the main material14.Chromium exposure can happen through food, water, and air contamination by inhalation, oral ways or through the skin. The average daily accumulation of chromium from the air, water, and foods is <0.2, 0.4, 2.0, and 60 micrograms depending on diet 15.The consumption of foods or medicinal plants which include chromium more than these levels can cause dangerous effects.
The Effects of Bioaccumulation of Medicinal Plants
Some herbs are used as medicine in the treatment of some diseases because of their pharmacologic effects; however, they can cause toxic effect if their heavy metal content is high. As a result of the consumption of the herbal remedies, the biological accumulation of HMs in human tissues causes negative health effects. In the USA, Europe, and Asia, the poisoning incidents,caused by the accumulation of toxic metals in medicinal herbs through the soil, water or air,are reported16-18.Moreover, the expansion of industrial areas, the use of agricultural pesticides (fertilizers containing cadmium, organic mercury or lead-based pesticides), contaminated waters are the significant reasons for the existence of the toxic metals19.WHO suggests controlling the medicinal plants in terms of various contaminants such as heavy/toxic metals, pesticides, fungi, and microorganisms20-22.There is very limited information about the potential effects of the metals in medicinal plants on the pharmacological activity of medicines. The toxic effects caused by HMs directly or indirectly appear pharmacologically by the attachment of the metals to the active substances or by transforming the pharmacokinetic of the active substances23.For that reason, the herbal medicines produced using the medicinal plants which grow on contaminated areas can cause serious problems for human health.
Materials and Methods
This research was conducted at Tekirdağ Namık Kemal University, Faculty of Agriculture, in the laboratories of Soil Sciences and Plant Nutrition Department in according to the randomized block pattern with 3 replicates. In the test, 30 mgkg-1 CrO3 (Chromium (IV)-oxide) was used as the contaminant. Then, the soils were left to the incubation for 30 days. The seeding of the medicinal plant C. vulgarewas completed in order to see its availability in phytoremediation method. To increase the dissolubility of the contaminant, 0, 3, 6, 8, and 10 mmol/ kg EDTA (Ethylene Diamine Tetra acetic Acid) doses were applied to the pots after 45 days of germination. Subsequent to the germination, 3 plants were left in the pots (Fig. 1). The plants were harvested 60 days later. The harvested plants were washed with distilled water, dried at 68°C for 48 hours before the wet digestion 21. The determination of total Cr concentration wavelength was 267.7 nm, and International Advanced Analytical and Life Science Solutions peak performance certified reference materials were used for Cr analysis with ICP-OES (Inductively Couple Plasma Spectrophotometer) device 24.
 |
Figure 1: Schematic of the proposed of phytoremediation. |
In the soil samples, pH, lime (CaO), organic substance, available phosphor (P), exchangeable potassium (K)25, extractable chromiummeasurements were made according to26. The research results were determined by variance analysis using the PASW® Statistics 18 for Windows software. The differences between groups were tested using Duncan multiple comparison testat p<0.01 level.
Results and Discussion
Some physical and chemical features of the testing soil which were gathered for the analyses are presented in Table 1.As it is observed in Table 1, the testing soil had argillaceous texture, insufficient organic substance (0.21%), and neutral pH (7.55). The testing soil which had acceptable saline structure was medium calcareous. Micro nutrient elements were sufficient considering the Fe, Cu, Zn, and Mn at the level of6.002 mgkg-1, 0.95 mgkg-1, 1.05 mgkg-1, and 14.23 mgkg-1, respectively. Especially chromium, which was the subject of this study, was identified as in trace quantity (0.060±0.1 mgkg-1).
Table 1: Some physical and chemical features of the testing soil
| Soil property | Value |
| pH | 7.55 |
| EC, (%) | 0.018 |
| Texture class | Clay |
| Organic matter (%) | 0.21 |
| P2O5 (kgda-1) | 13.54 |
| K2O (mgkg-1) | 485.02 |
| CaCO3, (%) | 5.92 |
| Fe (mg kg-1) | 6.002 |
| Zn (mgkg-1) | 1.05 |
| Cu (mg kg-1) | 0.95 |
| Mn (mgkg-1) | 14.23 |
| Cr(mgkg-1) | 0.060 |
The effects of 30 mg/ kg Cr contaminant which was applied to C. vulgare, and the application of EDTA doses which were used to increase the heavy metal absorption of the chromium heavy metal contents of the above-ground parts of the plant’s are explained below (Table 2).
Table 2: The average values (mg/kg) of the chrome heavy metal contents of the C. vulgare’s aboveground parts with the application of EDTA chelate and the significance groups.
| Plant
|
EDTA doses (mmol/kg) | |||||
| Control | 0 | 3 | 6 | 8 | 10 | |
| Chromium (Cr) (mg/kg) | 0.060±0.18a | 4.274±0.29b | 4.724±0.35c | 8.237±0.49d | 5.680±0.51e | 4.533±0.32c |
The values in the table with different uppercase letters (a, b, c, d, and e) are significantly different as per Duncan’s test (p<0.01)
Means ± standard deviation.
Many factors such as the physicochemical condition of the nutrient elements in soil, plant metabolism, and plant-root relations are effective in the absorption of the elements by the plants. Generally, the nutrient absorption system of the plant can change according to the soil’s element capacity and its solubility in the soil solution. Elements are held by the roots of the plant after their movement in soil. They first attach to the cell wall, then they reach to the cell by going into the plasma membrane and being regulated by transportation systems and intracellular attachment parts. Ions are taken by the canal proteins and/or carrier proteins 27,28.
In this study, only the chromium value was measured as heavy metal in above-ground biomass. The lowest Cr content in the above-ground parts of C. vulgarewas determined in 0.060 mgkg-1 control pot (Table 2). The highest Cr content was determined as 8.237 mgkg-1 in the pots on which the third dose (6 mmol/kg EDTA) was applied. This can explain theaugmentation in the solubility of the HMs which increased with the application of increasing doses of EDTA chelate. So, these resultsindicatedthat the EDTA applications increased the solubility of the Cr in soil (P<0.01) and thus, increased their absorption by the plantsfrom 0.060±0.1 mgkg-1 to 8.237±0.49 mgkg-1. When the plant was evaluated under significance groups, Cr heavy metal contents of the above-ground parts of C. vulgarewereobserved to be in different groups (Table 2 and Fig.2).
On the other hand, the argillaceous texture makes the cleaning process more difficult. Many medicinal aromatic plants are observed to be used in phytoremediation, which is applied in cleaning the dirty soils29,30.
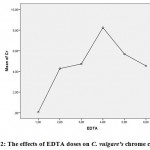 |
Figure 2: The effects of EDTA doses on C. vulgare’s chrome contents |
In research held by 31Luo et al, Cu, Zn, Pb, and Cdwere used as contaminants and corn plant was experimented. The researchers observed that the EDTA applications increased the Pb absorption of the corn plant and Pb’s transportation from roots to the trunk. Our findings were parallel with Luo et al.’s research results.
In another study conducted by Adiloğlu et al.32, 100 mgkg-1 Cr(NO3)3 chromium was used as a contaminant in the removing of the heavy metal contamination from the agricultural fields, and phytoremediation method was used on the growing canola plant. According to the research results, phytoremediation method can be used for removing of the chromium heavy metal from contaminated soils.
Besides, the medicinal C. vulgare’s Cr ions have higher mobility with EDTA chelate, therefore its bioaccumulation becomes easier. These plants offer a potential for the use of the cleaning of chromium in the soil. In the field studies, similar results were determined that the HMs were accumulated in each part of medicinal plants 33-35.
According to the statistical analysis results, the effects of different doses of EDTA chelate on C. vulgare’s absorption and solubility of chromium heavy metal were determined as P<0,01significant statistically (Table 3).
After the test, it was observed that the HMs remained in the pots (Table 2). This result was expectable because this test was conducted with highly concentrated HMs such as 30 mgkg-1. HMs of these high levels were impossible to be removed from the soil completely.
Table 3: Variant analysis results of Cirsium vulgare’s trunk parts that grew in chrome contaminated pots
| Cr | Sum of Squares | Df | MeanSquare | F | Sig. |
| Between Groups | 105.390 | 5 | 21.078 | 2106.179** | 0.000 |
| Within Groups | 0.120 | 12 | 0.010 | ||
| Total | 105.510 | 17 |
**: Highly significant on P≤ 0.01 level, *: significant on P≤0.05 level.
Conclusion
The contaminated soil, plant, water, and air are important environmental problems, and they are threat to the human health depending on the exposure level. It is possible to remove contaminants from the soil using phytoremediation method with appropriate plants. At the same time, it is dangerous to use the herbs that grow on contaminated soils for medical purposes, because the chronic absorption of chromium causes chromium toxicity. Medicinal plants should definitely be controlled forbeing sure that they grow on uncontaminated soils. For that reason, such medicinal plants should be produced on the soils far from industry, with uncontaminated air and water. As clearly understood from the chromium accumulation of this plant, it should not be used for medical purposes if phytoremediation will be applied. The results have shown that the medicinal plant C. vulgare is an effective accumulator plant for the phytoremediation of soils that are contaminated by chromium element. This situation should be remediation of low-level Cr toxicity in the soils.
Acknowledgment
This study was supported by Tekirdağ Namık Kemal University, Faculty of Agriculture, Soil Sciences and Plant Nutrition Department.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Lutts S., Lefevre I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Annals of botany,2015;115(3):509-28.
- Sarwar N, Imran M, Shaheen M.R, Ishaque W, Kamran M.A, Matloob A, Rehim A and Hussain S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chem.,2017; 171:710-721.
- Yinanc A, Adiloglu S. Use of Plants in Water Treatment: Models and Pilot Study Case in Kozan District. Journal of Tekirdag Agricultural Faculty,2017;14 (1):18-36.
- Parmar T.K, Rawtani D and Agrawal Y.K. Bioindicators: the natural indicator of environmental pollution, Frontiers in Life Science,2016;9(2): 110-118.
- Rascioa N, Navari-Izzo F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Science,2011;180(2):169-18.
- Adiloğlu S. The effects of manganese on the remediation of the heavy metal contaminated soil by using the dock (Rumex patientia L.) plant. Desalination and Water Treatment, 2017; 93:335-338.
- Ali H, Khan E and SajadM.A. Phytoremediation of heavy metals-Concepts and applications. Chem.,2013; 91(7): 869-88.
- Cheng S. Effects of heavy metals in plants and resistance mechanism. Environmental Science and Pollution Research,2003; 10: 250-264.
- Scott T.L, Buhner S.H. “Thistle (Cirsium spp.)”, Invasive Plant Medicine: The Ecological Benefits and Healing Abilities of Invasives. 2010; p:291. Toronto, Canada.
- United States Forest Service. Humboldt-Toiyabe National Forest (N.F.), Bridgeport Travel Management Project: Environmental Impact Statement. 2010; p:194.
- Dökmeci I, Dökmeci A.H. Toksikoloji, Zehirlenmeler de Tanı ve Tedavi. İstanbul Medikal Yayıncılık.; 2009; ISBN-978-9944- 211-43-7 (In Turkish).
- Madhavi V, Reddy A.V.B, Reddy K.G, Madhavi G and Prasad T.N.K.V. An Overview on Research Trends in Remediation of Chromium. Research Journal of Recent Sciences,2013; 2(1):71-83.
- USEPA IRIS, Toxicological Review of Hexavalent Chromium. 2010. External Review Draft). U.S. Environmental Protection Agency, Washington, DC EPA/635/R- 10/004A.
- Mitra G.N. “Uptake of heavy Metals”. Regulation of Nutrient Uptake by Plants: A Biochemical and Molecular Approach, Springer, India. 2015; p:92.
- ATSDR, (Agency for Toxic Substances and Disease Registry). 2017. ToxFAQs for Chromium, www.atsdr.cdc.gov/tfacts7.html (accessed on 10/8/18).
- Zhao M.Z, Chao-Sheng Z, Guang-Ming H and Dan-Lian C. Min. Toxicity and bioaccumulation of heavy metals in Phanerochaete chrysosporium. The Transactions of Nonferrous Metals Society of China,2016; 26:1410-1418.
- Zhang J, Wider B, Shang H, Li X and Ernst E. Quality of herbal medicines: challenges and solutions. Complementary Therapies in Medicine,2012; 20:100-6.
- Tripathy V, Basak B.B, Varghese T.S and Saha A. Residues and contaminants in medicinal herbs-a review. Phytochemistry Letters,2015;14:67-78.
- Dökmeci A.H, Yildiz T, Öngen A and Sivri N. Heavy metal concentration in deepwater rose shrimp species (Parapenaeus longirostris, Lucas, 1846) collected from the Marmara Sea Coast in Tekirdağ. Environmental Monitoring Assessment,2014;186:2449-2454.
- WHO (World Health Organization). 2003. “WHO Guidelines on Good Agricultural and Collection Practices (GACP) FOR Medicinal Plants”, WHO Press, Geneva, Switzerland.
- WHO (World Health Organization).2007. “Monographs on Selected Medicinal Plants”, Vol.1-3, WHO Press, Geneva, Switzerland.
- WHO (World Health Organization). 2008. “Quality Control Methods for Medicinal Plant Materials”, WHO Press, Geneva, Switzerland.
- Sadhu A, Upadhyay P,Singh P.K,Agrawal A, Ilango K, Karmakar D, Singh G.P andDubey G.P. Quantitative analysis of heavy metals in medicinal plants collected from environmentally diverse locations in India for use in a novel phytopharmaceutical product. Environmental Monitoring and Assessment,2015; 187(8): 542.
- Kacar B, İnal A. Plant Analysis, Nobel Publisher. 2010; No: 1241. Ankara.
- Sağlam M.T. Toprak ve Suyun Kimyasal Analiz Yöntemleri. Namık Kemal Üniversitesi, 2012; No: 2, Tekirdağ (In Turkish).
- Lindsay W.L, Norvell W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. The Soil Science Society of America,1978; 42: 421- 428.
- Clemens S, Palmgren M.G, Kraemer U. A long way ahead:Understanding and engineering plant metal accumulation.Trends in Plant Science,2002; 7:309–315.
- Karaman M.R, Adiloğlu A, Brohi R, Güneş A, İnal A,Kaplan M, Katkat V, Korkmaz A, Okur N, Ortaş İ, Saltali K, Taban S, Turan M, Tüfenkçi Ş, Eraslan F and Zengin M.Plant Nutrition. 2012; ISBN 978-605-87103-2-0, Dumat Ofset, Matba. San. Tic. Ltd. Şti., Ankara (In Turkish).
- Adiloğlu S. Using Phytoremediation with Canola to Remove Cobalt from Agricultural Soils.Polish Journal Environmental Study,2016;57:2251-2254.
- Adiloğlu S. Interaction of Manganese and Some Heavy Metals in Dock (Rumex patientia L.) Plant for Remediation of Contaminated Soils. Desalination and Water Treatment. 2017; 93: 335-338.
- Luo C, Shen Z, Lou L, Lix L. EDDS and EDTA-enhanced pyhtoextraction of metals from artificially contaminated soil and residual effects of chelant compounds. Environmental Pollution,2006;144: 862-871.
- Adiloğlu S, Sağlam M.T and Sümer A. Chromium (Cr) Pollution in Agricultural Areas Improvement by Phytoremediation Method with Canola (Brassica napus L.) Plant Growing. Journal of Essential Oil-Bearing Plants, 2015; 18;1180-1186.
- Maharia R.S, Dutta R.K, Acharya R and Reddy A.V.R. Heavy metal bioaccumulation in selected medicinal plants collected from Khetri copper mines and comparison with those collected from fertile soil in Haridwar, India. Journal of Environmental Science and Health, Part B2010; 45(2):174-181.
- Oyaro N, Makena B, Osano M.A andNyaigoti Omwoyo W. Determination of the Levels of selected Heavy Metals in Medicinal plants from Narok County, Kenya and variations in their levels due to hot water Infusion. International Journal of Environmental Science,2014; 3(12):5-10.
- Masvodza D.R, Dzomba P, Mhand F and Masamha B. Heavy Metal Content in Acacia saligna and Acacia polyacantha on Slime Dams: Implications for Phytoremediation. American Journal of Experimental Agriculture,2013; 3(4): 871-883.

This work is licensed under a Creative Commons Attribution 4.0 International License.





