How to Cite | Publication History | PlumX Article Matrix
Intestinal Macrophages and Intestinal Infection
Hadba Al-Amrah 1* Ahmad Bahieldin1,2, Dikhnah Alshehri1,3 Hanan Alatawi1,3 and Marfat Alatawy 1,3
Ahmad Bahieldin1,2, Dikhnah Alshehri1,3 Hanan Alatawi1,3 and Marfat Alatawy 1,3
1Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah-21589, Saudi Arabia.
2Department of Genetics, Ain Shams University, Cairo, Egypt.
3Department of Biological Sciences, Collage of Science, Tabuk University, Tabuk-74191, Saudi Arabia
Corresponding Author E-mail: hggaber@kau.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2885
ABSTRACT:
There has been increased interest in the role played by macrophages in the maintenance of an active immune system and intestinal homeostasis. Nonetheless, they are also responsible for the rise of chronic pathologies such as inflammatory bowel syndrome in the gut. The lack of differentiation of monocytes in the intestines due to disease conditions leads to a fall in the diversity of microbiota and subsequent gut inflammation. Macrophages play a central role in the homeostasis and immunity of the gut, making them potential sources of novel therapies or remedies for inflammatory bowel disease (IBD) patients. To explore this possibility, this research discusses their structure, differentiation, and functionality in an in-depth manner. It will also describe their role in the local intestinal environment and how it changes upon infection. Finally, the paper will outline its conclusions as well as comment on the future outlook of related research.
KEYWORDS: Anti-Inflammatory; Differentiation; Intestinal Homeostasis; Immunity; Inflammatory Bowel Disease; Monocyte; Microbiota
Download this article as:| Copy the following to cite this article: Al-Amrah H, Bahieldin A, Alshehri D, Alatawi H, Alatawy M. Intestinal Macrophages and Intestinal Infection. Biosci Biotech Res Asia 2020;17(4). |
| Copy the following to cite this URL: Al-Amrah H, Bahieldin A, Alshehri D, Alatawi H, Alatawy M. Intestinal Macrophages and Intestinal Infection. Biosci Biotech Res Asia 2020;17(4). Available from: https://bit.ly/394UNBC |
Introduction
A macrophage is a reference to a specific kind of white blood cell that forms part of the human body’s defense mechanism. It protects the body against foreign bodies, microbes, cellular debris, cancerous cells, and anything whose protein composition does not correspond to healthy body cell information on its surface. Macrophages are considered the sentinels of the intestinal immune system, and have a significant influence on the maintenance of homeostasis in the intestinal passage (1). Nonetheless, macrophages are also responsible for chronic ailments in the gastrointestinal tract, including inflammatory bowel disease. This research paper aims to perform an extensive discussion on intestinal macrophage and infection, with a special focus on intestinal infection by inflammatory bowel disease in human beings. Consequently, the essay will describe the structure, functions, as well as different types of macrophages. Moreover, it will discuss the role of macrophages in intestinal infection, especially inflammatory bowel disease(2, 3).
The gastro-intestinal tract houses the largest part of the immune system because it is highly prone to foreign antigens(1). It contains an extensive system of mono-nuclear phagocytes such as conventional dendric cells as well as macrophages. This network of cells has distinct roles that are complementary in the discrimination of potential pathogens, innocuous antigens, and subsequent corrective action (4). The breakdown of the efficiency of this process in the intestinal passage can result in the deployment of unsuitable defense mechanisms targeting commensal microbiota. This can cause chronic gastro-intestinal complications such as inflammatory bowel syndrome. Macrophages have wound-healing capabilities as well as plasticity that makes them potential novel remedies for the treatment of bowel inflammation. The role played by the macrophages in the intestinal tract also highly depends on their anatomical placement. Intestinal and colonic macrophages have a strategic location in the subepithelial lamina propria, where there is the largest concentration of antigenic stimulation.
Structure of Macrophage
Classification of macrophages has always been a difficult and ever-changing task, thanks to the difficulty of their identification. Traditionally, murine macrophages have been identified because they express the pan-macrophage marker F4/80 gene (1, 5). However, later research revealed that the gene could be expressed to some extent by eosinophils and conventional dendritic cells (cDCs). These cDCs have been previously known based on how they express MHCII as well as CD11c markers.
Nonetheless, these makers have also been extensively expressed by numerous macrophages, including gastro-intestinal ones. Superior markers of identifying macrophages in diverse tissues include FcγR1 (CD64) as well as the Mer tyrosine kinase (MERTK) (4). CD64 can be used together with CD11c and MHCII to differentiate macrophages and cDC in the intestinal tract.
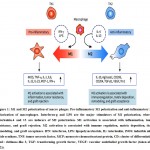
There are several types of macrophages based on their location and function in the human body. There are two dominant types of macrophages in the intestine: M1 and M2(6, 7). The former class secrete interleukin-12 (IL-12) in higher amounts as compared to IL-10, and are activated by interferon-gamma (IFN) as well as lipopolysaccharide (LPS). Their functions are phagocytic, pro-inflammatory, and bactericidal. M2 macrophages, on the other hand, engage in constructive activities such as regeneration and healing of inflamed muscles (8)(Shaw et al., 2018). By producing cytokines like IL-10 with inflammatory properties, they inhibit the activation of damaging the immune system(9). In the early stages of inflammation or wound healing, M1 macrophages are the dominant phenotype. They recede as the wound heals and are replaced by M2 macrophages which are necessary for the formation of collagen through re-epithelialization and revascularization (Figure 1)(4, 10, 11).
Fig.1. M1 and M2 polarization of macro phages. Pro-inflammatory M1 polarization and anti-inflammatory M2 polarization of macrophages. Interferon-g and LPS are the major stimulators of M1 polarization, whereas interleukin-4 and 13 are inducers of M2 polarization. M1 activation is associated with inflammation, tumor resistance, and graft rejection. M2 activation is associated with immune regulation, matrix deposition, tissue remodeling, and graft acceptance. IFN: interferon, LPS: lipopolysaccharide, IL: interleukin, iNOS: inducible nitric oxide synthase, TNF: tumor necrosis factor, MCP: monocyte chemoattractant protein, CD: cluster of differentiation, Ym1: chitinase-like 3, TGF: transforming growth factor, VEGF: vascular endothelial growth factor (taken after (12)).
Differentiation
Monocyte differentiation in the mucosa of healthy tissue results in the creation of anti-inflammatory macrophages. Differentiation allows macrophages to evolve specific functions and characteristics that mirror their environment(2, 13). Macrophages are characterized by high plasticity which aids their environmental differentiation. Intestinal macrophages exist in environments that contain other numerous microbiotas which cannot be identified as ‘self,’ and can, thus, be candidates for phagocytosis by macrophages (14, 15). To avoid the destruction of useful bacteria in the digestive tract, intestinal macrophages have evolved to suit their immediate environment.
Consequently, unlike tissue macrophages, intestinal macrophages do not secrete inflammatory cytokines. This change can be attributed to the production of TGF-β by intestinal epithelial cells which transform them into anti-inflammatory from pro-inflammatory (14). Nonetheless, phagocytosis in the gastro-intestinal tract is not affected by these environmental changes. Moreover, intestinal macrophages do not express receptors for IgA, IgG, or LPS to avoid the detection of gut microbiome molecular patterns(16, 17).
The differentiation of macrophages in the intestinal tract or lack of it is credited for the creation of pro-inflammatory conditions in the gut, which are a precursor for the onset of inflammatory bowel diseases (14). Monocytes in the gut undergo significant changes to their default functionality through a process of differentiation from the rest of tissue monocytes. Not only do they become hypersensitive to exogenous stimulation resulting in anti-inflammatory responses, but they also learn to distinguish microbiota in the gut to avoid their destruction. However, when the host is not in good physical health, the differentiation of these macrophages does not occur normally. The implication of this is the accumulation of macrophages in the gut and adverse influence on both the composition and diversity of commensals, thus leading to an inflamed gut(18, 19).
Functuion of Macrophages
The environment in which macrophages exist is very crowded, and, thus, immune responses must differentiate disease-causing micro-organisms from commensal ones. The epithelium of the gut acts as a structural inhibitor for both pathogens and commensals by producing a protective mucus coat containing antimicrobial elements (16). Gut macrophages play a phagocytic role in the intestinal tract, including scavenging dead cells or debris, preventing infection of the mucosa by disease pathogens, and controlling inflammatory reactions to the breach of the epithelial wall by pathogens and antigens (17, 20). Their phagocytic function is important to the regulation of inflammation because they ingest mature neutrophils which are responsible for initial inflammation. Fixed macrophages are located strategically in organs such as the liver, spleen, lungs, bones, and neural tissue where they ingest and destroy foreign micro-organisms as well as dead cells(21). Upon ingestion of these foreign materials by the phagocytes, they become surrounded by a phagosome. Consequently, fuses occur with a lysosome inducing the breakdown of the micro-organism by toxic peroxides and enzymes(22).
Besides, macrophages also participate in innate or non-specific immunity and the activation of adaptive or specific defense mechanisms. This is achieved through the recruitment of another immunity mechanism such as lymphocytes. Together with dendrites, these monocytes play an important role in the presentation of antigens and consequently initiating the body’s defense mechanisms against pathogens (16, 17). Upon digestion, the antigen of the pathogen is represented to the appropriate helper T-cell. This is achieved through cell membrane integration as well as by attachment to an MHC class II molecule (MHCII), which differentiates it from other antigens containing pathogens and white blood cells. The body produces antibodies for the attachment of antigens which aid in the adherence of macrophages to the pathogen cell membrane for phagocytosis(22).
Macrophages and monocytes regulate inflammation as well as immunity responses by the production of powerful secretions known as monokines. Such chemical substances include complement proteins, regulators such as interleukin-1, and enzymes (20, 23). They also have receptors for lymphokines, which once activated, relentlessly pursue tumor cells and micro-organisms.
Macrophages also play a vital role in muscle regeneration, growth, and repair. The onset of muscle injury stimulates the activation of two waves of macrophages: a phagocytic and regenerative wave. The first wave of phagocytes occurs during physical activity that can result in lysis and inflammation of the muscle membrane. These macrophages are at their peak during the first 24 hours and focus on the degradation of injured muscle tissue (20, 24). Upon their decline after 48 hours, the second wave of non-phagocytic macrophages occurs to stimulate muscle regeneration. This second wave occurs approximately 2-4 days after muscle injury, remaining in high concentration over a span of several days. The accumulation of macrophages in tissue regeneration is not muscle-specific but occurs in responses to an injury. Macrophages also influence wound healing by phagocytosis of pathogen and injured tissue as well as re-epithelialization. Growth factors secreted by cells such as platelets attract monocytes reserved in the spleen, which mature into macrophages upon reaching the wound site (20). Macrophages also secrete factors that participate in the proliferation of the healing stage.
Furthermore, macrophages participate in iron and tissue homeostasis. They play a crucial role in parenteral irons’ pharmacokinetics by virtue of their role in the destruction of erythrocytes, macromolecules, and other cellular debris (25). The iron that results from this process is either circulated into the body through ferroportin or stored in the ferritin. When high levels of systemic iron are present, hepcidin inhibits macrophage channels maintaining the homeostatic conditions. Resident as well as specialized populations of macrophages are also found in tissues. This non-migratory or sessile macrophages have vital growth factors that aid tissues in their physiological functions and protect them from excessive inflammation (25). Moreover, a sub-set of macrophages known as melanophages absorb both native as well as exogenous phagocytosed pigment.
Macrophages in Intestinal Infection
Research has shown that macrophages play an important part in the occurrence of the IBDs such as Crohn’s disease and ulcerative colitis (25, 26). Symptoms of IBD are rectal bleeding, diarrhea, weight loss, abdominal pain, and internal pelvic cramps(27). Ulcerative colitis only inflames the colon as well as the rectum, while Crohn’s disease affects numerous parts of the digestive tract, including the anus, ileum, colon, stomach, esophagus, and the mouth (28, 29)(Figure 2).
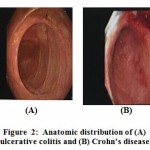 |
Figure 2: Anatomic distribution of (A) ulcerative colitis and (B) Crohn’s disease. |
Not only do they maintain tissue homeostasis, but they also resolve the onset of inflammation. Some individuals have an environmental or genetic predisposition that impairs their intestinal immunity in a chronic relapse of their immune system activation as well as gastro-intestinal infection, including but not limited to IBD (25, 30). When the gut is in a healthy condition, the inflammatory response is limited by intestinal macrophages. Disease-causing organisms alter intestinal macrophages extensively as far as diversity and numbers are concerned. The implication of this is an inflamed gut and subsequent IBD symptoms.
Macrophages have been touted in academic circles as potentially having novel treatment implications on patients suffering from inflammatory bowel syndrome. Recent studies have established a causal link between defective intestinal homeostasis and the differentiation of macrophages in the gut of IBD patients. The gut environment is symbiotic, and altering the composition as well as diversity of microbiota in the gut impacts immunity negatively and promotes the onset of IBD(14, 31). This intestinal ecosystem can be altered by oral medication such as antibiotics as well as environmental factors like concentrated milk fats often present in normal confectionery processed foods. Inflammation is generally resolved through the accumulation of externally activated pro-resolution macrophages as well as localized monocyte recruitment (4). Alternatively, activating differentiation of macrophages has resulted in successful therapeutic interventions targeting inflammatory bowel syndrome patients. Resolving intestinal inflammation and subsequent mucosal healing plays a vital part in the elimination of IBD signs and symptoms (25, 32, 33).
Conclusion
In conclusion, understanding the role of macrophages in the digestive tract is critical to the development of alternative therapies for IBD patients. Not only are they part of the intestinal immune system but they are also the maintainers of homeostasis in the gut. Homeostatic conditions in a healthy intestinal canal result in the downregulation of pathogen recognition receptors, preventing the destruction of microbiota and maintaining ecosystem balance. Epithelial breach, however, activates monocytes and lymphocytes from the bloodstream, which react protectively and in a pro-inflammatory manner. Failed resolution of the anti-microbial response and restoration of homeostasis can result in chronic inflammation. Nonetheless, numerous aspects of the biology, functionality, and differentiation are still not adequately understood. Case in point, human understanding of macrophage diversity in the intestinal ecosystem is still very limited.
Further research is necessary to better classification of macrophages and intra-system translation. Macrophage subpopulations that have been discovered will be critical in providing insight into mediating conditions that determine function, longevity, and phenotype of these macrophages. Such a new understanding will be invaluable in answering the question “why are some niches supportive of macrophage longevity while others are dependent on replenishment by monocytes?” There is also a need for further research on the impact of differences that exist along the digestive tract on the function and development of macrophages.
Acknowledgments
The authors would like to acknowledge the microbiome members, Department of Biological Science, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia for their expertise and assistance throughout all aspects of our study.
Conflict of Interests
The authors declare no conflicts of interest
References
- Scott CL, Bain CC, Wright PB, Sichien D, Kotarsky K, Persson EK, et al. CCR2+ CD103− intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal immunology. 2015;8(2):327.
CrossRef - Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nature Reviews Gastroenterology & Hepatology. 2019:1.
CrossRef - Hine AM. Intestinal macrophages in resolving inflammation. The Journal of Immunology. 2019;203(3):593-9.
CrossRef - Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, Zangerle-Murray T, et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. Journal of Experimental Medicine. 2018;215(6):1507-18.
CrossRef - Zhu Y, Zhang L, Lu Q, Gao Y, Cai Y, Sui A, et al. Identification of different macrophage subpopulations with distinct activities in a mouse model of oxygen-induced retinopathy. International Journal of Molecular Medicine. 2017;40(2):281-92.
CrossRef - Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6.
CrossRef - Mihic-Probst D, Reinehr M, Dettwiler S, Kolm I, Britschgi C, Kudura K, et al. The role of macrophages type 2 and T-regs in immune checkpoint inhibitor related adverse events. Immunobiology. 2020;225(5):152009.
CrossRef - Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. International journal of molecular sciences. 2018;19(6):1801.
CrossRef - Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Critical Reviews™ in Immunology. 2012;32(1).
CrossRef - Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Frontiers in physiology. 2018;9:419.
CrossRef - Kotwal GJ, Chien S. Macrophage differentiation in normal and accelerated wound healing. Macrophages: Springer; 2017. p. 353-64.
CrossRef - Lee K. M1 and M2 polarization of macrophages: a mini-review. Medical Biological Science and Engineering. 2019;2:1-5.
CrossRef - Grainger JR, Konkel JE, Zangerle-Murray T, Shaw TN. Macrophages in gastrointestinal homeostasis and inflammation. Pflügers Archiv-European Journal of Physiology. 2017;469(3-4):527-39.
CrossRef - Bernardo D, Marin A, Fernández-Tomé S, Montalban-Arques A, Carrasco A, Tristán E, et al. Human intestinal pro-inflammatory CD11c high CCR2+ CX3CR1+ macrophages, but not their tolerogenic CD11c− CCR2− CX3CR1− counterparts, are expanded in inflammatory bowel disease. Mucosal immunology. 2018;11(4):1114.
CrossRef - Bain CC, Schridde A. Origin, differentiation, and function of intestinal macrophages. Frontiers in immunology. 2018;9:2733.
CrossRef - Kim M, Galan C, Hill AA, Wu W-J, Fehlner-Peach H, Song HW, et al. Critical role for the microbiota in CX3CR1+ intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity. 2018;49(1):151-63. e5.
CrossRef - Shalhoub J, Falck-Hansen MA, Davies AH, Monaco C. Innate immunity and monocyte-macrophage activation in atherosclerosis. Journal of inflammation. 2011;8(1):9.
CrossRef - Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121-41.
CrossRef - Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Research. 2020:1-15.
- Panea C, Farkas AM, Goto Y, Abdollahi-Roodsaz S, Lee C, Koscsó B, et al. Intestinal monocyte-derived macrophages control commensal-specific Th17 responses. Cell reports. 2015;12(8):1314-24.
CrossRef - Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews immunology. 2011;11(11):723-37.
CrossRef - Walters M-I, Papadimitriou JM, Spector W. Phagocytosis: a review. CRC critical reviews in toxicology. 1978;5(4):377-421.
CrossRef - Spiering MJ. Primer on the immune system. Alcohol research: current reviews. 2015;37(2):171.
- Huen SC, Cantley LG. Macrophages in renal injury and repair. Annual review of physiology. 2017;79:449-69.
CrossRef - Bain CC, Schridde A. Origin, differentiation, and function of intestinal macrophages. Frontiers in immunology. 2018;9.
CrossRef - Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nature microbiology. 2017;2(5):1-7.
CrossRef - Fakhoury M, Coussa-Charley M, Al-Salami H, Kahouli I, Prakash S. Use of artificial cell microcapsule containing thalidomide for treating TNBS-induced Crohn’s disease in mice. Current Drug Delivery. 2014;11(1):146-53.
CrossRef - Alshehri D, Saadah O, Mosli M, Edris S, Alhindi R, Bahieldin A. Dysbiosis of gut microbiota in inflammatory bowel disease: Current therapies and potential for microbiota-modulating therapeutic approaches. Bosnian Journal of Basic Medical Sciences. 2020.
CrossRef - Al-Amrah H, Saadah O, Mosli M, Edris S, Alhindi R, Bahieldin A. Alteration Of The Gut Microbiome For Patients With Inflammatory Bowel Disease: A Review. Applied Ecology And Environmental Research.18(5):7379-92.
CrossRef - Alatawi H, Mosli M, Saadah O, Dulai P, Al-Hindi R, Bahieldin A, Et Al. Primary Non-Response In Inflammatory Bowel Disease, Definition, Potential Causes, Therapeutic Drug Monitoring And Microbiota–A Review. Applied Ecology And Environmental Research. 2020;18(4):5505-25.
CrossRef - Lozupone C, Lladser Me, Knights D, Stombaugh J, Knight R. Unifrac: An Effective Distance Metric For Microbial Community Comparison. The Isme Journal. 2011;5(2):169-72.
CrossRef - Sommer F, Rühlemann MC, Bang C, Höppner M, Rehman A, Kaleta C, et al. Microbiomarkers in inflammatory bowel diseases: caveats come with caviar. Gut. 2017;66(10):1734-8.
CrossRef - Sokol H, Leducq V, Aschard H, Pham H-P, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039-48.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.