How to Cite | Publication History | PlumX Article Matrix
Spore Morphology of Selected Pteridophytes Found in the Western Ghats of India
Shaiesh Morajkar1,2 , Sudha Sajeev1
, Sudha Sajeev1 and Smitha Hegde3
and Smitha Hegde3
1Department of Postgraduate Studies and Research in Biotechnology, St Aloysius College (Autonomous), Mangalore – 575003, Karnataka, India.
2Goa State Wetland Authority, O/o Goa State Biodiversity Board, DSTE complex, Saligao-403511, Bardez, Goa, India.
3Nitte University Centre for Science Education and Research, Nitte Deemed to be University, Derelakatte, Mangalore- 575018, Karnataka, India.
Corresponding Author E-mail: smitha.hegde@nitte.edu.in
DOI : http://dx.doi.org/10.13005/bbra/2899
ABSTRACT:
The currents study evaluated the morphology (apperture, size, perine structures and surface ornamentation) of treated spores of 45 selected fern species from the Western Ghats of India, using Scanning Electron Microspcopy (SEM). Twenty-six species of fern spores were trilete type, while 19 of them had monolete aperture types. The size of the spore were found to be highly variable (20X20µm to 60X60µm) with an average mean spore size of 44 µmX38µm. Further more the spores were found to have a highly diverse perine ornementaion with 11 different types of perine structures. Gammate and psilatetype of perine ornamentation,and Globose and ellipsoidal spore shape were found to be the most common within the studies fern spore samples.The variability found in the spore ultra structure and perispore ornamentation of the selected pteridophytes species reflects the morphological differences observed in the sporophyte. The spores could be an important source of characteristics with systematic value in fern taxonomy.The spore morphology of the examined pteridophytes studied common, endemicor otherwise will find a significant role in future taxonomic surveys, and other morphology, Palynology, discrimination, and identification studies of pteridophytesin the Western Ghats.
KEYWORDS: Endemic; Ferns; Perine Ornamentation; SEM; Spore Apertures
Download this article as:| Copy the following to cite this article: Morajkar S, Sajeev S, Hegde S. Spore Morphology of Selected Pteridophytes Found in the Western Ghats of India. Biosci Biotech Res Asia 2021;18(1). |
| Copy the following to cite this URL: Morajkar S, Sajeev S, Hegde S. Spore Morphology of Selected Pteridophytes Found in the Western Ghats of India. Biosci Biotech Res Asia 2021;18(1). Available from: https://bit.ly/2Stqmzt |
Introduction
There are approximately 1100 species of pteridophytes within 70 families and 192 genera distributed in India, with more than 349 species occurring in the Western Ghats1. With such high diversity of pteridophyte species occurring in the biodiversity hot spot of the Western Ghats,numerous of which are endemic, rare and endangered, it is challenging to get the appropriate information required for species identification and discrimination with the desired speed2. The easiest and one of the most efficient solution to this is classical taxonomic, and morphological data compendia of the extant fern diversity.
Palynology of ferns has proved to be very useful in the identification and discrimination of various fern taxa3. There have been several Palynology studies of pteridophytes that examined the characteristics of spore samples using basic microscopic staining techniques2, 4-9 to the modern use of scanning electron microscope (SEM)10-16. Palynology data depositories have been very useful for taxonomic purposes in ferns and have been previously used for identifying palynological fern sediments17, interspecies discrimination18-19, relatedness and phylogeny of the fern species20-24.This study aims to investigate the spore morphology and perine ultra structure of selected fern species found in the Western Ghats of India by using SEM. The results of the current study may provide a key for future studies in general fern morphology, palynology, fern identification and discrimination of pteridophytes in the Western Ghats.
Materials and methods
Forty-five different species of fern spore samples collected in June 2016, from the herbarium collection of Morajkar25,of Kudremukh National Park (13°1′ to 13°29′ N latitude and 75°0′ to 75°30′ E longitude), Western Ghats, thatwere made available at St Aloysius College (Autonomous) Mangalore, Karnataka. These fern samples were considered in the study as Morajkar25 has reported ferns that are rare, endemic and threatened in the Western Ghats. The spore surface was studied using Scanning Electron Microscope (SEM) after treating the spores in ultrasonic wave bath (50-60Hz) and subsequent washing with ethanol in a three-step process as elaborated by Hu et al.26. The spore structures and ornamentation were observed and photographed under a tabletop SEM.Two SEM models were utilized in the study namely, Hitachi TM 3030 and JOEL JFC-1600. For each pteridohyte species, multiple spore samples were examined from two different herbarium accession. The spore appertures, size, perine structures and surface variations were recorded as per Tschudy27.
Results and Discussion
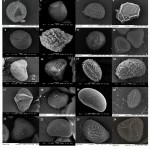
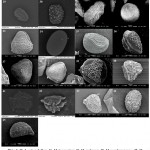
All 45selected fern spore samples were successfully treated and spore morphology was examined in details (Table1, Fig 1 and Fig 2 Plate 1 and Plate 2). The size of the spores were found to be highly variable and ranged from 20 X 20µm to 60 X 60µm with an average mean of 44µm X 38µm. Angiopteris helferiana and Nephrolepis hirsutula had the smallest spore, while Lygodium flexuosum and Osmunda huegeliana were found to have large spores. Similar observations of these fern spore sizes were also made by Makgomol11, Zenkteler2, and Shaikh & Madhav28.
Table 1: Characteristic spore morphology of 45 selected pteridophytes examined in the current study.
| Sr. No. | Fern species | Voucher no. | Avg. Size (µm) | Spore morphology | ||
| Aperture Type | Shape | Perispore Surface | ||||
| 1. | Acrositucum Aaureum L. | 54 KNP | 40×45 | Trilete | Globose | Verrucate |
| 2. | Adiantum philippense L. | 26 KNP | 50×45 | Trilete | Tetrahedral | Psilate |
| 3. | Adiantum raddianum Presl | 01 KNP | 40×30 | Trilete | Tetrahedral | Faveolate |
| 4. | Aleuritopteris anceps (Blanf.) Panigrahi | 27 KNP | 55×50 | Trilete | Globose | Striate |
| 5. | Angiopteris helferiana C. Presl. | 02 KNP | 20×20 | Trilete | Globose | Rugulate |
| 6. | Arachniodes tripinnata (Goldm.) Sledge | 10 KNP | 35×35 | Trilete | Globose | Baculate |
| 7. | Araiostegia pulchra (D. Don) Copel. | 21 KNP | 48×36 | Monolete | Globose | Verrucate |
| 8. | Asplenium yoshinagae makino Subsp. Indicum (Sledge) Frazer-Jenk. | 18 KNP | 50×40 | Monolete | Ellipsoidal | Rugulate |
| 9. | Blechnum orientale L. | 11 KNP | 30×28 | Monolete | Spheroidal | Psilate |
| 10. | Bolbitis semicordata (Baker) Ching | 37 KNP | 40×50 | Monolete | Globose | Echinate |
| 11. | Bolbitis subcrenatoides Fraser-Jenk. | 29 KNP | 30×35 | Monolete | Globose | Scabrate |
| 12. | Cheilanthes tenuifolia (Burn. F.) Sw. | 51 KNP | 45×45 | Trilete | Globose | Psilate |
| 13. | Cyathea gigantea (Wall. ex Hook.) Holttum | 12 KNP | 35×30 | Trilete | Globose | Rugulate |
| 14. | Cyathea nilgirensis Holttum | 13 KNP | 50×45 | Trilete | Globose | Psilate |
| 15. | Cyclosorus (Christella) dentata (Forssk.) Brownsey& Jermy | 30 KNP | 45×30 | Monolete | Ellipsoidal | Reticulate |
| 16. | Cyclosorus (Christella) parasitica (L.) H. Lev. | 14 KNP | 50×30 | Monolete | Ellipsoidal | Striate |
| 17. | Dicranpteris linearis (Born. f.) Underwood | 31 KNP | 55×50 | Trilete | Spheroidal | Psilate |
| 18. | Diplazium esculentum (Retz.) Sw. | 53 KNP | 30×20 | Monolete | Ellipsoidal | Reticulate |
| 19. | Drynaria quercifolia (L.) J. Sm. | 48 KNP | 65×40 | Monolete | Ellipsoidal | Scabrate |
| 20. | Lepisorous nudus (Hook.) Ching | 43 KNP | 50×25 | Monolete | Ellipsoidal | Gemmate |
| 21. | Lindsaea heterophylla Dryand. | 33 KNP | 35×33 | Trilete | Globose | Gemmate |
| 22. | Lindsaea ensifolia Sw. | 16 KNP | 40×36 | Trilete | Globose | Psilate |
| 23. | Lycopodiella cernua (L.) Pic. Ser. | 07 KNP | 54×40 | Monolete | Ellipsoidal | Verrucate |
| 24. | Lygodium flexuosum (L.) Sw. | 04 KNP | 60×60 | Trilete | Globose | Gemmate |
| 25. | Lygodium microphyllum (Cav.) R. Br. | 15 KNP | 55×40 | Trilete | Globose | Reticulate |
| 26. | Macrothelypteris torrensiana (Gaudich.) Ching | 35 KNP | 45×40 | Monolete | Spheroidal | Faveolate |
| 27. | Microlepia speluncae (L.) T. Moore | 17 KNP | 25×30 | Trilete | Tertrahedral | Pisilate |
| 28. | Microsorum membranaceum (Don) Ching | 34 KNP | 60×40 | Monolete | Ellipsoidal | Faveolate |
| 29. | Microsorum punctatum (L.) Copeland | 44 KNP | 40×30 | Monolete | Ellipsoidal | Gammate |
| 30. | Nephrolepis hirsutula (G. Frost.) C. Presl | 36 KNP | 28×17 | Monolete | Ellipsoidal | Verrucate |
| 31. | Odontosoria chinensis (L.) J. Smith | 41 KNP | 32×40 | Monolete | Ellipsoidal | Gammate |
| 32. | Osmunda huegeliana C. Presl | 08 KNP | 60×60 | Trilete | Spheroidal | Baculate |
| 33. | Pityrogramma calomelanos (L.) Link | 19 KNP | 50×45 | Trilete | Tetrahedral | Reticulate |
| 34. | Pteridium aquilinum (L.) Kuhn | 06 KNP | 25×30 | Trilete | Tetrahedral | Gemmate |
| 35. | Pteris argyraea T. Moore | 03 KNP | 50×50 | Trilete | Tetrahedral | Reticulate |
| 36. | Pteris biaurita L. | 38 KNP | 60×45 | Trilete | Tetrahedral | Gemmate |
| 37. | Pteris camerooniana Kuhn | 40 KNP | 54×55 | Trilete | Tetrahedral | Clavate |
| 38. | Pteris confusa T.G. Walker | 39 KNP | 40×35 | Trilete | Tetrahedral | Striate |
| 39. | Pteris quadriaurita Retz. | 20 KNP | 50×35 | Trilete | Tetrahedral | Gemmate |
| 40. | Pteris vittata L. | 45 KNP | 45×35 | Trilete | Tetrahedral | Rugulate |
| 41. | Selaginella tenera (Hook. &Grev.) Spring | 22 KNP | 33×30 | Trilete | Globose | Winged / Gemmate |
| 42. | Sellaginella delicatula (Desv.) Alston | 42 KNP | 40×32 | Trilete | Globose | Winged / Psilate |
| 43. | Tectaria coadunata (J. Sm.) C. Chr. | 24 KNP | 30×25 | Monolete | Ellipsoidal | Echinate |
| 44. | Tectaria polymorpha (Wall. ex Hook.) Copel. | 09 KNP | 65×40 | Monolete | Ellipsoidal | Baculate |
| 45. | Thelypteris (metathelypteris) flaccida (Bl.) Ching | 52 KNP | 54×45 | Monolete | Ellipsoidal | Scabrate |
The SEM results divide the spores of 45 fern species into 2 aperture types, trilete type and monolete type. 26 species of fern spores were found to be trilete, while 19 species had monolete aperture types. The majority of the trilete spores were globose (13), followed by11 species with tetrahedral and two with spheroidal type of spore shape. Spores with monolete apperture type were dominated by ellipsoidal shape constituting 14 species while spheroidal and globose spores were found in two and three species respectively. The apertures of most of the fern spores were found to be in accord with the studies of Vijayakanth & Sathish7 and Vijayakanth et al.29 It was also observed that the fern ultrastructure, apperture type and to much extent the shape of the spore were found to be similar within a fern genus.
The perispore forms the outer surface and often the characteristic contours of the spores.The most common ornamentation of perispore in the studied pteridophytespore samples was found to be gammate and psilate, with nine and eightfern species having the respective spore ornamentation. Among all the spores examined, Pteris camerooniana was the only fern with clavate type of perine ornamentation. Echinate type of perine ornamentation was seen in only two fern species namely Bolbitis semicordata and Tectaria coadunate. B. semicordata is known to be endemic to the Western Ghats.Additionally, seven species namely S. tenera, Osmunda huegeliana, Pteris quadriaurita, Bolbitis subcrenatoides, Cyathea nilgirensis, Cyclosorus parasitica, and Tectaria polymorpha are also known to be endemic to the Western Ghats. Perine structure of these species are very important for accurate identification and differentiation in future studies. As noted (Table 1), Perine structure of S. tenera and P. quadriaurita had gammate while T. polymorpha and O. huegelianahadbaculate type of perine ornamentation. Other endemic fern species namelyB. subcrenatoides, C. nilgirensis, and C. parasitica had scabrate, psilateandstriate type of perine ornamentation respectively.In addition to the perine surface structures fern species namely, Sellaginella tenera and Sellaginella delicatula had winged perispore. Earlier study such byZhou et al.30 has also reported winged perispores in Sellaginella species. The perispore surfaces observed in the current study were mostly in agreement with earlier studies2,7,17,29, this suggests that perispore morphology is generally consistent within species. A similar observation was also made by Moran et al23 in his study of perispore structures in Dryopteridaceae.
The spore structure given by Zenkteler2, describes the spore of P. aquilinum as tetrahedral and trilete with verrucae and baculate structures depicting an irregularly granulate perine structure. A similar spore structure with an uniform gammate perine structure is observed in the current study. It was noted by Zenkteler2 that P. aquilinum had the potential to release large numbers of spores which are known to be toxic and carcinogenic 31-32 and are dispersed by wind. This fern also known as the bracken fern, grows like a weed that can be an immense threat to the ecosystem.There has been confusion with this fern with P. revolutum 33 and both have been misidentified with each other.Even thought here are studies that report the distribution of this fern in various regions, such as Eastern Ghats7,The Western Ghats 34-36 and other parts of India37, there are also claims that P. aquilinum is not to be found in the Indian sub continent 38.Hence future comprehensive taxonomic studies with a standard method, along with molecular taxonomy are required in this regard, considering the toxicity and weed ability of this fern species.
Based on the results its found that all the pteridophytes within each family have the same aperture type, with the exception of Dryopterideceae. Both the species of Tectaria were found to have monolete aperture type, while A. tripinnata who belongs to the same family had a trilete spore aperture. In most cases the shape of spore were same within genus, but differed distintictively when perispore surface was compared. These results significantly elaborates on the importance of spore ultra structure and other features in identification and discrimination of pteridophytes. Contrary to this the findings of Yañez et al.,15 suggested that the spores with similar characteristics in phylogenetically unrelated families do not alow palynological features to have an evolutionary value in determining relationships between groups above the genus level. But the study on spore morphology and charaters by Passarelli et al.,39 suggest that perispore characters have distinct diagnostic value, since different combinations of ornamentation/structure were found to vary considerably among the pteridophytes.He also inferred that, when spore ornamentation is useful complementary feature at the specific level identification and discrimination when used in combination with other morphological traits of fern sporophytes. Additionally, the study undertaken by Chao and Huang 24 to investigate spore morphology evolution in Pteris species, revealed that spore characters, similar to leaf morphologies, reversed several times, but the combination of both characters could be useful in identification and discrimination and to establish more natural relationships within this group of fern species. Hence fern spore morphology is an important source of charateristics with systematic value in fern taxonomy.
Conclusion
The variability found in the spore ultra structure and perispore ornamentation reflects the morphological differences observed in the sporophyte of the selected fern species. This study will be an important source of characteristics with systematic value in fern taxonomy. The spore appertures, size, perine structures and surface variations observed within the selected pteridophytes will help future taxonomic studies of ferns in th Western Ghats of India. The ultara structure of the spores will suppliment other morphological characters and advance molecular tools,to enableprecise and standard taxanomic identification and differentiation in other fern species in question, such as P. aquilinum and P. revolutum debate.
Acknowledgement
Our sincere thanks to Rev Fr Swebert D’Silva SJ, Principal St Aloysius College (Autonomous), Dr Asha Abraham, HOD, Department of Post Graduate Studies and Research in Biotechnology, St Aloysius College and Rev Fr Dr Leo D’Souza SJ, Director, Dr Küppers Laboratory of Applied Biology, St Aloysius College for providing the research facilities.
Conflict of Interest
No potential conflict of interest is reported by the authors
Funding Source
None
References
- Manickam V. S., Irudayaraj V. (eds): Pteridophyte flora of the Western Ghats – South India. New Delhi: BI Publications Pvt Ltd, 1992.
CrossRef - Zankteler E. Morphology and peculiar features of spores of ferns species occurring in Poland. Acta Agrobot., 2012; 65 (2): 3-10.
CrossRef - Kramer K. U., Tryon R. M. Introduction to the Treatment of Pteridophytes. In: Pteridophytes and Gymnosperms (Kramer K. U, Green P. S, eds). The Families and Genera of Vascular Plants, vol 1. Berlin, Heidelberg: Springer,1990; pp 12-13. https://doi.org/10.1007/978-3-662-02604-5_3
CrossRef - Nayar B. K., Devi S. Spore Morphology of Indian Ferns. Grana, 1965; 6(1): 121-127. doi:10.1080/ 00173136 509429139
CrossRef - Irudayaraj V.,Bir S. S. Cytology of some ferns from the Nilgiris, South India – II. Fern Gazette. 1994; 14:301–312.
- Sorsa P. Studies on the spore morphology of Fennoscandian fern species. Annales Botanici Fennici, 1964; 1(3): 179-201. http://www.jstor.org/stable/23724600
- Vijayakanth P., Sathish S. S. Studies on the spore morphology of pteridophytes from Kolli hills, Eastern Ghats, Tamil Nadu, India. IJREB, 2016; 4(1): 1-12.
- Lashin G. Palynological Studies of Some Species of Aspleniaceae-Pteridophyta. American J. Plant Sci., 2012; 3: 397-402.
CrossRef - Sofiyanti N., Iriani D., Wati F., Marpaung A. A. Morphology, palynology, and stipe anatomy of four common ferns from Pekanbaru, Riau Province, Indonesia. Biodiversitas, 2019; 20(1): 327-336. doi: 10.13057/biodiv/d200138
CrossRef - Liu Y. C., Kuo C. M., Liu H. Y. SEM studies on spore in Taiwanese Fern Genera I. Athyriods. Taiwania, 2000; 45(2): 181-200.
- Makgomol K. Morphology of Fern Spores from Phu Phan National Park. Kasetsart J. (Nat. Sci.), 2006; 40: 116 – 122.
- Misra P. C., Tiwari S. Palynological studies of Nepalese fern- family Adiantaceae. Indian J.Sci.Res., 2016; 7(1): 77-79.
- Vaganov A. V., Gureyeva I. I., Kuznetsov A. A., Shmakov A. I., Romanets R. S., König V. A. Spore morphology of the representatives of the subfamily Ceratopteridoideae (J. Sm.) R.M. Tryon from the family Pteridaceae E.D.M. Kirchn. (Pteridophyta). J. Ecol., 2017; 7(2), 124-129. doi: 10.15421/2017_29
CrossRef - Vieira Jr. N. P., Schroeder G. D., Dec E., Mouga D. Palynological characterization of ferns of Acarai State park, São Francisco Do Sul, Santa Catarina State, Southern Brazil. J. Curr. Res., 2019; 11(9): 7060-72.
- Yañez A., Marquez G. J., Morbelli M. A. Palynological analysis of Dennstaedtiaceae taxa from the Paranaense Phytogeografic Province that produce monolete spores and its systematic implications (I): Blotiella lindeniana, Histiopteris incisa and Paesia glandulosa. An Acad Bras Cienc, 2017; 89(4): 2731-2748. http://dx.doi.org/ 10.1590/0001-3765201720170400
CrossRef - Pérez-Jiménez J. C., Eslava-Silva F. de J., Jiménez-Dúran K., Gómez-Noguez F., Muñiz-Díaz de León M. E. Palynological study of the ferns and lycophytes of the core west zone of the Ecological Reserve of the Pedregal of San Angel, Mexico City, Mexico. Botanical Sciences, 2020; 98(3), 517-532. https://doi.org/ 10.17129/ botsci.2424
CrossRef - Bouchal J. M., Zetter R., Grímsson F., Denk T. The middle Miocene palynoflora and palaeoenvironments of Eskihisar (Yatagan basin, south-western Anatolia): a combined LM and SEM investigation. J. Linn. Soc., 2016; 182, 14–79.
CrossRef - Márquez G. J., Morbelli M. A., Giudice G.E. Spore morphology and ultrastructure of Cyathea (Cyatheaceae, Pteridophyta) species from southern South America. Grana, 2010; 49(4): 269-280.doi: 10.1080/ 00173134.2010.517270
CrossRef - Ramos Giacosa J. P., Morbelli M. A., Giudice G. E. Spore morphology and wall ultrastructure of Blechnum L. species from North West Argentina. Review of Palaeobotany and Palynology, 2009; 156(1-2), 185–197. doi:10.1016/j.revpalbo.2008.11.002
CrossRef - Vaganov A.V. A comparative study of spore morphology of the subfamily Cryptogrammoideae genera. Biological Bulletin of Bogdan Chmelnitskiy Melitopol State Pedagogical University, 2016; 6(3), 333–346.http://dx.doi.org/10.15421/2016103
CrossRef - Moy C. J. Variations of fern spore ultrastructure as reflections of their evolution, Grana, 1988; 27(1): 39-51.doi: 10.1080/00173138809427731
CrossRef - Kuznetsov A. A., Vaganov A. V., Skapcov M. V., Erst A. S. A. Comparative Study of Spore Morphology of Some Pteridoideae Subfamily Genera. Biotechnol. Res. Asia, 2014; 11(Spl. Edn.): 17-25.
CrossRef - Moran R. C., Hanks J. G., Rouhan G. Spore morphology in relation to phylogeny in the fern genus Elaphoglossum (Dryopteridaceae). J. Plant Sci. 2007; 168(6): 905–929.
CrossRef - Chao Y-S., Huang Y-M. Spore morphology and its systematic implication in Pteris (Pteridaceae). PLoS ONE, 2018; 13(11): e0207712. https://doi.org/10.1371/journal.pone.0207712
CrossRef - Morajkar S. Biodiversity of ferns of Western Ghats using molecular markers (Doctoral dissertation thesis, Mangalore University, Mangalore, India). Shodhganga, 2018; http://hdl.handle.net/10603/249856
- Hu X. Y., Zhai J. W., Wang F. G., Xu X. L. Simple treatment to investigate spore ornamentation of ferns for SEM observation from herbarium specimens. J. Bot., 2010; 42: 2335-2338.
- Tschudy R. H. The plant kingdom and its palynological representation. In: Aspects of Palynology (Tschudy RH. ed) Wiley-interscience. 1969; pp 32-34.
- Shaikh S. D., Madhav N. A. Spore morphology of four species of pteridophytes from Northern Western Ghats of Maharashtra (India). Indian Fern J., 2019; 36: 89-94.
- Vijayakanth P., Sathish S., Palani R., Thamizharasi T., Vimala A. Palynomorphic studies on the pteridophytes of Kolli Hills, Eastern Ghats, Tamil Nadu. Disc., 2017; 8(4): 752-761
- Zhou X., Jiang L., Zhang L., Gao X., He Z., Zhang L. Spore morphology of Selaginella (Selaginellaceae) from China and its systematic significance. Phytotaxa, 2015; 237(1): 1. doi: 10.11646/phytotaxa.237.1.1
CrossRef - Page CN (ed): The Ferns of Britain and Ireland. Cambridge: Cambridge University Press. 1997; pp 344-370.
- Siman S. E., Povey A. C., Sheffield E. Human health risks from fern spores? – a review. Ferngaz, 1999; 15: 275 -287.
- Fraser-Jenkins C. R. (ed): Taxonomic revision of three hundred Indian sub-continental pteridophytes with a revised census list – a new picture of fern-taxonomy and nomenclature in the Indian subcontinent. Dehradun: Bishen Singh Mahendra Pal Singh Publishers, 2008.
- Benjamin A., Manickam V. S. Medicinal pteridophytes from Western Ghats. IJTK, 2007; 6(4): 611-618.
- Hegde S., Sajeev S. (eds):A collection of selected ferns of Karnataka. Karnataka: Karnataka Forest Department, Government of Karnataka, India, 2013.
- Shaikh S. Status of some important pteridophytes from the parts of Northern Western Ghats of Maharashtra, India. Res. J. Biol. Sci., 2017; 6(1): 47-49.
- Behera S. K., Khare P. B. First report of pteridophytes from Govind Wildlife Sanctury, Uttarkashi, Uttarakhand, India. Plant Res., 2014; 1(2): 37-47.
- Ranil R. H. G., Pushpakumara G., Fraser-Jenkins C. R., Wijesundara S. Misidentification of Pteridium revolutum (Blume) Nakai as an invasive alien in Sri Lanka. In: Invasive Alien Species in Sri Lanka – Strengthening Capacity to Control Their Introduction and Spread (Marambe B., Silva P., Wijesundara S., Atapattu N. eds), Biodiversity Secretariat, Ministry of Environment, Colombo, Sri Lanka, 2010; pp141-149.
- Passarelli L. M., Galán J. M. G., Prada C., Rolleri C. H. Spore morphology and ornamentation in the genus Blechnum (Blechnaceae). Grana, 2010; 49(4): 243-262. https://doi.org/10.1080/00173134.2010.524245
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.