How to Cite | Publication History | PlumX Article Matrix
Adipokines in Insulin Resistance: Current Updates
Department of Biochemistry, Assistant Professor Dr Ambedkar College, Deekshabhoomi, Nagpur 440010, Maharashtra, India
Corresponding Author E-mail: utpal24dongre@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2922
ABSTRACT:
Obesity is a chronic metabolic disease that affects both the pediatric and adult populations. Adipose tissue acts as an endocrine organ which secretes various adipokines involved in fat mass regulation and energy balance via modulating the metabolic signalling pathways. Altered secretion of adipokines promotes multiple complications, including insulin resistance. The primary mechanism of action that underlines the involvement of adipokines in the development of insulin resistance includes phosphorylation/de-phosphorylation of insulin receptor substrate-1 (IRS-1) facilitate by other signalling molecules like a suppressor of cytokine signalling 1 (SOCS-1). Adipokines mediated insulin resistance further contribute to the development of atherosclerosis, dyslipidemia, fatty liver disease, cancer etc. Thus, this review provides recent updates on the role of resistin, lipocalin-2, RBP-4, chemerin, TNF-alpha and IL-6 adipokines in the progression of insulin resistance.
KEYWORDS: Adipose Tissue; Adipokines; Fatty Liver Diseases; Insulin resistance; Insulin Receptor Substrate-1 (IRS-1)
Download this article as:| Copy the following to cite this article: Dongre U. J. Adipokines in Insulin Resistance: Current Updates. |
| Copy the following to cite this URL: Dongre U. J. Adipokines in Insulin Resistance: Current Updates. Available from: https://bit.ly/2UALFQu |
Introduction
Obesity is emerging as an epidemic in both developed and developing countries. As per the current statistics, in the United States, approximately 42.4% adult men and women are affected with obesity 1, while for India a rise of 30.5% from the prevailing percentage has been forecasted by the year 2040 2. Obesity usually promotes type 2 diabetes mellitus, hypertension, atherosclerosis and cardiovascular diseases 3. But, amid them, type 2 diabetes mellitus can be more severe due to insulin resistance in the liver, muscle cells and other peripheral tissues 4. In this regard, it can be assumed that obesity is a metabolic disorder that further increases the diabetes burden apart from the pathophysiology of insulin. Over nutrition or high-calorie intake deposits lipids in the form of triglycerides in adipose tissue, resulting in obesity 5. The severity of obesity is often accompanied by low exercise and stressful lifestyle 6. In mammals, adipose tissue cluster pre-adipocytes, mature adipocytes, stromal vascular cells, macrophages and endothelial cells. However, adipose tissue is no longer considered as a mere fat storage depot; instead, it is now considered as an endocrine organ due to the secretion of adipokines such as adiponectin, leptin, visfatin, omentin etc., which regulate energy/metabolic homeostasis 7,8. Deposition of excess energy causes adipose tissue dysfunction, which usually exhibits a low-grade chronic inflammation due to higher secretion of inflammatory and pro-inflammatory cytokines like IL-6 and TNF-alpha. This chronic inflammation favours insulin resistance by modulating various metabolic pathways 9. The present review discusses the basic pathophysiology of adipokines with their current updates in the aetiology of insulin resistance.
Adipose Tissue Physiology
Adipose tissues are classified as brown adipose tissue (BAT) and white adipose tissue (WAT), originated from mesoderm and the mesenchymal stem cells during embryogenesis. BAT is rich in mitochondria; hence appear brown and predominantly involved in thermogenesis (heat production) via uncoupling proteins 7. Conversely, WAT is organ-specific and is further divided into visceral (mesenteric, retroperitoneal, omental and pericardial) and subcutaneous (beneath the skin) adipose depots; thus obesity-related consequences are primarily regulated by WAT 10. To store extra energy pre-adipocyte differentiates into mature adipocytes under the strict regulation of CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor-gamma (PPARγ) transcriptional factors 11. This causes WAT expansion through a rise in adipocyte number (hyperplasia) and/or increasing adipocyte volume/size (hypertrophy). The rise in adipocyte number favours severe obesity, while increased adipocytic volume contributes to obesity, overweight and diabetes. In- vivo studies showed that in adults, adipocytic numbers are usually constant; however, adipocytic volume increases 12. This suggests the severity of obesity in the onset of type 2 diabetes mellitus in adult patients. Earlier, adipose tissue was considered as an inert fat storage organ, but the discovery of leptin revealed the endocrine functions of this organ, which secrete proteins/hormones/factors/cytokines, collectively called as “adipokines” 13. Obesity promotes altered secretion of adipokines, which work as endocrine, paracrine and autocrine way and modulate lipid (lipogenesis and lipolysis) and glucose metabolism 14. A growing body of evidence proves the role of various adipokines in the pathophysiology of insulin resistance, which includes, resistin, lipochalin-2, retinol-binding protein-4 (RBP-4), chemerin, TNF-alpha and IL-6 15,16.
Resistin
Resistin a 114 amino acid (10 kDa) containing adipokine also called as an adipose tissue-specific secretory factor (ADSF) was discovered by Dr Mitchell A. Lazar in the year 2001 17,18. Resistin is a member of a cysteine-rich protein termed as resistin like molecule (RELM) and circulates as a hexamer and trimer. Hexameric form of this adipokine is more abundant, while trimeric form induces severe insulin resistance 19. The mechanism by which resistin causes the insulin resistance includes the activation of suppressor of cytokine signalling-3 (SOCS-3), which attenuates insulin-arbitrate signalling in adipocytes 20. In association with the toll-like receptor (TLR-4), resistin stimulates insulin resistance in different cells. In the hypothalamus, resistin directly binds with the TLR-4, which suppresses signalling pathways via stimulation of MyD88 and TIRAP adaptor protein accumulation and debilitates insulin response in the hypothalamus by phosphorylation of insulin receptor, AKT and ERK1/2. The activation of Resistin/TLR-4 pathway also upregulated the activity of SOCS-3 and protein-tyrosine phosphatase 1B (PTP1B) and thereby promote insulin resistance 21.
The fibroblast growth factor (FGF)-21 is an important hormone that regulates many metabolic activities. The FGF-21 works as insulin-sensitizing hormone as like of adiponectin. Study on the chronic intracerebroventricular mechanism revealed that resistin infusion in the brain of mice downregulates adiponectin synthesis via regulating its adaptor protein known as APPL1 in both hypothalamus and liver. Resistin also inhibits expression of FGF-21 receptors on the hypothalamus and the peripheral tissues, resulting in FGF-21 resistance. This effect of resistin was abolished in TLR4 knockout mice, suggesting the role of Resistin/TLR-4 pathways in FGF-2 resistance/ insulin sensitivity 22. Further, studies on mice reported resistin/TLR-4 pathway for increased hypertension, insulin resistance 23 and breast cancer progression 24. It has been evidenced that aerobic exercise prevents insulin resistance in type 2 diabetes mellitus via miR-382-3p/Resistin25 and miR-492/Resistin axis 26. Recent studies exhibit the role of resistin in endothelial related insulin resistance. Treatment of resistin on human umbilical vein endothelial cells (HUVC) showed that oxidative stress in the endoplasmic reticulum promotes insulin resistance and impairment in the endothelium 27. Also, tunicamycin induced oxidative stress in endoplasmic reticulum reported increased resistin mRNA in human THP-1 monocytes 28.
Lipocalin-2
The lipocalin-2 (Lcn2) is a 25 kDa adipokine also known as neutrophil gelatinase-associated lipocalin (NGAL), sidrocalin and 24p3 belong to the lipocalin superfamily and reported for altered glucose metabolism and insulin resistance 29. Lcn2 is highly expressed in adipocytes, liver, kidney and on macrophages and regulates apoptosis and innate immunity 30. The primary mechanism underlines the effect of Lcn2 on insulin resistance include the modulation of 12-lipoxygenase activity and TNF-α levels in adipose tissue 31. The level of this adipokine increases during pre-adipocyte differentiation into mature adipocyte. With the help of a small cavity like hydrophobic structures Lcn2 binds and transport distinct lipophilic compounds like steroids, retinoids and arachidonic acids 32. Study on LCN2−/− mice showed increased hepatic gluconeogenesis, debilitate lipid metabolism, impaired oxidation capacity of mitochondria, elevated inflammation favouring dyslipidemia due to diet-induced obesity, fatty liver disorders and insulin resistance 33.
Systems genetics analyses studies revealed the sex-specific role of Lcn-2. Overexpression of this adipokine in adipose tissue has been reported for elevated fat mass, glucose intolerance and insulin resistance only in females via mitochondrial dysregulation 34. Studies using synthetic glucocorticoids and dexamethasone in the regulation of Lcn-2 expression in adipose tissue explore the role of sex steroids 35. In postmenopausal women, 17-β-estradiol (E2) increases the Lcn-2 expression in subcutaneous adipose tissue. There are two estrogen receptors (ERα and ER-β), which facilitates the effects of steroids on adipose tissue, however, among them; ERβ plays a significant role in the binding of β-estradiol36. Synthetic dexamethasone increases ERβ pathway and decreases the ERα pathway and thus responsible for glucocorticoid-induced insulin resistance in human adipose tissue via Lcn-2 adipokine 37. Many other studies also implicate the role of steroids in the induction of Lcn-2 induced insulin resistance 38,39. Moreover, Lcn-2 showed inhibition of autophagy and insulin resistance induction in H9c2 cells derived from rat heart ventricle 40.
Retinol Binding Protein-4 (Rbp-4)
Retinol binding protein-4 (RBP-4) is another crucial adipokine that attributes in insulin resistance. Apart from adipocyte RBP-4 is also expressed in liver and macrophages 41. Higher expression of this adipokine in the adipocyte is inversely associated with the GLUT-4 expression in the adipocyte. Thus, decreased GLUT-4 in adipocytes promotes higher expression of RBP-4, which inhibits insulin-mediated insulin receptor substrate-1 (IRS-1) phosphorylation that can contribute to insulin resistance 42. During obesity, RBP-4 is preferentially produced by visceral fat depot than subcutaneous fat depot, suggesting the role of intra-abdominal adipose tissue in insulin resistance 43. The thiazolidinedione, a peroxisome proliferator-activated gamma (PPARγ) stimulating drug suppresses RBP-4 production in adipose tissue and thereby stimulate insulin sensitivity of tissues (skeletal muscle) 44. However, RBP-4 mediated insulin resistance also play a pivotal role in the development of cardiovascular diseases (CVDs). Increased production of RBP-4 in adipose tissue stimulates the higher production of adhesion molecules like vascular cell adhesion molecule-1 (VCAM), intercellular adhesion molecule-1 (ICAM) and E-selectin in the endothelial cells, resulting in atherosclerosis-related CVDs and hypertension 45.
Of note, the prevalence of RBP-4 related insulin resistance is considered as a significant risk factor for the pediatric cardiometabolic system 46. Also, RBP-4 in association with adiponectin and Fatty Acid–Binding Protein 4 (FABP-4) are reported to be associated with the increased rate of CVDs in male patients of type 2 diabetes mellitus 47, while in adolescent girls the increased CVDs are associated with waist circumference in overweight/ obese 48. Thus, RBP-4 and insulin resistance suggest the role of sex hormones in the progression of CVDs and coronary artery diseases 49. Furthermore, assessment of the levels of RBP-4 for ten years during childhood can help in the prediction of future cardiometabolic risks 50. Apart from the CVDs elevated RBP-4 related insulin resistance can be correlated with the progression of rheumatoid arthritis 51 and non-alcoholic fatty liver disease 52.
Chemerin
Chemerin is secreted from adipose tissue as an inactive pre-pro chemerin (163 amino acids). After the intracellular hydrolytic cleavage of N‑terminal polypeptide (20‑amino‑acid), it releases in the serum as 18-kDa inactive pro-protein, which then converts into 16-kDa active chemerin by serine protease cleavage of the C-terminal portion 53. The estimated concentration of chemerin in plasma and serum of mice was reported 0.6 and 0.5 nM, respectively, while in human 3.0 and 4.4 nM, respectively 54. This adipokine is also called as tazarotene-induced gene 2 (TIG2) encoded by the retinoic acid receptor responder 2 (Rarres2) gene and acts as an endocrine, paracrine as well as an autocrine way 55. Chemerin is a pro-inflammatory adipokine predominantly produced by white adipose tissue (WAT) and act as a ligand for G-protein-coupled receptor CMKLR1 56. Chemerin regulates the immune system (adaptive and innate), adipogenesis and metabolic homeostasis 57. Overexpression of chemerin in adipose tissue causes insulin resistance in human skeletal muscles by modulating IRS-1, glucose uptake, Akt, glycogen synthase kinase 3 phosphorylation (GSK3P), nuclear factor-κB (NF-kB), p38 mitogen-activated protein kinase and extracellular signal-regulated kinase (ERK)-1/2 58.
Non-alcoholic fatty liver disease (NAFLD) is a common phenomenon in obesity, which is closely associated with chemerin induced increased insulin resistance 60. However, outdoor aerobic exercise improves the status of chemerin induced insulin resistance and thereby NAFLD 61. Overexpression of CMKLR1 promotes insulin resistance. During low-grade inflammation (a common feature of obesity), both chemerin and CMKLR1 exhibit inverse expression, manifesting in the progression of insulin resistance 62. Tumour necrosis factor-alpha (TNF-alpha) is a potent inflammatory cytokine that in association with chemerin induces insulin resistance. This effect of chemerin-TNF-alpha is overcome by the high-intensity interval training (HIIT), further suggesting the role of exercise in the prevention of insulin resistance 63. However, another study in women with multiple sclerosis proves that the continuous chronic aerobic exercise lowers the chemerin, insulin and thereby suppresses insulin resistance 64. Chemerin can be used as an adipokine marker for uremic insulin resistance in chronic kidney diseases at stages 3, 4, and 5 65. Moreover, a growing body of recent evidences revealed the role of chemerin induced insulin resistance in the development and the prognosis of polycystic ovary syndrome (PCOS) in adult women. A recent study on 45 patients with PCOS showed higher chemerin levels in obese PCOS group as compared to the lean PCOS, obese and the non-obese groups 66. These finding described above and other recent studies 67-68 signify the role of chemerin induced insulin resistance in the progression of PCOS.
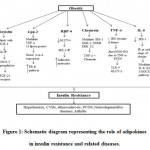 |
Figure 1: Schematic diagram representing the role of adipokines in insulin resistance and related diseases.Click here to view figure |
Adipokines such as resistin, Lpn-2, RBP-4, chemerin, TNF-α and IL-6 secreted during obesity altered numerous metabolic signalling pathways that result in insulin resistance and related diseases. TLR-4: Toll-like receptor-4, Irs-P: Insulin receptor phosphorylation, SOCS-3: suppressor of cytokine signalling-3, FGF-21: Fibroblast growth factor, Lpn-2: Lipocalin-2, TNF-α: Tumour necrosis factor-α, ER-β: Estrogen receptor-β, RBP-4: Retinol binding protein-4, GLUT: Glucose transporter, IRS-1-P: Insulin receptor substrate-1-phosphorylation, GSK-3: Glycogen synthase kinase 3 phosphorylation, NF-kB: Nuclear factor-κB (NF-kB), ERK: Extracellular signal-regulated kinase, NOS: Nitric oxide synthase, NO: Nitric oxide, PTEN: Phosphatase and tension homologue, IR: Insulin resistance, IL-6: Interleukin-6, STAT-3: signal transducer and activator of transcription 3.
TNF-α
Tumour necrosis factor-α (TNF-α) is secreted by adipose tissue often considered as an adipocytokine. A 26 kDa transmembrane monomer of this adipokine converts into 17-kDa soluble TNF-α molecule by an enzyme TNF-α converting enzyme (TACE). It acts as an inflammatory cytokine that affects distinct cellular and biological functions including apoptosis, cell differentiation, energy metabolism and immune system 69. TNF-α adipocytokine induces insulin resistance via decreasing the tyrosine kinase activity of the insulin receptor 70. This causes altered signalling pathways that can induce insulin resistance and related diseases. One of such signalling pathway that modulated by TNF-α is Akt/eNOS (nitric oxide synthase)/NO. In mice with fed by a high-fat diet, overexpression of TNF-α in adipose tissue positively modulates phosphatase and tension homologue (PTEN) and suppresses Akt/eNOS/NO signalling pathways in a vascular wall, leading to insulin resistance 71. Thus, TNF-α/PTEN pathway can be targeted as a therapeutic to treat insulin resistance and vascular complications in obesity. During diabetes, in hepatic cells, TNF-attenuation improves the insulin receptor substrate 1 (IRS-1) via phosphorylation 72 at serine residues 636/639 and inhibiting the tyrosine phosphorylation of IRS-1. This phosphorylation/dephosphorylation is governed by c-jun N-terminal kinase (JNK) and an extracellular signal-regulated kinase (ERK) phosphorylation 73. Apart from the IRS-1, toll-like receptors TLRs and GLUT-2 pathways modulate other signalling pathways that results in insulin resistance 74,75. Further, the PPAR α/γ agonist aleglitazar76, GW501516 77 and TACE selective inhibitor JTP-96193 78 have been reported for the inhibition of TNF-α arbitrate inflammatory reactions and insulin resistance. However, through independent pathways, insuin resistance due to this adipocytokine induces neuroinflammation in immortalised hypothalamic neuronal cells, which may promote neurodegenerative diseases 79.
IL-6
Interleukin-6 is a pro-inflammatory cytokine secreted by many different cell types and tissues, including adipose tissue, which regulates growth and development of distinct tissues and plays a significant role in the immune response 80. This adipocytokine contributes to low-grade chronic inflammatory state responsible for adipose tissue dysfunction via altered lipid and carbohydrate metabolism, coronary artery diseases (atherosclerosis), CVDs diabetes and insulin resistance 81. The mechanism of action by which this cytokine imparts its role in insulin resistance involves inhibitory effects on the gene transcription of PPAR gamma, GLUT-4 and IRS-1. This causes a reduction in IRS-1, insulin-stimulated tyrosine phosphorylation. In hepatocytes, SOCS inhibit insulin receptor signalling that stimulates insulin resistance 82, 83. Obesity-related insulin resistance due to IL-6 promotes impaired adipogenesis in subcutaneous fat in humans, suggesting the role of IL-6 in the modulation of signalling pathways 84. By emphasizing the role of IL-6 in T cells, it has been corroborated that through a classical signalling pathway IL-6 stimulates inflammation and insulin resistance at the early stages of obesity development 85. However, studies in humans showed an association between the amount of IL-6 and the size of the visceral adipose tissue favours insulin resistance 86. Further, IL-6 in association with signal transducer and activator of transcription 3 (STAT3) modulates a variety of signalling pathways that imparts their role in insulin resistance/sensitivity and related diseases. It has been reported that the treatment of myo-Inositol in rat PCOS model downregulates the insulin resistance in association with IL-6-STAT-3 signalling pathway 87. Whereas, atmospheric fine particles (PM2.5) increases the IL-6 levels in rat liver and suggest an essential role in the regulation of type 2 diabetes mellitus through IL-6/STAT3/SOCS3 pathway 88. Pu-erh tea extract mitigates insulin resistance and non-alcoholic steatohepatitis through IL-6/STAT3 signalling pathway in mice 89. However, the blocking of IL-6 receptor improves insulin sensitivity in patients with rheumatoid arthritis and non-diabetic 90.
Conclusion
Dysfunctional adipose tissue secretes altered levels of adipokines that are associated with many health problems, including insulin resistance. Adipokines imparts their deleterious effects in the development and the progression of insulin resistance mostly through IRS-1/STAT-3/SOCS signalling pathways. Recent findings exhibit the role of adipokine induced insulin resistance as a major risk factor for the development of chronic diseases like neurodegenerative diseases, non-alcoholic fatty liver disease, chronic kidney diseases, cardiovascular diseases etc. However, determining the role of adipokines in the aetiology of insulin resistance may provide new opportunities for developing novel therapeutics for obesity arbitrates insulin resistance.
References
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief. 2020;(360):1-8.
- Luhar S, Timæus IM, Jones R, et al. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS One. 2020;15(2):e0229438.
CrossRef - Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7):s176-s185.
CrossRef - Li Y, Ding L, Hassan W, Abdelkader D, & Shang J. Adipokines and hepatic insulin resistance. Journal of diabetes research, 2013, 170532.
CrossRef - Sacks G, Vanderlee L, Robinson E, et al. BIA-Obesity (Business Impact Assessment-Obesity and population-level nutrition): A tool and process to assess food company policies and commitments related to obesity prevention and population nutrition at the national level. Obes Rev. 2019;20 (2):78-89.
CrossRef - van den Berk-Clark C, Secrest S, Walls J, et al. Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: A systematic review and meta-analysis. Health Psychol. 2018;37(5):407-416.
CrossRef - Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018;27(1):68-83.
CrossRef - Bozkurt L, Göbl CS, Rami-Merhar B, et al. The Cross-Link between Adipokines, Insulin Resistance and Obesity in Offspring of Diabetic Pregnancies. Horm Res Paediatr. 2016;86(5):300-308.
CrossRef - Stolarczyk E. Adipose tissue inflammation in obesity: a metabolic or immune response?. CurrOpinPharmacol. 2017;37:35-40.
CrossRef - Hou B, Zhao Y, He P, et al. Targeted lipidomics and transcriptomics profiling reveal the heterogeneity of visceral and subcutaneous white adipose tissue. Life Sci. 2020;245:117352.
CrossRef - Kuri-Harcuch W, Velez-delValle C, Vazquez-Sandoval A, Hernández-Mosqueira C, Fernandez-Sanchez V. A cellular perspective of adipogenesis transcriptional regulation. J Cell Physiol. 2019;234(2):1111-1129.
CrossRef - Cox AR, Chernis N, Masschelin PM, Hartig SM. Immune Cells Gate White Adipose Tissue Expansion. Endocrinology. 2019;160(7):1645-1658.
CrossRef - Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf). 2012;205(2):194-208.
CrossRef - Francisco V, Pino J, Gonzalez-Gay MA, et al. Adipokines and inflammation: is it a question of weight?. Br J Pharmacol. 2018;175(10):1569-1579.
CrossRef - Monzillo LU, Hamdy O, Horton ES, et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res. 2003;11(9):1048-1054.
CrossRef - Cătoi AF, Suciu Ş, Pârvu AE, et al. Increased chemerin and decreased omentin-1 levels in morbidly obese patients are correlated with insulin resistance, oxidative stress and chronic inflammation. Clujul Med. 2014;87(1):19-26.
CrossRef - Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem. 2001;276(14):11252-11256.
CrossRef - Barnes KM, Miner JL. Role of resistin in insulin sensitivity in rodents and humans. Curr Protein Pept Sci. 2009;10(1):96-107.
CrossRef - Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307-312.
CrossRef - Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25(4):1569-1575.
CrossRef - Benomar Y, Taouis M. Molecular Mechanisms Underlying Obesity-Induced Hypothalamic Inflammation and Insulin Resistance: Pivotal Role of Resistin/TLR4 Pathways. Front Endocrinol (Lausanne). 2019;10:140.
CrossRef - Benomar Y, Amine H, Crépin D, et al. Central Resistin/TLR4 Impairs Adiponectin Signaling, Contributing to Insulin and FGF21 Resistance. Diabetes. 2016;65(4):913-926.
CrossRef - Jiang Y, Lu L, Hu Y, et al. Resistin Induces Hypertension and Insulin Resistance in Mice via a TLR4-Dependent Pathway. Sci Rep. 2016;6:22193. Published 2016 Feb 26.
CrossRef - Wang CH, Wang PJ, Hsieh YC, et al. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene. 2018;37(5):589-600.
CrossRef - Liu SX, Zheng F, Xie KL, Xie MR, Jiang LJ, Cai Y. Exercise Reduces Insulin Resistance in Type 2 Diabetes Mellitus via Mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol Ther Nucleic Acids. 2019;18:34-44.
CrossRef - Cai Y, Xie KL, Zheng F, Liu SX. Aerobic Exercise Prevents Insulin Resistance Through the Regulation of miR-492/Resistin Axis in Aortic Endothelium. J Cardiovasc Transl Res. 2018;11(6):450-458.
CrossRef - Luo J, Huang L, Wang A, et al. Resistin-Induced Endoplasmic Reticulum Stress Contributes to the Impairment of Insulin Signaling in Endothelium [published correction appears in Front Pharmacol. 2018 Dec 10;9:1446]. Front Pharmacol. 2018;9:1226. Published 2018 Oct 26.
CrossRef - Hamada J, Onuma H, Ochi F, et al. Endoplasmic reticulum stress induced by tunicamycin increases resistin messenger ribonucleic acid through the pancreatic endoplasmic reticulum eukaryotic initiation factor 2α kinase-activating transcription factor 4-CAAT/enhancer binding protein-α homologous protein pathway in THP-1 human monocytes. J Diabetes Investig. 2016;7(3):312-323.
CrossRef - Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56(10):2533-2540.
CrossRef - Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917-921.
CrossRef - Law IK, Xu A, Lam KS, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59(4):872-882.
CrossRef - Esteve E, Ricart W, Fernández-Real JM. Adipocytokines and insulin resistance: the possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009;32 Suppl 2(Suppl 2):S362-S367.
CrossRef - Guo H, Jin D, Zhang Y, et al. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59(6):1376-1385.
CrossRef - Chella Krishnan K, Sabir S, Shum M, et al. Sex-specific metabolic functions of adipose Lipocalin-2. Mol Metab. 2019;30:30-47.
CrossRef - Li D, Yan Sun W, Fu B, Xu A, Wang Y. Lipocalin-2-The myth of its expression and function. Basic Clin PharmacolToxicol. 2020;127(2):142-151.
CrossRef - Kamble PG, Pereira MJ, Almby K, Eriksson JW. Estrogen interacts with glucocorticoids in the regulation of lipocalin 2 expression in human adipose tissue. Reciprocal roles of estrogen receptor α and β in insulin resistance?. Mol Cell Endocrinol. 2019;490:28-36.
CrossRef - Kamble PG, Pereira MJ, Sidibeh CO, et al. Lipocalin 2 produces insulin resistance and can be upregulated by glucocorticoids in human adipose tissue. Mol Cell Endocrinol. 2016;427:124-132.
CrossRef - Bhusal A, Lee WH, Suk K. Lipocalin-2 in Diabetic Complications of the Nervous System: Physiology, Pathology, and Beyond. Front Physiol. 2021;12:638112.
CrossRef - Park YM, Pereira RI, Erickson CB, Swibas TA, Cox-York KA, Van Pelt RE. Estradiol-mediated improvements in adipose tissue insulin sensitivity are related to the balance of adipose tissue estrogen receptor α and β in postmenopausal women. PLoS One. 2017;12(5):e0176446. Published 2017 May 4.
CrossRef - Chan YK, Sung HK, Jahng JW, Kim GH, Han M, Sweeney G. Lipocalin-2 inhibits autophagy and induces insulin resistance in H9c2 cells. Mol Cell Endocrinol. 2016;430:68-76.
CrossRef - Wolf G. Serum retinol-binding protein: a link between obesity, insulin resistance, and type 2 diabetes. Nutr Rev. 2007;65(5):251-256.
CrossRef - Kojta I, Chacińska M, Błachnio-Zabielska A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients. 2020;12(5):1305. Published 2020 May 3.
CrossRef - Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184-6223. Published 2014 Apr 11.
CrossRef - Majerczyk M, Olszanecka-Glinianowicz M, Puzianowska-Kuźnicka M, Chudek J. Retinol-binding protein 4 (RBP4) as the causative factor and marker of vascular injury related to insulin resistance. PostepyHig Med Dosw (Online). 2016;70(0):1267-1275.
- Sun X, Zhang Z, Ning H, Sun H, Ji X. Sitagliptin down-regulates retinol-binding protein 4 and reduces insulin resistance in gestational diabetes mellitus: a randomized and double-blind trial. Metab Brain Dis. 2017;32(3):773-778.
CrossRef - Lin WT, Lin PC, Lee CY, et al. Effects of insulin resistance on the association between the circulating retinol-binding protein 4 level and clustering of pediatric cardiometabolic risk factors. Pediatr Diabetes. 2018;19(4):611-621.
CrossRef - Liu G, Sun Q. Response by Liu and Sun to Letter Regarding Article, “Plasma Levels of Fatty Acid-Binding Protein 4, Retinol-Binding Protein 4, High-Molecular-Weight Adiponectin, and Cardiovascular Mortality Among Men With Type 2 Diabetes: A 22-Year Prospective Study”. ArteriosclerThrombVasc Biol. 2017;37(5):e57.
CrossRef - Klisić A, Kavarić N, Bjelaković B, Soldatović I, Martinović M, Kotur-Stevuljević J. The Association Between Retinol-Binding Protein 4 and Cardiovascular Risk Score is Mediated by Waist Circumference in Overweight/Obese Adolescent Girls. Acta Clin Croat. 2017;56(1):92-98.
CrossRef - Wang H, Zhou P, Zou D, Liu Y, Lu X, Liu Z. The role of retinol-binding protein 4 and its relationship with sex hormones in coronary artery disease. BiochemBiophys Res Commun. 2018;506(1):204-210.
CrossRef - Li G, Esangbedo IC, Xu L, et al. Childhood retinol-binding protein 4 (RBP4) levels predicting the 10-year risk of insulin resistance and metabolic syndrome: the BCAMS study. Cardiovasc Diabetol. 2018;17(1):69. Published 2018 May 14.
CrossRef - Wei Y, Xia N, Zhang W, et al. Serum retinol-binding protein 4 is associated with insulin resistance in patients with early and untreated rheumatoid arthritis. Joint Bone Spine. 2019;86(3):335-341.
CrossRef - Boutari C, Perakakis N, Mantzoros CS. Association of Adipokines with Development and Progression of Nonalcoholic Fatty Liver Disease. Endocrinol Metab (Seoul). 2018;33(1):33-43.
CrossRef - Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282(38):28175-28188.
CrossRef - Zabel BA, Zuniga L, Ohyama T, et al. Chemoattractants, extracellular proteases, and the integrated host defense response. Exp Hematol. 2006;34(8):1021-1032.
CrossRef - Helfer G, Wu QF. Chemerin: a multifaceted adipokine involved in metabolic disorders. J Endocrinol. 2018;238(2):R79-R94.
CrossRef - Kennedy AJ, Yang P, Read C, et al. Chemerin Elicits Potent Constrictor Actions via Chemokine-Like Receptor 1 (CMKLR1), not G-Protein-Coupled Receptor 1 (GPR1), in Human and Rat Vasculature. J Am Heart Assoc. 2016;5(10):e004421. Published 2016 Oct 14.
CrossRef - Stojek M. The role of chemerin in human disease. PostepyHig Med Dosw (Online). 2017;71(0):110-117. Published 2017 Feb 15.
CrossRef - Sell H, Laurencikiene J, Taube A, et al. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes. 2009;58(12):2731-2740.
CrossRef - Zhang Z, Wang J, Wang H. Correlation of blood glucose, serum chemerin and insulin resistance with NAFLD in patients with type 2 diabetes mellitus. Exp Ther Med. 2018;15(3):2936-2940.
CrossRef - Ren SM, Mei L, Huang H, Cao SF, Zhao RH, Zheng PY. Zhonghua Gan Zang Bing Za Zhi. 2019;27(5):369-375.
- Kim DI, Lee DH, Hong S, Jo SW, Won YS, Jeon JY. Six weeks of combined aerobic and resistance exercise using outdoor exercise machines improves fitness, insulin resistance, and chemerin in the Korean elderly: A pilot randomized controlled trial. Arch GerontolGeriatr. 2018;75:59-64.
CrossRef - Corona-Meraz FI, Navarro-Hernández RE, Ruíz-Quezada SL, et al. Inverse Relationship of the CMKLR1 Relative Expression and Chemerin Serum Levels in Obesity with Dysmetabolic Phenotype and Insulin Resistance. Mediators Inflamm. 2016;2016:3085390.
CrossRef - Skuratovskaia D, Zatolokin P, Vulf M, Mazunin I, Litvinova L. Interrelation of chemerin and TNF-α with mtDNA copy number in adipose tissues and blood cells in obese patients with and without type 2 diabetes. BMC Med Genomics. 2019;12(Suppl 2):40. Published 2019 Mar 13.
CrossRef - Lakhdar N, Landolsi M, Bouhlel E, Tabka Z. Effect of diet and diet combined with chronic aerobic exercise on chemerin plasma concentrations and adipose tissue in obese women. Neuro Endocrinol Lett. 2019;40(6):262-270.
- El-Khashab SO, Gamil M, Ali AY, et al. Chemerin level and the relation to insulin resistance in chronic kidney disease. Saudi J Kidney Dis Transpl. 2019;30(6):1381-1388.
CrossRef - Yang X, Quan X, Lan Y, et al. Serum chemerin level in women with PCOS and its relation with the risk of spontaneous abortion. Gynecol Endocrinol. 2018;34(10):864-867.
CrossRef - Foda AA, Foda EA, El-Negeri MA, El-Said ZH. Serum chemerin levels in Polycystic Ovary Syndrome after metformin therapy. Diabetes MetabSyndr. 2019;13(2):1309-1315.
CrossRef - Abruzzese GA, Gamez J, Belli SH, et al. Increased chemerin serum levels in hyperandrogenic and normoandrogenic women from Argentina with polycystic ovary syndrome. Gynecol Endocrinol. 2020;36(12):1057-1061.
CrossRef - Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582(1):117-131.
CrossRef - Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271(5249):665-668.
CrossRef - da Costa RM, Neves KB, Mestriner FL, Louzada-Junior P, Bruder-Nascimento T, Tostes RC. TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc Diabetol. 2016;15(1):119.
CrossRef - Alipourfard I, Datukishvili N, Mikeladze D. TNF-αDownregulation Modifies Insulin Receptor Substrate 1 (IRS-1) in Metabolic Signaling of Diabetic Insulin-Resistant Hepatocytes. Mediators Inflamm. 2019;2019:3560819.
CrossRef - Takaguri A. YakugakuZasshi. 2018;138(11):1329-1334.
CrossRef - Tian S, Wang M, Liu C, Zhao H, Zhao B. Mulberry leaf reduces inflammation and insulin resistance in type 2 diabetic mice by TLRs and insulin Signalling pathway. BMC Complement Altern Med. 2019;19(1):326.
CrossRef - Xuguang H, Aofei T, Tao L, Longyan Z, Weijian B, Jiao G. Hesperidin ameliorates insulin resistance by regulating the IRS1-GLUT2 pathway via TLR4 in HepG2 cells. Phytother Res. 2019;33(6):1697-1705.
CrossRef - Massaro M, Scoditti E, Pellegrino M, et al. Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)α/γ agonist aleglitazar in attenuating TNF-α-mediated inflammation and insulin resistance in human adipocytes. Pharmacol Res. 2016;107:125-136.
CrossRef - Kim WJ, Lee W, Jung Y, Jang HJ, Kim YK, Kim SN. PPARβ/δ agonist GW501516 inhibits TNFα-induced repression of adiponectin and insulin receptor in 3T3-L1 adipocytes. BiochemBiophys Res Commun. 2019;510(4):621-628.
CrossRef - Maekawa M, Tadaki H, Tomimoto D, et al. A Novel TNF-α Converting Enzyme (TACE) Selective Inhibitor JTP-96193 Prevents Insulin Resistance in KK-AyType 2 Diabetic Mice and Diabetic Peripheral Neuropathy in Type 1 Diabetic Mice. Biol Pharm Bull. 2019;42(11):1906-1912.
CrossRef - Clemenzi MN, Wellhauser L, Aljghami ME, Belsham DD. Tumour necrosis factor α induces neuroinflammation and insulin resistance in immortalised hypothalamic neurones through independent pathways. J Neuroendocrinol. 2019;31(1):e12678.
CrossRef - Keller C, Keller P, Marshal S, Pedersen BK. IL-6 gene expression in human adipose tissue in response to exercise–effect of carbohydrate ingestion. J Physiol. 2003;550(Pt 3):927-931.
CrossRef - Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48(9):e12997.
CrossRef - Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278(46):45777-45784.
CrossRef - Senn JJ, Klover PJ, Nowak IA, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278(16):13740-13746.
CrossRef - Almuraikhy S, Kafienah W, Bashah M, et al. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia. 2016;59(11):2406-2416.
CrossRef - Xu E, Pereira MMA, Karakasilioti I, et al. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat Commun. 2017;8:14803.
CrossRef - Kuo FC, Huang YH, Lin FH, et al. Circulating Soluble IL-6 Receptor Concentration and Visceral Adipocyte Size Are Related to Insulin Resistance in Taiwanese Adults with Morbid Obesity. MetabSyndrRelatDisord. 2017;15(4):187-193.
CrossRef - Zhang Y, Li C, Zhang W, Zheng X, Chen X. Decreased Insulin Resistance by Myo-Inositol Is Associated with Suppressed Interleukin 6/Phospho-STAT3 Signaling in a Rat Polycystic Ovary Syndrome Model. J Med Food. 2020;23(4):375-387.
CrossRef - Long MH, Zhang C, Xu DQ, et al. PM5aggravates diabetes via the systemically activated IL-6-mediated STAT3/SOCS3 pathway in rats’ liver. Environ Pollut. 2020;256:113342.
CrossRef - Cai X, Fang C, Hayashi S, et al. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J Gastroenterol. 2016;51(8):819-829.
CrossRef - Cai X, Fang C, Hayashi S, et al. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J Gastroenterol. 2016;51(8):819-829.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.







