How to Cite | Publication History | PlumX Article Matrix
Artificial Sweeteners and their Health Implications: A Review
Tejashree Anil More1  , Zoya Shaikh1
, Zoya Shaikh1  and Ahmad Ali2*
and Ahmad Ali2*
1Department of Life Sciences, University of Mumbai, Vidyanagari, Santacruz (East), Mumbai – 400098, India.
2Department of Hematogenetics, ICMR- National Institute of Immunohaematology, 13th Floor, NMS Building, KEM Hospital Campus, Parel, Mumbai, Maharashtra, 400012, India.
Corresponding Author E-mail: ahmadali@mu.ac.in
DOI : http://dx.doi.org/10.13005/bbra/2910
ABSTRACT: Background: Sugar is an inevitable part of our diet. Since ages, sweeteners have been used to enhance the flavour and appearance of food products. Sweeteners may be natural or synthetically produced. Those that are synthetic, as a whole, are referred to as artificial sweeteners. This review aims at highlighting the characteristics and health implications of artificial sweeteners. Methodology: In this review, the physical and chemical characteristics of artificial sweeteners are highlighted. Also, the impact of artificial sweeteners on human health is discussed in detail. The data has been collected using standard search engines like PubMed, Google scholar and websites of publishing houses like Elsevier and springer. Results and Discussion: Today, due to high calorie content, natural sweeteners are getting replaced by artificial ones. The US Food and Drug Administration(USFDA) has approved utilization of five artificial sweeteners namely, saccharin, sucralose, aspartame, neotame and cyclamate. However, artificial sweeteners should be consumed carefully and in limited quantities. This is because the consumption of artificial sweeteners is controversial owing to their effects on health ranging from mild headache to dreadful cancer risks. Conclusion: Hence, long term study of these sweeteners for further safety evaluation on health risks is essential.
KEYWORDS: Artificial Sweeteners; Calorie; Diabetes,Obesity; Sugars
Download this article as:| Copy the following to cite this article: More T. A, Sheikh Z, Ali A. Artificial Sweeteners and their Health Implications: A Review. Biosci Biotech Res Asia 2021;18(2). |
| Copy the following to cite this URL: More T. A, Sheikh Z, Ali A. Artificial Sweeteners and their Health Implications: A Review.Biosci Biotech Res Asia 2021;18(2). Available from: https://bit.ly/3jb7ArY |
Introduction
Sugar is an inextricable part of our diet. From centuries, sweeteners have been used for making foodstuffs more delicious and tempting. Sweeteners of natural origin are either mono- or disaccharides with some nutritional value[1]. It is inherent nature of humans to like sweet food. Previously published studies have reported the inclination of new-borns towards sweet-tasting nutrition. Therefore, sweetening agents has always been popular in man-made food preparations[2].
The energy disparity observed between the calorie intake and its expenditure, as a result of luxurious lifestyle proceeding urbanisation in the Indian population, is priming their way towards obesity. Among the various food sources, sugar-sweetened beverages have been significantly highlighted as a cause for obesity. Recently, the harmful effect of high intake of sugars is steadily reported. On the other hand, the increasing exposure to databases through internet sources and media has significantly amplified health awareness among the masses in recent years. Therefore,better alternatives to the existing food products are required for the maintenance of health and fitness in the society[3].Some debated recommendations indicate that excessive sugar may cause certain neuro degenerative diseases. Keeping this in mind, consumers started using artificial sweeteners as a replacement for common sugar. In view of customer satisfaction, the pressure to meet dietary requirements with caloric restrictions and competition between factors like taste and appearance, the food industries are continuously in search of low calorie and intense artificial sweeteners. A number of marketed low-calorie products are now available as healthier food choices. Artificial sweeteners also classified as food additives for man indispensable part of modern food industry. They are utilised globally not only for taste enhancement but also for maintenance of food quality and safety[4]. As the name suggests, sweeteners are used in desserts, beverages and similar preparations. However, they are around 200 times sweeter and contain lesser calories than sugar. The sweeteners may be natural or synthetically produced. Those that are synthetic, as a whole, are called artificial sweeteners. These are consumed as universal additives in confection aries and pharmaceutical preparations. However, the safety of these additives must be approved by the European Union (EU) and Food and Drug Administration (FDA) under the Generally Recognized as Safe (GRAS) category.Till date, five artificial sweeteners have been approved by the FDA i.e., aspartame, saccharin, sucralose, neotame and acesulfame-K on the basis of several toxicological and clinical trials studies. The European Food Safety Authority (EFSA)has also considered these compounds as safe for human consumption in limited amount of intake[5].
However, from a metabolic point of view, artificial sweeteners cannot simply replace natural sugars. The physical characteristics of a natural sweetener in terms of sweetness intensity, quality, degradability and abundance in nature are much superior to that of artificial sweeteners. Moreover, they are easily metabolised in humans. In contrast, the artificial sugars provide low calories because they are not metabolised completely in the human body. The use of artificial sweeteners has become more prevalent to improve insulin resistance in those with diabetes, obesity, and metabolic syndrome, although the evidence does not support this result. There are however some promising data to suggest that natural alternative sweeteners may be a better alternative to sugar and artificial sweeteners[6]. While they may reduce the caloric intake, per se they may not have any beneficial effects on control of diabetes because they may themselves alter the insulin sensitivity. Whether artificial sweetener can lead to reduced obesity &diabetes mellitus is still controversial. Some data also suggest that they may have other safety concerns like cancer and are also emerging as an environmental pollutant due to their presence in waste waters[7, 8]. The aim of this review is to discuss the physical and chemical characteristics of artificial sweeteners along with their health implications.
Artificial Sweeteners
The substitutes of sugar, which are usually non-sugar alternatives are known as artificial sweeteners. They are widely used in the food industry[4]. Depending on the source of calories, artificial sweeteners are classified as nutritive and non-nutritive. The polyols are the nutritive sweeteners having equivalent sweetness as sucrose. The non-nutritive sweeteners, well recognized as high-intensity sweeteners or artificial sweeteners, consist of substances from various chemical groups that interact with taste receptors. Theyare about 30 to 13,000 times as sweet as natural sugar sucrose [9]. They have negligible nutritional value. In other words, artificial sweeteners have higher degree of sweetness, implying that the same amount of sweetness can be attained by less of the compound. Hence, caloric intake can be restricted in many products anddental cavities can also be avoided.Among the widely used artificial sweeteners include acesulfame-K, sodium cyclamate, aspartame, and sodium saccharin (Table1).These are legalised in about 90 countries. Consumers opt for such product to cut energy or sugar intakes for health reasons.Artificial sweeteners also help in enhancing the flavour and improving the shelf life of products.
Table 1: Examples of some artificial sweeteners
| Artificial Sweeteners | X Sweeter than sugar | Brand names | ADI (mg/kg body weight per d) |
| Aspartame | 200 | Nutrasweet | 50 |
| Acesulframe-K | 200 | Sweet One | 15 |
| Saccharin | 600 | Sweet N’ Low | 5 |
| Sucralose | 300 | Splenda | 5 |
| Neotame | 8000 | Newtame | 2 |
| Cyclamate | 30 | – | 1 |
| Alitame | 2000 | – | 0-1 |
| Advantame | 37000 | – | 5 |
Saccharin
Saccharin (Fig. 1)is the oldest and first artificial sweetener.Its acceptable daily intake (ADI) is lowest among the four sweeteners according to the formulation of the World Health Organization (WHO). It is used in products like carbonated and noncarbonated, beverages, dairy products, table top sweeteners, juice, jams, chewing gum, confections, desserts, puddings and jellies[2].
Saccharin is formed by electrochemical oxidation of o-toluenesulfonamide to the corresponding carboxylic acid. This is done with the help of different agents like potassium permanganate [10], and chromic acid [11, 12]. This gives rise to an ortho isomer which undergoes dehydration producing the sweetener. In the second method, diazotization of methyl anthranilate occurs. This is followed by treatingdiazonium salt with sulphur dioxide and chloride gas, resulting insulfonyl chloride which undergoes ammonification to give saccharin. Saccharin does not get absorbed or metabolized in our body, but is excreted through kidneys without any modification.
Experimental studies suggested that saccharin shows both positive and negative outcomes in inducing cancer in rats, dogs and humans. In 1997, saccharin was banned by the FDA based on the results of animal studies linking saccharin to cause bladder cancer in rats. After that, several studies performed on the same line revealed no clear association between utilisation of saccharin and hazardous health effects. At present, saccharin is allowed to be used with provisional regulation which implies the amount of saccharin added in processed food. It is also necessary to mention the presence of saccharin in the product label along with the declaration of ingredient and indicate the quantity used[2].Analysis in a study concluded that the expression of emotional behaviours is linked to the voluntary utilisation of saccharin[13]. It is speculated that the increase in fluid retention or decrease in energy expenditure might be the cause of weight gain due to the consumption of saccharin, in comparison with sucrose[14]. Further studies and long-term clinical trials are requisite to analyse the energy expenditure and weight gain after NNSs (Non-nutritive Sweeteners) exposure in rats and humans.
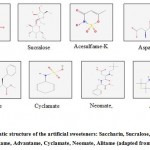 |
Figure 1: Schematic structure of the artificial sweeteners: Saccharin, Sucralose, Acesulfame-K, Aspartame, Advantame, Cyclamate, Neomate, Alitame (adapted from [15]) |
Sucralose
Sucralose (Fig. 1) is made from sucrose by substituting the 3 hydroxyl groups on the sucrose molecule with 3 chloride atoms. Sucralose is 600 times as sweet as sugar with no calories. It is marketed under the registered trademark Splenda since its approval by the FDA in 1998. It is used as a sweetening agent in bakery items. Carbonated beverages, dairy product, desserts, jams, pie fillings, juices, frozen desserts, other processed food products and gelatine also contain sucralose. In 1999, the usage of sucralose was extended by FDA as a table-top sweetener in all food products [9].Sucralose can be used in cooking and baking due to its immense stability. It has an ADI of 5 mg/kg BW. [16].
Sucralose is synthesised bychlorinating the three hydroxyl groups of sucrose. In spite of being produced from sucrose, it is non-nutritive because of its poor absorption in our body. However, sucralose is a stable molecule due to its hydrophilic nature, limited metabolism of the fraction absorbed, rapid elimination and lack of bio accumulative potential. In view of the fact that sucralose remains undigested in our body, it is excreted in the faeces without any modifications [16].
Current studies report shrinking of thymus glands on the intake of 5% sucralose in diet. However, proper assessment of the records and specific immunotoxicity studies evidently cleared the misconception proving that the involution process of the thymus gland was mainly due to nutritional deficit [17].Migraine attacks were reported upon increased incidence of sucralose ingestion[18]. The tolerance studies along withextensive animal safety database indicate that it does not have absence of adverse effects on human health due to long-term or frequent intake of sucralose at the maximum amount of ingestion estimated[19].Toxicological studies established the non-toxicity of sucralose in rodents upon acute oral administration. It was convincingly suggested that higher intake of sucralose triggered decrease in food intake sequentially, reducing food conversion efficiency and body weight gain [20].
Acesulfame-K
It is potassium salt of 6-methyl-1, 2, 3-axathiazine-4 (3H)-one 2, 2-dioxide with molecular formulaC4H4KNO4S and molecular weight of 201.24. It is a white crystalline powder, soluble in water (Fig. 1). It is a high intensity sweetener, 200 times as sweet as sucrose and devoid of calories. Acesulfame-K is mostly used to cutback the bitter aftertaste of aspartame. It is sold by the trade name of Sunett and Sweet One. Acesulfame-K is heat stable; hence, can be used in cooking and baking. Due to its stability, it is used as a sweetening agent in frozen desserts, baked foods,candies, beverages, cough drops, and beverages. It may have a bitter after taste when used by itself, so to get rid of it, Ace-K is often combined with other sweeteners like sucralose or aspartame, wherein a synergistic effect is displayed, one sweetener balances the other after taste making the amalgamation sweeter than its individual constituents. In addition, acesulfame-K is used in nearly 100 countries and its ADI value is 0–15 mg/kg in China. In lacti-beverage the extreme amount of acesulfame-K is 0.3 mg/kg [4].
In early days, Ace-K is synthesized using chlorosulfonyl or fluro sulfonyl isocyanate with propyne acetone to form N-chloro or N- (fluro-sulfonyle) acetoacetamide[21], it is then degraded by potassium hydroxide to give Ace-K. The other unconventional method used is by treating acetoacetamide with a minimum of two equivalents of sulphur trioxide leading to the formation of N-sulfoacetoacetamide, this further undergoes dehydration by sulphur trioxide to form oxathiaazinone dioxide following its neutralization with potassium hydroxide gives Ace-K. In human body, acesulfame-K is not metabolized. In spite of its potassium content, it has no effect on the potassium intake. Acetoacetamide, a by-product of ace-K can be toxic if utilised in high amounts[21]. Genotoxic and clastogenic studies performed on acesulfame-K showed that it has no toxic effects and hence safe for use[22, 23].
Aspartame
Aspartame (Fig. 1) is a dipeptide of the amino acids aspartic acid and phenylalanine joined by a methyl ester (L-aspartyl-L phenylalanine methyl ester). It gets hydrolysed to form methanol under acidic or alkaline conditions. The peptide bonds under stern conditions get hydrolysed giving rise to aspartic acid and phenylalanine. It is slightly soluble in water. There is a liner relationship of solubility of aspartame with the temperature and pH. Aspartame has maximum stability at pH 4.3. It is marketed as Candere, Equal and Nutrasweet. Aspartame cannot be utilised in cooking and baking as it is not heat stable.Owing to its clean and good sweet taste profile, aspartame has been used as a sweetening agent in more than 6000 different type of food products like dessert mixtures, chewable multi-vitamins, frozen desserts, and table top sweeteners.
People suffering from the hereditary disease of phenylketonuria (PKU)are sensitive to phenylalanine which is a metabolite of aspartame. In many countries, it is necessary for all products that contain ASP to be stamped for phenylalanine. It is permitted to be used in about 100 countries, with the ADI value of 40 mg/kg. In consequence of the formation of diketopiperazine (DKP), a minor cyclic dipeptide derivative of aspartame, formed in some aqueous solutions, an ADI of 7.5 mg/kg BW was also fixed, to prevent the health risks[24].
The two major processes for chemical synthesis of aspartame are referred to as Z- and F- processes depending on the presence of the protecting group on the aspartyl moiety. Along with formation of the anticipated α-aspartame, some undesirable β-coupled products are also produced in the process. In the Z-process, benzyloxy carbonyl-L-aspartic acid is dehydrated using acetic anhydride; the resultant dehydrated product is then fixed with the methyl ester of L-phenylalanine in toluene giving a mixture of benzyloxy carbonyl α-and β aspartames. Further, the process ofhydrogenolysis eliminates the protecting groups yielding a mixture of aspartame isomer, which in turn, on crystallization give off aspartame[25]. The synthesis of aspartame by F-process protects the amino group of aspartic acid with a formyl group and is simultaneously dehydrated to form anhydride. Subsequently, the formed anhydride is further treated either with L-phenylalanine or its methyl ester and the protecting groups are eliminated by acid hydrolysis[26]. Later on, the resultant mixture of α and β products are esterified with aqueous methanol. This mixture then undergoes crystallization followed by neutralization to yield aspartame.Recently, the method for chemical synthesis of aspartame has been significantly studied owing to the increasing development in the field of biotechnology as well as use of enzymes as a catalyst. Two Japanese companies presented one novel method for the direct synthesis of aspartame by incubating L- aspartic acid and methyl ester of phenylalanine with microorganisms[25].
Aspartame has low calorie content; hence it is a non-nutritive sweetener. Aspartame is assimilated in our body and degrades into natural constituents, including phenylalanine, aspartic acid, methanol which further simplifies into products such as formic acid, formaldehyde, and diketopiperazine. Each of this above mentioned breakdown products are digested in similar manner as derived from other nutritive sources and hence are considered as safe[24].Since its approval by the FDA in 1974, aspartame has been the controversial topic in aspect of its safety. People born with PKU; a rare inherited disease need to be cautious while consuming products containing aspartame as an ingredient because one of the major constituents of aspartame is phenylalanine.The consumption of aspartame at higher doses may leads to hepatocellular injury and affects the liver antioxidant grade by causing changes in the glutathione dependent system[27]. The NIH-AARP Diet and Health Study showed no relation between aspartame and haematopoietic neoplasms. Many case control studies suggested no association of aspartame to cancer risks, vascular events and preterm deliveries [24].Aspartame and acesulfame-K, two sweeteners belonging to different groups of chemicals, when used in combination exhibit a synergistic sweetening effect. Statistical analysis of the results indicate that aspartame in blend with acesulfame-K is not genotoxic[28, 29].
Cytogenetic studiesin animal models emphasized that substantial increase in the enzymatic activity in protein carbonyl, lipid peroxidation levels, glutathione-S-transferase, superoxide dismutase, glutathione peroxidase and catalase activity may occur due to long term exposure to aspartame. The results clearly showed that at higher doses, aspartame could cause changes in the brain antioxidant system inducing apoptotic alterations in brain[30].
It was demonstrated in a study, that N-acetyl cysteine (NAC) has the potential to reverse the neurotoxicity induced by aspartame thereby reducing cortical inflammation and oxidative stress. The data suggests that the ability of NAC to temper the aspartame-induced brain pathophysiology and diminish the oxidative stress may be promising approach for the treatment of aspartame-induced toxicity[31].
Advantame (Fig. 1), a derivative of aspartame is formed by an N-substitution in the aspartic acid portion. It is a high-intensity sweetener similar in taste to aspartame. It does not have any bitter or sour after taste. Advantame is about 100 sweeter than aspartame and 20,000 times sweeter than that of sucrose. Owing to its stability at low pH and also at high temperature, advantameserves as a sweetening agent in a wide range of foods and beverages. Advantame was confirmed to perform very well as a sweetener in iced tea, coffee (hot and warm), and powdered beverage formulations. It is used to enhance the flavour in beverages, chewing gum and yogurt[32].
Toxicological studies indicate that advantame has no teratogenic potential. A study which involved two-generation reproductive toxicity in rats clearly showed no involvement of aspartame in any of the adverse effects on reproduction or offspring development. The animal toxicology studies and human trial records confirmed that it is safe to use advantame in food products [32, 33].
Cyclamate
Cyclamate (Fig. 1) is a cyclohexylsulfamic acid salt. Sodium cyclamate is utilised as non- nutritive sweetener.Its equivalent calcium salt is used particularly in low sodium diets. Cyclamate is 30 times sweeter than sucrose. Owing to its heat-stable properties, it is perfect for cooking and baking. The molecular formula of sodium salt is C6H12NNaO3S, and its molecular weight is 201.22 g/mol. Some individuals noticed a bitter after taste on the ingestion of cyclamate but not as much as that in the case of Ace-K. To avoid the aftertaste, cyclamate is used in synergism with sucralose. It is used as a table top sweetener, in diet beverages, and in other low-calorie foods. Also, cyclamate is used to enhance the flavour in many pharmaceuticals and toiletries [4, 9].The chemical synthesis of cyclamate begins with the chemical chlorination of trisaccharideraffinose to form tetrachlororaffinose (TCR), which is then enzymatically treated with a galactosidase to shift the 6-chloro-6-deoxygalactosyl moieties from the 6th position to yield cyclamate [11].
Cyclamate is metabolized in our body by the gut bacteria to form cyclohexylamine which exhibits higher toxicity. Many recent studies indicatednovel information about the conversion of cyclamate to cyclohexylamine during long term intake of cyclamate. Another study concluded that the exposure to cyclohexylamine from cyclamate metabolism in humans over a period can be lethal, this was considered and applied for the establishment of ADI for cyclamate [34].
Neotame
Neotame (Fig. 1) is a derivative of aspartame produced by N-alkylating aspartame. Depending on the kind of food and composition of its blend, its degree of sweetness varies. It is 7000 to 13,000 times and about 30 to 60 times sweeter than sugar and aspartame respectively. It has no calories. Since its approval by the FDA in 2002, it serves as a sweetener in beverages, baked foods, gelatines, chewing gum, jams, jellies, and many other foodstuffs as flavour enhancer. Neotame is an odourless, white to grey-white powder. It is freely soluble in alcohols and slightly soluble in water. The 0.5% aqueous solution of neotame is weakly acidic having pH 5.8 [9].
Neotame is synthesized by chemoenzymatic method by preparing N-[N-(3-3dimethylbutyl)-L-aaspertyl]-L-phenylamine 1- methyl ester. It involves the enzymatic regioselective hydrolysis of neotame ester either by lipases or estarases. Another method involves hydrogenation of L-α- aspartyl –L- phenylalanine I methyl ester and 3–3 dimethylbutyraldehyde by the hydrolysis of a 3-3-dimethylbutyraldehyde precursor resulting in the production of neotame. To the free amino group of aspartic acid, a hydrophobic t-butyl group is added forming neotame.Neotame is poorly absorbed, entirely eliminated and does not build up in the body. Neotame is quickly metabolized in our body by the hydrolysis of the methyl ester by esterase yielding deesterifiedneotame along with a small quantity of methanol. The presence of the 3-3-di-methylbutyl group does not allow thepeptidases to break the peptide bond between the two constitutive amino acids, this reduces the phenylalanine availability. JECFA has favourably evaluated neotame and established an ADI of 2mg/kg BW[35].
Neotame was permitted for use by the FDA in 2002, but still it is hardly used. Studies revealed that neotame effects body weight. These effects are not due to the toxic nature of neotame, instead it relates to the indigestibility of foods containing neotame as e sweetener. Due to this reason, animal models showed less weight gain or loss in body weight on long-term exposure to neotame. Safety studies demonstrated that neotame has no hostile effects in clinical observations, physical examinations, pathophysiological finding, health, toxicological, macroscopic, microscopic orpost-mortem findings[36].
Alitame
Alitame (Fig. 1) is about 2000 times sweetener than sucrose. The terminal amide of 2, 2, 4, 4- tetramethyl thietanylamine, (TTA) is responsible for the sweet taste as well as stability of alitame. The aspartic acid moiety of alitame resembles that of aspartame. The D-alanine interchanged L-phenylalanine and the methoxy group of the ester part was substituted by the TTA group in the aspartame to form alitame[37].Alitame is synthesized by reacting (S)-[2-5-dioxo-(4-thiazolidine)] acetic acid with (R) –2- amino-N-(2, 2, 4-4-tetramethyl-3-thietanyl) propanamides. The resultant product is separated and purified by crystallizing an alitame−4-methylbenzenesulfonic acid following its subsequent purification steps and then ultimately recrystallized by using water[38].
Alitame is easily absorbed and undergoes fast metabolism in the gastrointestinal (GI) tract and then excreted. It breaksdown into its two primary constituents, aspartic acid and alanine amide. The component of aspartic acid is metabolized easily while alanine amide undergoes slight metabolic alterations. The glucoronic derivative of D-alanine tetramethylthietane amide is the prime urinary metabolite in humans. In 2002, JEFCA evaluated safety data on alitame. It was concluded that no evidence exists to prove alitameas carcinogenic. An ADI of 0–1 mg/kg BW has been fixed for the use of alitame. Mexico, Colombia China, Australia and New Zealand have approved the usage of alitame[4].
Rare sugars and Polyols
Sugars which are found in very small amounts in nature are called rare sugars. These rare sugars are recently grabbing more attention due to their characteristics and hence have a high demand in the market. These rare sugars are not digested in our body attributing to its low caloric value. They are monosaccharides exhibiting similar sweetness and bulkiness as sugar and do not have any known harmful effects on human health. Rare sugars do not possess any ADI value and are chiefly synthesized through bioreactor [4].
Many of the rare sugars are available in the market, such as such as D-tagatose and D-sorbose. L-enantiomers are metabolic stable, have a good toxicological profile and antiviral activity. L-sugars such as L-glucose, L-xylose, and L-galactose can be used to produce L-nucleosides analogues like lamivudine, which has antiviral property. L-Sugars, on their own, can be used as pesticides and to inhibit glycosidase activity[39].Among the rare sugars available, D-psicose and D-tagatose were recognized as Generally Recognized As Safe: these compounds elicit a sweet taste without undesirable qualities.This study showed that rare sugar syrup containing D-allulose, D-sorbose, and D-tagatose can maintain glucose homeostasis and insulin release during a glucose tolerance test in rats by enhancing the blood glucose to glycogen conversion in the liver via glucokinase translocation between the nucleus and cytoplasm[40].
D-tagatose is similar to D-fructose in structure except for an inverted optically active center. Since its approval by the USFDA as a food additive, it has achieved and gained much more interest due to its beneficial characteristics. It has no glycaemic effect, promoting weight loss, and can be used in dental caries and prebiotic. It has same metabolic fate as fructose but has very poor absorption. Due to its low glycaemic index; it can be used as an alternative sweetener by diabetic patients.Another well-known rare sugar is D-psicose, which isa C-3 epimer of D-fructose. D-psicose has 70% of the sweetness of sucrose. It is a no-calorie sweetener; has high solubility making it appropriate for food processing. It improves the gelling behaviour and also enhances the flavour and texture of food products. In addition to this, it has good antioxidant property[4]. D-allose, an isomer of D-psicose, is a cis-aldohexose exhibiting good antioxidant properties. It is used as a cryopreservative and also shows anti-inflammatory properties. It is anti-carcinogenic, inhibitingcancer proliferation. Moreover, it is used as an immunosuppressant in transplantation and surgical operations.
Trehalose is a non-reducing disaccharide consisting of two glucose units linked by a 1, 1-glycosidic bond. It is with a relatively 40–45% sweeter as that of sucrose (Fig. 2). Trehalose is directly produced from food-grade starch by a multi-enzymatic process and has good antioxidant property [4, 39].
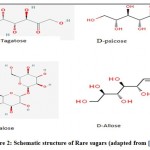 |
Figure 2: Schematic structure of Rare sugars (adapted from [15]) |
Polyols are low digestible carbohydrates existing either in a variety of crystalline forms or as liquid syrups. Polyols are bulk sweeteners having clean, pleasant and good taste profile and can be compared to those of sucrose. They are poorly absorbed by the small intestine attributing to its low-calorie content. Recent research directed that polyols have shown good antioxidant properties reducing stress responses. The most commonly reported polyols are the six-carbon sugar alcohols, sorbitol and mannitol. Other polyols which are used are erythritol, lactitol, xylitol, maltitol (Fig.3).
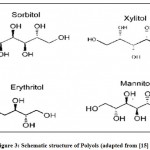 |
Figure 3: Schematic structure of Polyols (adapted from [15] ) |
Sorbitol is obtained by hydrogenating glucose in the presence of catalyst. The FDA allows the value of 2.6 calories per gram. It is 60% sweet as that of sucrose and used widely in confectionery, food, oral care, pharmaceutical, and industrial applications. Mannitol, also known as mannite, is manufactured either by hydrogenating fructose obtained from sucrose or starch or by the yeast fermentation in aerobic conditions.It is about 0.7 times sweeter than sucrose and readily soluble in water.It also has slight solubility in ethanol. It is poorly absorbed in the body and hence remained unchanged. In powdered foods it is used as a bulking agent and also used to “dust” chewing gum. The FDA permits the caloric value of 1.6 per gram [42, 43].
Erythritol is four carbon sugar alcohol (or polyol) produced by fermenting glucose and sucrose by Trichosporonoides mega chiliensis (Trichosporonceae).It has sweetness approximately 60–80% of sucrose. It is also manufactured from wheat or corn starch by enzymatic hydrolysis, by yeast fermentation[26, 43]. Studies indicate that depending on dosage, most of ingested erythritol is rapidly metabolised in the body and excreted unaffected in the urine in animals and humans. The remaining unabsorbed erythritol either formed short-chain fatty acids by microbial fermentation or is defecated in the faeces. Xylitol is another naturally occurring five carbon sugar equivalents to sucrose in sweetness. The production of xylitol involves the extraction of xylan from plant cell wall or some algae by the treatment of acid hydrolysis, which further undergoes hydrogenation to yield the desired product. It has a standard glycaemic index of 8 and have a caloric value of 2.4 calories/gm. Being not fermented by the microorganisms, xylitol is used in reducing dental caries. Studies involving human tolerance showed that a consumption of more than 50 g of xylitol per day leads to diarrhoea [4, 39, 42, 43].
Lactitol, obtained by catalytic hydrogenation of lactose, is not metabolized in the small intestine and is acted upon by the microflorapresent in large intestine.It is 30-40% as sweet as sucrose. It. It is used as a sweetening agent in confections, baked goods, frostings, gum, frozen dairy desserts mixes, jams and jelliesimproving the taste as well as texture of the food products. The FDA permitted use of 2 calories per gram. Maltitol (4-O-α-D-glucopyranosyl- D-sorbitol) is about0.9 times sweeter than sucrose having a caloric value of 3.0 per gram as allowed by the FDA. It is specifically suitable for candy coatings. Isomalt is a combination of α-D-glucopyranosyl-1, 6-mannitol (GPM) and α-D-glucopyranosyl-1, 6-sorbitol (GSM) having approximately 45-65% sweetness as sucrose. It possesses clean, pleasant taste profile and gelling properties. As it is heat stable, it can be used in baking. The FDA has allowed its usage of 2 calories per gram[43].
Conclusion
Sweet is an inevitable part of our daily diet. Since ages, people are using natural sweeteners. But in the recent times, to avoid obesity and other health risks occurring due to their high calorie content, artificial sweeteners are replacing them rapidly. The FDA has approved utilisation of 5 artificial sweeteners namely, saccharin, sucralose, aspartame, neotame and
cyclamate. Alitame is still in the waiting list. The above-mentioned artificial sweeteners have no calories; theyare non-nutritive. They are poorly absorbed in the body attributing no changes in body weight gain. It has been noticed that synergism of two different classes of artificial sweeteners exhibit higher degree of sweetness in comparison to its individual components. This helps in masking the bitter or sour after taste of certain sweeteners. Since the discovery of the artificial sweeteners, the subject of their consumption is controversial owing to their effects on health ranging from mild headache to dreadful cancer risks. In spite of the daily consumption of artificial sweeteners in almost every foodstuff, the present studies demonstrate that artificial sweeteners are unhealthy. Artificial sweeteners should be consumed carefully and in limited quantities. Long term intake of artificial sweeteners above their ADI level is not recommended.Long term study of these sweeteners for further safety evaluation on health risks is essential. Further research is desirable to interpret the safety and metabolic effects of artificial sweeteners and better understand the possible consequence of these frequently used sweeteners on human health.In conclusion, to live a healthier life, it is best to use natural sweeteners or to consume artificial sweeteners taking into account its acceptable daily intake.
References
- Sudan, P., et al., A critical review on natural and artificial sweeteners. The Pharmaceutical and Chemical Journal, 2016. 3(1): p. 21-29.
- Weihrauch, M. and V. Diehl, Artificial sweeteners—do they bear a carcinogenic risk? Annals of Oncology, 2004. 15(10): p. 1460-1465.
CrossRef - Popkin, B.M. and S.J. Nielsen, The sweetening of the world’s diet. Obesity Research, 2003. 11(11): p. 1325-1332.
CrossRef - Chattopadhyay, S., U. Raychaudhuri, and R. Chakraborty, Artificial sweeteners–a review. Journal of Food Science and Technology, 2014. 51(4): p. 611-621.
CrossRef - Lorenzo, R., et al., Artificial sweeteners in beverages by ultra performance liquid chromatography with photodiode array and liquid chromatography tandem mass spectrometry. Food Control, 2015. 47: p. 43-52.
CrossRef - Mejia, E. and M. Pearlman, Natural alternative sweeteners and diabetes management. Current Diabetes Reports, 2019. 19(12): p. 142.
CrossRef - Purohit, V. and S. Mishra, The truth about artificial sweeteners–are they good for diabetics? 2018, Elsevier.
CrossRef - Ali, A. and J. Devrukhkar, In vitro study on glycation of plasma proteins with artificial sweeteners. Acta Biologica Szegediensis, 2016. 60(1): p. 65-70.
- Whitehouse, C.R., J. Boullata, and L.A. McCauley, The potential toxicity of artificial sweeteners. Aaohn Journal, 2008. 56(6): p. 251-261.
CrossRef - Tarbell, D.S. and A.T. Tarbell, The Discovery of Saccharin. Journal of Chemical Education, 1978. 55(3): p. 161-2.
CrossRef - Bennett, C., et al., Biocatalytic synthesis of disaccharide high‐intensity sweeterner sucralose via a tetrachlororaffinose intermediate. Biotechnology and Bioengineering, 1992. 39(2): p. 211-217.
CrossRef - Drasar, B., A. Renwick, and R. Williams, The role of the gut flora in the metabolism of cyclamate. Biochemical Journal, 1972. 129(4): p. 881-890.
CrossRef - VanderWeele, D.A., N.K. Dess, and T.W. Castonguay, Ingestional responses to metabolic challenges in rats selectively bred for high and low saccharin intake. Physiology &Behavior, 2002. 75(1-2): p. 97-104.
CrossRef - de Matos Feijó, F., et al., Saccharin and aspartame, compared with sucrose, induce greater weight gain in adult Wistar rats, at similar total caloric intake levels. Appetite, 2013. 60: p. 203-207.
CrossRef - NCBI. PubChem. [cited 2021; Available from: https://pubchem.ncbi.nlm.nih.gov/.
- Rocha, G., et al., Sucralose sweetener in vivo effects on blood constituents radiolabeling, red blood cell morphology and radiopharmaceutical biodistribution in rats. Applied Radiation and Isotopes, 2011. 69(1): p. 46-51.
CrossRef - Grice, H. and L. Goldsmith, Sucralose-an overview of the toxicity data. Food and Chemical Toxicology, 2000. 38(suppl 2).
CrossRef - Bigal, M.E. and A.V. Krymchantowski, Migraine triggered by sucralose—a case report. Headache: The Journal of Head and Face Pain, 2006. 46(3): p. 515-517.
CrossRef - Baird, I.M., et al., Repeated dose study of sucralose tolerance in human subjects. Food and Chemical Toxicology, 2000. 38: p. 123-129.
CrossRef - Goldsmith, L., Acute and subchronic toxicity of sucralose. Food and Chemical Toxicology, 2000. 38: p. 53-69.
CrossRef - George, V., et al., Analysis of multiple sweeteners and their degradation products in lassi by HPLC and HPTLC plates. Journal of Food Science and Technology, 2010. 47(4): p. 408-413.
CrossRef - Mukherjee, A. and J. Chakrabarti, In vivo cytogenetic studies on mice exposed to acesulfame-K—a non-nutritive sweetener. Food and Chemical Toxicology, 1997. 35(12): p. 1177-1179.
CrossRef - Ali, A., et al., Antiglycating potential of acesulfame potassium: an artificial sweetener. Applied Physiology, Nutrition, and Metabolism, 2017. 42(10): p. 1054-1063.
CrossRef - Marinovich, M., et al., Aspartame, low-calorie sweeteners and disease: regulatory safety and epidemiological issues. Food and Chemical Toxicology, 2013. 60: p. 109-115.
CrossRef - Ager, D.J., et al., Commercial, synthetic nonnutritive sweeteners. Angewandte Chemie International Edition, 1998. 37(13‐14): p. 1802-1817.
CrossRef - Hill, J.B., et al., One-pot process for the preparation of α-L-aspartyl-L-phenylalanine methyl ester hydrochloride. 1991, Google Patents.
CrossRef - Abhilash, M., et al., Effect of long term intake of aspartame on antioxidant defense status in liver. Food and Chemical Toxicology, 2011. 49(6): p. 1203-1207.
CrossRef - Mukhopadhyay, M., A. Mukherjee, and J. Chakrabarti, In vivo cytogenetic studies on blends of aspartame and acesulfame-K. Food and Chemical Toxicology, 2000. 38(1): p. 75-77.
CrossRef - Ahire, K., D. Kumar, and A. Ali, Differential glycation of arginine and lysine by glucose and inhibition by acesulfame potassium. Journal of BioScience and Biotechnology, 2018. 7(1): p. 11-15.
- Ashok, I. and R. Sheeladevi, Biochemical responses and mitochondrial mediated activation of apoptosis on long-term effect of aspartame in rat brain. Redox Biology, 2014. 2: p. 820-831.
CrossRef - Saleh, A.A.S., Anti-neuroinflammatory and antioxidant effects of N-acetyl cysteine in long-term consumption of artificial sweetener aspartame in the rat cerebral cortex. The Journal of Basic & Applied Zoology, 2015. 72: p. 73-80.
CrossRef - Otabe, A., et al., Advantame–an overview of the toxicity data. Food and Chemical Toxicology, 2011. 49: p. S2-S7.
CrossRef - Otabe, A., T. Fujieda, and T. Masuyama, A two-generation reproductive toxicity study of the high-intensity sweetener advantame in CD rats. Food and Chemical Toxicology, 2011. 49: p. S70-S76.
CrossRef - Renwick, A., Postscript on advantame–a novel high-potency low-calorie sweetener. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association, 2011. 49: p. S1.
CrossRef - Nofre, C. and J.-M. Tinti, Neotame: discovery, properties, utility. Food Chemistry, 2000. 69(3): p. 245-257.
CrossRef - Mayhew, D.A., C.P. Comer, and W.W. Stargel, Food consumption and body weight changes with neotame, a new sweetener with intense taste: differentiating effects of palatability from toxicity in dietary safety studies. Regulatory Toxicology and Pharmacology, 2003. 38(2): p. 124-143.
CrossRef - Kholeif, S. and G. Anderegg, Stability constant determinations of alitame with H (I) and Cu (II) under physiological conditions using potentiometric measurements. Food Chemistry, 1999. 64(3): p. 397-401.
CrossRef - Abbott, P.J., R. Walker, and C. Leclercq, 3.1 Screening by the budget method.
- Beerens, K., T. Desmet, and W. Soetaert, Enzymes for the biocatalytic production of rare sugars. Journal of Industrial Microbiology &Biotechnology, 2012. 39(6): p. 823-834.
CrossRef - Shintani, T., et al., Rare sugar syrup containing d-allulose but not high-fructose corn syrup maintains glucose tolerance and insulin sensitivity partly via hepatic glucokinase translocation in Wistar rats. Journal of Agricultural and Food Chemistry, 2017. 65(13): p. 2888-2894.
CrossRef - Al-Asadi, A. K., G. H. Majeed, S. T.Al-Sahlany, Purification and identification of mannitol producing from the isolation of locally isolated bacteria Leuconostoc mesenteroides. Iraqi Journal of Biotechnology, 2010. 9(1): p. 48-55
CrossRef - Mortensen, A., Sweeteners permitted in the European Union: safety aspects. Scandinavian Journal of Food and Nutrition, 2006. 50(3): p. 104-116.
CrossRef - Zumbe, A., A. Lee, and D. Storey, Polyols in confectionery: the route to sugar-free, reduced sugar and reduced calorie confectionery. British Journal of Nutrition, 2001. 85(S1):p. S31-S45.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





