How to Cite | Publication History | PlumX Article Matrix
Nataliya Denysenko* and Alexander Sklyarov
and Alexander Sklyarov
Department of Biological Chemistry, Danylo Halytsky Lviv National Medical University,Lviv, Ukraine. 69 Pekarska str, Lviv, Ukraine, 79010.
Corresponding Author E-mail: denysenko.natalka@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2918
ABSTRACT: Introduction. L-arginine is a semi-essential amino acid and a precursor of many biologically active compounds. Polyamines and NO produced from L-arginine take part in the regulation of biochemical processes in colon mucosa. Emotional stress, nonsteroidal anti-inflammatory drugs (NSAIDs) and their combined action can change the activity of L-arginine metabolizing enzymes. The aim of this study was to investigate the single action of NSAIDs with different mechanisms of action and their combination with acute stress on L-arginine metabolism in colon mucosa of rats. Methods. Animals were divided into 8 groups: control group (1), administration of nonselective, COX-2 selective and dual COX-2/5-LOX inhibitors (groups 2-4), acute stress group (5), administration of same NSAIDs as in groups 2-4 under the conditions of acute stress (groups 6-8). The activity of iNOS, cNOS, arginase, concentration of L-arginine, nitrite and nitrate was measured in colon mucosa. Results. Nonselective COX inhibition by naproxen caused the increase in iNOS and decrease in cNOS activity in colon mucosa. Both COX-2 (celecoxib) and dual COX-2/5-LOX (2A5DHT) inhibitors enhanced cNOS and arginase acting in combination with acute stress. The concentration of L-arginine remained unchanged in most of the groups, but combination of dual COX-2/5-LOX inhibitor and acute stress raised this parameter.
KEYWORDS: Arginase; COX/LOX-Inhibitors; Colon Mucosa; L-arginine; NO-synthase; Stress
Download this article as:| Copy the following to cite this article: Denysenko N, Sklyarov A. Changes of L-Arginine Metabolism in Rat`S Colon Mucosa Under the Conditions of COX/LOX Inhibition and Acute Stress Action. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Denysenko N, Sklyarov A. Changes of L-Arginine Metabolism in Rat`S Colon Mucosa Under the Conditions of COX/LOX Inhibition and Acute Stress Action. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/35rrSW9 |
Introduction
L-arginine and its metabolites are important regulators of biochemical processes in colon mucosa (CM) and whole gastro-intestinal (GI) tract.The turnover of L-arginine in CM takes place in properly colonocytes1, it regulates the proliferation of intestinal stem cells2. Exertive metabolism of L-arginine was also identified in macrophagespresent in lamina propria3and in CM biofilm, where it is metabolized by bacteria1. Intracellular pool of L-arginine is formed by few factors – protein degradation4, transport from intracellular space by amino acid and L-arginine-containing peptides transporters5 and endogenous synthesis via intestinal-renal axis6. Cationic amino acids transporters (CAT) – CAT-1 and CAT-2are responsible for L-arginine and other cationic amino acids transport7. Endogenous synthesis of L-arginine by intestinal-renal axis covers few steps: L-citrulline is synthesized from glutamine, glutamate and proline in the mitochondria of enterocytes, released from the small intestine and taken up by kidneys for L-arginine production via argininosuccinate synthase and argininosuccinate lyase8.
There are four main pathways of L-arginine metabolism – oxidative NO-synthase/NO pathway and non-oxidative arginase/L-ornithine, arginine decarboxylase/agmatine and arginine:glycine amidinotransferase/creatine pathways9 (Fig. 1). More than 1% of metabolized L-arginine is utilized for polyamine synthesis and near 2% for constitutive NO production in mammalian cells6. All the rest L-arginine can be included into protein biosynthesis6. L-ornithine can be used by ornithine transaminase to provide L-proline10, which takes part in collagen and other proteins biosynthesis. Arginine: glycine amidinotransferase takes part in creatine synthesis mainly in kidneys, but is also present in intestines11(Fig. 1).
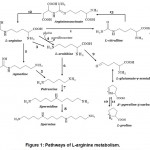 |
Figure 1: Pathways of L-arginine metabolism. |
Notes: 1 – NO-synthase, 2 – arginine decarboxylase, 3 – arginase, 4 – arginine:glycine amidinotransferase, 5 – agmatinase, 6 – ornithine decarboxylase, 7 – spermidine synthase, 8 – spermine synthase, 9 – ornithine aminotransferase, 10 – Δ1-pyrroline-5-carboxylate reductase, 11 – L-proline oxidase, 12 – argininosuccinate synthase, 13 – argininosuccinate lyase
NO-synthase (NOS) and arginase are the most active L-arginine metabolizing enzymes of CM12. There are three isoforms of NOS – neuronal (NOS-1), inducible (NOS-2, iNOS) and endothelial (NOS-3). NOS-1 and NOS-3 are constitutive (cNOS) isoforms; all of them are detected in CM13. Both isoforms of arginase– arginase-1 (cytoplasmic isoform) and arginase-2 (mitochondrial isoform)are also found in CM14. The utilization of L-arginine by NOS/NO pathway in CM is highly augmented in state of stress15 and inflammation16 due to infiltration of CM with iNOS-expressing macrophages17. Polyamines like putrescine, spermidine and spermine protect CM from endotoxins and activate protein synthesis18, induce the secretion of immunoglobulin A, maintain barrier integrity19, DNA stabilization, activate ion channel transport,scavenge free radicals and induce cell proliferation20. However,polyamines synthesis can be disturbed under the conditions of elevated consumption of L-arginine by iNOS1.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are still one of the most wide-used medications21. They act by inhibition of cyclooxygenase (COX) pathway of arachidonic acid (AA) metabolism.It results in down regulation of prostaglandins (PGs), mainly prostaglandin E2 (PG E2) synthesis22. Basal level of PG E2 synthesis is carried out by constitutive isoform of COX (COX-1) in epitheliocytes, located in lower half of crypts23. PG E2 binds with PGE2 receptors and activates the production of mucusby CM via cAMP dependent mechanism24. Thromboxane A2 (TX A2) and prostacyclin (PG I2) synthesis is also catalyzed by COX-1, they ate blood flow and thrombocytes aggregation25. Expression of inducible COX-2 is activated in the apical epitheliocytes of villi and inflammatory cells of lamina propria in case of inflammation26. The large intestine shows relatively low levels of COX-1 expression compared with thatof other parts in GI tract27. Sharply increased synthesis of PG E2by COX-2 can determine further inflammation reactions after binding with PGE2 receptors located on mast cells, T-helpers28 and other immune cells. Thus, inhibition of excessive PG E2 synthesis is the base of anti-inflammatory action of NSAIDs.
However, it is well-known that long-term and/or high-dose administration of NSAIDs can cause ulcers of GI mucosa29. Ulcerogenic action of NSAIDs is connected with mechanism of their action, mainly with the COX selectivity30.Declined activity of COX-1 leads to lower the production of mucus31, so CM becomes more sensitive to endotoxins, flagellins and bacterial lipopolysaccharides (LPS) action. In this regard, COX-2 selective NSAIDs are actively synthesized and studied nowadays. However, COX inhibition can cause the shift of AA metabolism on lipoxygenase (LOX) pathway32, which flows out in raised production of proinflammatory leukotriene B4 (LT B4)by 5-LOX isoform33. Dual COX-2/5-LOX inhibitors(darbufelone, licofelone, 2,3-diarylxanthones, chebulagic acid etc.) are considered to be more effective NSAIDs, as theyblock both pathways of AA metabolism34, while LOX inhibitors can cause the switchover of AA metabolism on COX pathway.
Emotional stress is another, but no less important factor of modern life. Like NSAIDs, stress can cause ulcers in GI mucosa35. The mechanism of its action is associated with adrenaline36, which can cause the vasoconstriction, hypoxia and activation of nitroso-oxidative processes37. On the other hand, slow influence of stressis mediated by activation of hypothalamic-pituitary-adrenal axis via hyperproduction of corticotropin releasing hormone, corticotropin and cortisol38. Cortisol activates the expression of lipocortin-1, which inhibits phospholipase A2 – a key enzyme of AA synthesis39.So, both COX and LOX pathways become inactivated like under the action of some NSAIDs.
Taking into account cytoprotective and proinflammatory pathways of L-arginine and AA metabolism, the purpose of this study was to find the cross-links between these pathways.The main parameters of L-arginine metabolism pathways in CM in the normal state, under the conditions of acute stress and action of different COX/LOX inhibitors were defined.
Materials and methods
Animals
All animal procedures were carried out in accordance with international guidelines for the use and care of laboratory animals.The permission was granted by Bioethics Committee of Danylo Halytsky Lviv National Medical University (Protocol №3, 16.03.2015).Total number of 80 12-week-old male out bred albino rats (Rattus norvegicus domestica), 200-240 g body weight were obtained from Danylo Halytsky Lviv National Medical University vivarium. All rats were housed in an environmentally controlled room under a 12 h light/dark cycle.There was ad libitum access to water and animals were kept on the standard dietin groups of five in cages.All the experiments were performed at the light cycle.
Experimental groups
Animals were randomly divided into 8 groups (Fig. 2), 10 rats in every group.The first group was control, animals were administered NaCl solution and taken out of the experiment after 1 hour. Rats of groups 2-4 were receiving COX/LOX inhibitors by the next scheme: 2nd was administered by non selective COX inhibitor (naproxen), 3rd– COX-2 selective inhibitor (celecoxib), 4th– dual COX-2/5-LOX inhibitor (2A5DHT) and were taken out of the experiment after 1 hour. The remaining 40 rats were fasting for 24 hours.Water-immersion stress (WIS) was carried out on animals of group 5,after that, they were taken out the experiment. Animals of groups 6-8 were administered the same COX/LOX inhibitors in the same dose and way as rats of groups 2-4. Animals of groups 6-8 were exposed to the WIS action after 30 min, then they were taken out the experiment. Euthanasia was done by urethane injection.
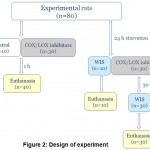 |
Figure 2: Design of experiment. |
WIS model
The WIS model was executed as described by K. Takagi40, with 5-hours modification41. Rats were fasting for 24 hours before WIS modeling and had free access to the water. Animals were fixed in plastic boxes in such a way that their body remained stationary. The boxes with rats were immersed into a water bath (t=23 °C), so that their heads were upon the water surface. Animals were kept in this position during 5 hours, water temperature was controlled every 30 min. Euthanasia was performed immediately after the experiment was finished.
Tested compounds, doses and ways of administration
Sterile sodium chloride solution in a concentration 0.9% (Arterium, Lviv, Ukraine) was administered intragastrically in a volume of 1 mL to a control group rats. Three COX/LOX inhibitors were used: non selective COX-inhibitor naproxen sodium salt (Sigma-Aldrich, St. Louis, MO, USA), selective COX-2 inhibitor celecoxib (American Custom Chemicals Ltd., San Diego, CA, USA), dual COX-2/5-LOX inhibitor 2A5DHT (2-amino-5-(3,5-ditertbutyl-4-hydroxy benzylidene)-thiazol-4-one)– a structural analogue of darbufelone. Compound 2A5DHT(Fig. 3) was synthesized in the laboratory of Department of organic, bioorganic and pharmaceutical chemistry (Danylo Halytsky Lviv National Medical University, Lviv, Ukraine) by method described previously15. All the tested compounds were administered intragastrically in a single dose of 10 mg/kg, in a volume of 1 mL. Naproxen sodium was dissolved in distilled water, while celecoxib and 2A5DHT were dissolved in water with addition of polysorbate-80 (10% of the total solution volume) for emulsification. Urethane(Sigma-Aldrich, St. Louis, MO, USA) was used for euthanasia in a single dose 4 g/kg, injected intraperitoneally, dissolved in a distilled water.
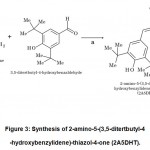 |
Figure 3: Synthesis of 2-amino-5-(3,5-ditertbutyl-4-hydroxybenzylidene)-thiazol-4-one (2A5DHT). |
Reagents, conditions and yields: (a) AcONa, AcOH, reflux 3h, 68%
Tissue homogenates preparation
After euthanasia colons were taken, cut along, washed with 0.9% sodium chloride solution, extra moisture was taken away with filter paper. CM was scraped with glass microscope slides, weighed and put into a test tubes for further homogenization. Cold (0-4 °C) 0.9 % sodium chloride solution was added to CM in a proportion 1:5 (tissue:solution) and CM was homogenized with homogenizer HS-30E (5000 g). Obtained homogenates were additionally centrifuged (1500 g) on cooling (0-4 °C) for 15 min.
NOS assay
NOS activity was evaluated by the activity of L-citrulline production42 using the reaction with diacetyl monoxime, antipyrine and iron (II) sulfate diluted in sulfuric acid. Two incubation solutions (pH=7.4)with the same composition, which differed with the presence of Ca2+ (for cNOS activity) and sodium ethylenediaminetetraacetate (for iNOS activity)were used.The concentration of L-citrulline was calculated using a calibration curve. Activity of NOS was expressed in μmol of L-citrulline/min·mg of protein.
Arginase assay
Total arginase (both arginase-1 and arginase-2) activity was checked by an approach, based on the activity of urea production43. The reaction with α-isonitrosopropiophenone, diluted in ethanol was usedfor urea detection. The concentration of urea was revealed using a calibration curve. Activity of arginase was expressed in μmol of urea/min·mg of protein.
L-arginine assay
Sakaguchi reaction was used for L-arginine concentration determination44. The concentration of L-arginine was found using a calibration curve, it was expressed in μmol/g of tissue.
Nitrite-, nitrate-anion assay
The reaction with Griess reagent for nitrite-anion determination and zinc powder to reduce nitrate-anion into nitrite-anion45 was used.All the reagents were dissolved in water for injections to avoid the false results, as distilled water may contain nitrite-/nitrate-anion. Absorbance (λ=550) was estimated and nitrite-anion concentration was calculated using a calibration curve. The values in tubes with zinc corresponded to the total concentration of nitrite-/nitrate-anions concentration, which was expressed in μmol/g of tissue.
Protein assay
A modified assay for protein determination was used, which is based on a biuret reaction46. The concentration of protein was distinguished using a calibration curve. Concentration of protein was expressed in μmol/L.
Statistical analysis
All experimental values are expressed as the mean ± standard deviation. Descriptive statistics and normality test (Shapiro-Wilk test) were complied, using BioStat Pro Version v6 (BioStat, AnalystSoft Inc., Walnut, CA, USA). The distribution was normal in all the tested groups. Unequal-variance t-test was performed to designate reliability using Excel (Microsoft Office, Microsoft, Redmond, WA, USA). Values p<0.05, p<0.01, p<0.001 were considered statistically significant.
Results
Administration of COX/LOX inhibitors did not change significantly the concentration of L-arginine and nitrite-anionin CM, itaffected mainly on NOS and arginase activity.
Thus, nonselective COX inhibitor naproxen administration augmented the iNOS activity by 19% anddecreased cNOS activity by 12% (p<0.01)(Fig. 4)in CMcompared with that ofa control group.Tendency of arginase activity to be lower and nitrate-anion to be higher(p>0.05) was noted.
Celecoxib, like naproxen, did not change the concentration of L-arginine and nitrite-anion.However, it caused iNOS activation by 22% (p<0.01) on the one hand, and tendency of cNOS and arginase activity to be lower (p>0.05) in CM compared to that of acontrolgroup (Fig. 4).Like naproxen, celecoxib caused the tendency of nitrate-anion concentration to be elevated (p>0.05).
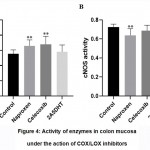 |
Figure 4: Activity of enzymes in colon mucosa under the action of COX/LOX inhibitors. |
There were no significant changes of studied parameters in CMunder the action of dual COX/LOX inhibitor A25DHT.However,we noted the tendency of arginase activity and nitrite-anion to be lower (p>0.05) compared with that ofa control group.
Action of WIS caused the diminishment of L-arginine concentration by 27%, constitutive enzymes`activity – cNOS by 22% and arginase by 24% (p<0.001)in CM compared with that of a control group (Fig. 5).At the same time iNOS activity was2.3 times higher (p<0.001), concentration of nitrite- and nitrate-anion increased by 20% and 27% (p<0.05) compared with that ofa control group (Fig.5).
Administration of COX inhibitors in case of WIS tended to raise L-arginine concentration in CM. Though, the general enzymatic activity did not change significantlycompared with that ofa WIS group, some interesting changes of enzymes` activity ratio were noted.
Action of naproxen under the conditions of WIS caused the tendency of L-arginine concentration to be elevated (p>0.05), activity of iNOS was loweredby 9% (p<0.05) in CMcompared with that ofWIS group (Fig. 5).Activity of cNOS, arginase, concentration of nitrite- and nitrate-anion did not change significantly in this case.
The linked action of celecoxibcaused the tendency of L-arginine concentration to be augmented (p>0.05), arginase activitywas higher by 12% and iNOS activity – lower by 14% (p<0.05) in CM compared with that of WIS group.Nitrite- and nitrate-anion concentration, cNOS activity remained unchanged(Fig. 5).
Dual COX/LOX inhibitor – 2A5DHT – was the only compound, which increased L-arginine concentration by 17% (p<0.05); iNOS activity was loweredby 15% (p<0.01), while arginase activity was higher by 12% (p<0.05) in CM compared with that ofa WIS group (Fig. 5).The tendency of cNOS to be higher (p>0.05) and no changes of nitrite- and nitrate-anion concentration were confirmed.
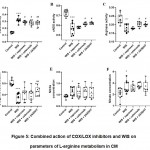 |
Figure 5: Combined action of COX/LOX inhibitors and WIS on parameters of L-arginine metabolism in CM. |
A – activity of iNOS, B – activity of cNOS, C – activity of arginase, D – concentration of L-arginine, E – concentration of nitrite-anion, F – concentration of nitrate-anion. Note. * – p<0.05, *** – p<0.001 compared to control group, # – p<0.05, ## – p<0.01 compared to WIS group
Discussion
It has to be noted that CM consists of different cell types, where expression of main L-arginine and AA-metabolizing enzymesis not equable. Moreover, CM is covered by a biofilm, which is inhabited by lots of bacteria capable of metabolizing L-arginine47. We consider the inability to study the L-arginine metabolism separately in every cell type and bacterium species as the main limitation of our study.
The first step of this investigation was to find out the influence of a single action of COX/LOX inhibitors on L-arginine metabolism in CM. In general, three possible levels of studied compound saction can be regarded: their action as COX/LOX inhibitors, their topical action as compounds were injected intragastrically and other mechanisms not related to COX/LOX inhibition. All the tested compounds are inhibitors of PG E2 synthesis, so AA was metabolized by other non-inhibited enzymes. None of studied compounds changed the concentration of L-arginine, nitrite- and nitrate-concentration in CM, so our focus was targeted on enzymes activity.
As a result of a nonselective mechanism of naproxen action, it causes a decrease in all the PGs concentration.This results in anelevation of LT B4 concentration according to excess of AA and ability of LOX, mainly 5-LOX, to metabolize it48. PG E2 is responsible for mucus and bicarbonates secretion in GI mucosa31. PG I2 and TX A2 are local regulators of the blood stream49. LT B4 is considered to be a potent proinflammatory agent50. The transition of balance between proinflammatory and cytoprotective mediators can cause the activation of inflammation by two mechanisms. The first one is related with macrophages activation by LT B4via LTB4-receptors51and later raise in their number in lamina propria52. Thus, enhanced activity of iNOS in this group may emerge from macrophages activation53. The second mechanism may be associated with lowered mucus production by cause of PG E2 deficiency.Mucus protects CM against bacterial LPS, flagellins and toxins supplied by rat`s own cells54. Moreover, due to the half maximal inhibitory concentration (IC-50) of naproxen, it shows higher affinity to COX-1 (IC-50 35.48 μmol/l) compared with that of COX-2 (IC-50 64.62 μmol)55. According to our previous investigation, naproxen did not cause histological changes in CM, but the augmentation of free radical oxidation and antioxidant enzymes activity was noted56. This fact supports the idea about proinflammatory action of naproxen. The reduction of cNOS and arginase activity may occur by reason of the competition with iNOS for a substrate or as a result of direct action of naproxen on their activity. More expressed changes were obtained in a gastric mucosa – activation of iNOS in gastric mucosa under the action of naproxen led to increased nitrite-anion concentration57.
The action of celecoxib – a selective COX-2 inhibitor – was accompanied byan elevationof iNOS activity and tended to decline cNOS activity, which may be caused by competition for L-arginine. As in a case with naproxen, the activity of 5-LOX was not inhibited, the AA was accumulating, and this underlies the raised iNOS activity. However, no changes of arginase activity were noted. It can be explained by not inhibited COX-1 activity, which contributed the normal production of other PGs. Thus, action of constitutive eicosanoidson arginase activity needs further investigation. Selective COX-2 inhibition demonstrated that the competition between NOS and arginase enzymes is not the only one reason of diversity in inducible and constitutive enzymes` activity. The investigation of celecoxib action on the gastric mucosa showed more expressed changes – enhanced iNOS activity and nitrite-anion concentration, diminished or tended to be diminished cNOS and arginase activity57.
Dual COX/LOX inhibitor 2A5DHT caused the tendency of arginase activity to be fallen off, while the activity of NOS isoforms did not change. According to its mechanism of action, 2A5DHT is inhibiting the production of PG E2 and LT B4.In this case AA metabolism may becrossed to the local vasoactive PGs production. Both PG I2 and TX A2 can be produced, so it remains unclear which one could decrease arginase activity and is this effect related to eicosanoids. We do not connect the lowering of arginase activity with competition for the substrate as the concentration of L-arginine and the activity of NOS isoforms did not change.
By cause ofpharmaсokinetic properties of tested compounds both naproxen58 and celecoxib59areabsorbedin 2-4 hours in upper GI tractwhen administered per os.Pharmacokinetic parameters of2A5DHT are not well-studied. Giving this, we do not support the idea of topical action of naproxen and celecoxib on CM, whereas 2A5DHT theoretically could have this property. Our previous investigation showed that neither celecoxib, nor 2A5DHT cause histological changes or prooxidant action, unlike naproxen56. We suppose that the proinflammatory impact of COX/LOX inhibitors can be observed in case of both iNOS activation and cNOS/arginase inhibition, while one of these actions separately does not cause the inflammation.
Action of WIS caused typical changes of studied parameters57, 60.The decline of L-arginine concentration in blood plasma was also noted57. We did not measure the concentration of L-arginine in blood plasma as there is a constant flux between the intracellular and extracellular space for L-arginine61. L-arginine concentration falling off in CM may be caused by a sharp activation of iNOS.Activation of this enzyme is mediated by CM infiltration with macrophages and activation of nonspecific antibacterial action. This process could be triggered by the action of adrenaline, which causes vasoconstriction62. Activation of brain-gut axis cause the inhibition of phospholipase A2by cortisol63and augments visceral sensitivity bycorticotropin releasing factor64. Glucocorticoidsaffect the sympathetic nervous system, mediating sensitivity of adrenoreceptors to catecholamines65, what may increase their effect.According to the literature data,expression of COX-2 and increase of PG E2concentration are raised during the acute stress in rats66. Decrease in L-arginine concentration also can be caused by the defect in CAT-2 function in CMin state of inflammation61. The deficiency of L-arginine is contributing toimpairment in epithelial restitution, protein translation, phagocytosis, and innate immunity, as well as dysregulation of apoptosis61. Defective L-arginine uptake can lead to the diminished ability of arginase/ornithine decarboxylase pathway to generate polyamines61. Polyamines have many biological functions in CM, including inhibition of Th1 cytokines production, monocyte activation, stimulation of epithelial restitution by enhancing cell migration and proliferation, regulation of apoptosis61. Literature data are showing controversary affection of glucocorticoids on arginase activity – it can be downregulating67 and upregulating68.Although it was reported about the co-induction between both arginase-1, arginase-2 and iNOS in macrophages68, we noted the opposite action. It can be caused by determination of enzymatic activity in CM homogenates, which consist of different cell types, and macrophages were not the most significant among them. Going back to NOS activity, it can synthesize not only NO, but also N-hydroxyarginine and superoxide-anion69.The last oneactivates arginase, whereas N-hydroxyarginine – inhibits69.Sharp elevationof NO production by iNOS in CM caused the elevated concentration of nitrite-/nitrate-anionrespectively, what was also noted in gastric57and colon70 mucosa. Increased concentration of NO can lead to enhanced formation of peroxinitrite anion, nitration of protein tyrosine, activate nitrosation processes. The last one is regarded to be one of the factors leading to augmented expression of some proinflammatory genes like tumor necrosis factor-α and p38 mitogen-activated protein kinases69.
Action of COX/LOX inhibitors in case of WIS action was different compared to their single action. Naproxen did not show pro-inflammatory impact, moreover the iNOS activity tended to decrease, although it was not enough to shift the L-arginine metabolism on constitutive pathways. We suppose that the reduction of iNOS activity in this case is associated with inhibition of both COX isoforms. In case of a single action, naproxen is supposed to inhibit COX-1 mainly, as the activity of COX-2 is minor. On the other hand, inhibition ofCOX-1 by naproxen could block the biosynthesis of TXs more than PG I2, thus increasing the blood flow in the ischemic CM. Related effect of combined action of naproxen and WIS was noted in gastric57 and small intestine mucosa60.
The coupled action of celecoxib and WIS cannot be considered proinflammatory. However, as described above, the single action of celecoxib enhanced the activity of iNOS. This may point to the cytoprotective role of COX-1 in course of inflammation. On the other hand, WIS actionelevates COX-2 activity, which is the main target for celecoxib action.Single administration of celecoxib could partially inhibit COX-1 also, thus causing biochemical changes that led to iNOS activation.Acting on the gastric mucosa under the conditions of WIS, celecoxib declined iNOS activity and did not change cNOS and arginase activity57 or diminished arginase activity70.
Dual COX/LOX inhibitor 2A5DHT showed the most active regulation of L-arginine-metabolizing enzymes activity in CM in state of WIS action. Uptake of L-arginine and its metabolism by arginase to L-ornithine and conversion to L-proline by ornithine aminotransferase is essential for colonic epithelial lesion repair71. Moreover, this compound was the only one which increased the concentration of L-arginine in CM. As it was mentioned, WIS may cause ischemia of CM due to augmented adrenaline production. Synthesis of PG I2, TXs and some other PGs was not inhibited by 2A5DHT. By reason ofthe reversed vasoactive influence of PG I2 and TX A2 and cytoprotective impact of 2A5DHT administration we suppose that AA was metabolized by a prostacyclin synthase mainly.
Interestingly, that the action of all the tested COX/LOX inhibitors did not change the total activity of L-arginine-metabolizing enzymes. Only the administration of dual COX/LOX inhibitor raised the concentration of L-arginine, which can be used in anabolic pathways. Moreover, according to experimental data, supplementation of rats with L-arginine attenuated the degree of tissue damage in the intestinal ischemia and promoted healing of intestinal mucosa61. Colonic epithelial restitution is dependent on L-arginine transport into the cells by CAT-2, which is interruptedin case of inflammation71. According to the literature data, global protein translation is low in the absence of L-arginine6. Most studies have demonstrated the beneficial action of L-arginine on GI function improving gastric ulcer healing, accelerating intestinal mucosal regeneration, enhancing bacterial clearance, and reducing histological bowel necrosis6. Like NOS isoforms, arginase-1and CAT-2 are also regarded as inducible, while arginase-2 and CAT-1 – constitutive isoforms72. It was reported, that iNOS and argininosuccinate synthetase activities can be co-induced in LPS and interferon-γstimulated macrophages72. This underlies the re-synthesis of L-arginine from L-citrulline. We did not observe this hypothetical elevation of L-arginine concentration under the single action of naproxen and WIS, which caused the highest augmentation of iNOS activity. That is why we speculate that L-arginine increasing effect of 2A5DHT may be mediated by PG I2. It serves for vasodilation, so the concentration of extracellular L-arginine becomes higher. As CAT-2 is an inducible L-arginine transporter localized on macrophages, it is possible that during WIS the transport of L-arginine was increased in all the groups, but catabolized immediately by activated iNOS. The argument for this assumption is lower value of Michaelis constant (Km) of iNOS (13 μM)73 compared with that of arginase-1 (3.3 mM) and arginase-2 (1.9 mM)74.
Our basic assumption is related to the shift of AA metabolism to different directions under the action of COX/LOX inhibitors, which defines proinflammatory or cytoprotective action.An alternative version is associated with accumulation of AA under the action of COX/LOX inhibitors.AA activates calcium entry into the cell, which can stimulate cNOS activity without iNOS inhibition69.However, literature data suggest that iNOS and arginase activities are regulated reciprocally in macrophages by cytokines, and this may guarantee the efficient production of NO75.
Conclusions
The single action of nonselective COX inhibitor naproxen can be considered proinflammatory to colon mucosa as it enhances iNOS activity and decreases cNOS/arginase activity. Action of selective COX-2 (celecoxib) and dual COX-2/5-LOX (2A5DHT) inhibitors in combination with acute stress decreased iNOS activity andupregulated cNOS and arginase activity as well. 2A5DHT raised the concentration of L-arginine, showing the most pronounced anti-inflammatory effect. The transition of arachidonic acid metabolism from inducible to constitutive pathways of metabolism can cause a complementary transition of L-arginine metabolism.
Acknowledgements. The authors would like to thank Prof. J. L. Wallace (Inflammation Research Network, University of Calgary, Canada) for providing us with naproxen and celecoxib and Prof. R. B. Lesyk (Department of organic, bioorganic and pharmaceutical chemistry, Danylo Halytsky Lviv National Medical University, Ukraine) for providing us with 2A5DHT for our investigations.
Conflict of Interests
The authors declare that they have no competing interests.
Funding Source
This work was supported by Danylo Halytsky Lviv National Medical University. The authors read and approved the final manuscript.
References
- QihangH., QinghuaY., YuanyangD., XiaoxiL., LuluY., BoW., Yuming G., Le S.,Bingkun Z.L-arginine enhances intestinal stem cell function [Internet]. SSRN (2019) [cited 2020Mar25]. Available from:https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3347896.
CrossRef - Blachier F., Davila A. M., Benamouzig R., Tome D.Channelling of arginine in NO and polyamine pathways in colonocytes and consequences. Front.Biosci.2011;16:1331-43.
- Young R., Bush S. J., Lefevre L., Mcculloch M. E. B., Lisowski Z. M., Muriuki C.,Waddel L. A., SauterK. A., PridansC., ClarkE. L., HumeD. A.Species-specific transcriptional regulation of genes involved in nitric oxide production and arginine metabolism in macrophages. ImmunoHorizons.2018;2:27-37.
CrossRef - Morris S. M. Arginine metabolism revisited. J.Nutr. 2016;146:2579S-86S.
CrossRef - Closs E. I., Simon A., Vékony N., Rotmann A. Plasma membrane transporters for arginine.J.Nutr. 2004;134:2752S-9S.
CrossRef - Wu G., Bazer F. W., Davis T. A., Kim S. W., Li P., Rhoads J. M., Satterfield M. C., Smith S. B., Spencer T. E., Yi Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153-68.
CrossRef - Yuan C., Zhang X., He Q., Li J., Lu J., Zou X.L-arginine stimulates CAT-1-mediated arginine uptake and regulation of inducible nitric oxide synthase for the growth of chick intestinal epithelial cells.Mol. Cell Biochem.2015;399:229-36.
CrossRef - Marini J. C., Agarwal U., Robinson J. L., Yuan Y., Didelija I. C., Stoll B., Burrin D. G. The intestinal-renal axis for arginine synthesis is present and functional in the neonatal pig.Am J. Physiol.-Endoc. M.2017;313:E233-E42.
CrossRef - Morris S. M. Enzymes of arginine metabolism.J.Nutr.2004;134:2743S-7S.
CrossRef - Pestour S., Couchet M., Breuillard C., Corne C., Mathieu N., Lamarche F., Fontaine E., Coëffier M., Moinard C. An in vitro explant model for studies of intestinal amino acid metabolism. Clin.Nutr. Exp. 2020; 29:1-9.
CrossRef - Garcia-Miranda P., Garcia-Delgado M., Peral M. J., Calonge M. L., Ilundain A. A. Ontogeny regulates creatine metabolism in rat small and large intestine.J. Physiol.Pharmacol. 2009;60:127-33.
- Wu G. Intestinal mucosal amino acid catabolism. J.Nutr, 1998;128:1249-52.
CrossRef - 1 Aoi Y., Terashima S., Ogura M., Nishio H., Kato S., Takeuchi K. Roles of nitric oxide (NO) and NO synthases in healing of dextran sulfate sodium-induced rat colitis.J. Physiol.Pharmacol. 2008;59:315-36.
- Choi S., Park C., Ahn M., Lee J. H., Shin T. Immunohistochemical study of arginase 1 and 2 in various tissues of rats.Acta Histochem.2012;114:487-94.
CrossRef - Fomenko I., Bondarchuk T., Emelyanenko V., Denysenko N., Sklyarov P., Ilkiv I., Lesyk R., Sklyarov A. Changes of nitric oxide system and lipid peroxidation parameters in the digestive system of rats under conditions of acute stress, and use of nonsteroidal anti-inflammatory drugs. Current Issues in Pharmacy and Medical Sciences.2015;28:37-41.
CrossRef - Sklyarov A. Y., Panasyuk N. B., Fomenko I. S. Role of nitric oxide synthase and cyclooxigenase/lipooxigenase systems in development of experimental ulcerative colitis.J. Physiol.Pharmacol. 2011;62:65-73.
- Xu J., Wu L., Yu P., Sun Y., Lu Y. Effect of T. spiralis Serine protease inhibitors on TNBS-induced experimental colitis mediated by Macrophages. Sci. Rep.2020;10(1):3147.
CrossRef - Bekebrede A. F., Keijer J., Gerrits W. J. J., Boer V. C. J. D. The molecular and physiological effects of protein-derived polyamines in the intestine. Nutrients. 2020;12:197.
CrossRef - Khan M. A. W., Ologun G., Arora R., Mcquade J. L., Wargo J. A. Gut microbiome modulates response to cancer immunotherapy.Dig. Dis. Sci. 2020;65:885-96.
CrossRef - Tofalo R., Cocchi S., Suzzi G. Polyamines and gut microbiota.Front.Nutr. 2019;6:16.
CrossRef - Ghlichloo I., Gerriets V. Nonsteroidal Anti-inflammatory Drugs (NSAIDs) [Internet]. Europe PMC. StatPearls Publishing; (2019) [cited 2020Mar25]. Available from: https://europepmc.org/article/NBK/NBK547742
- Wang D., Mann J. R., Dubois R. N. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128:1445-61.
CrossRef - Peng X., Li J., Tan S., Xu M., Tao J., Jiang J., Liu H., Wu B.COX-1/PGE2/EP4 alleviates mucosal injury by upregulating β-arr1-mediated Akt signaling in colitis. Sci. Rep. 2017;7:1055.
CrossRef - Gorman H., Moreau F., Chadee K. A281 Does muc2 mucin regulate mucus associated proteins and other goblet cell innate defense molecules? J. Can. Assoc. Gastroenterol. 2018;1:406.
CrossRef - Gunaydin C., Bilge S. S. Effects of nonsteroidal anti-inlammatory drugs at the molecular level. Eurasian J. Med. 2018;50:116-21.
CrossRef - Fratila O. C., Ilias T. I. COX-2 and Ki-67 immunohistochemical markers in the assessment of long-standing ulcerative colitis associated dysplasia. Rom. J.Morphol.Embryol.2013;54:143-9.
- Haworth R., Oakley K., Mccormack N., Pilling A. Differential expression of COX-1 and COX-2 in the gastrointestinal tract of the rat.Toxicol.Pathol.2005;33:239-45.
CrossRef - Kawahara K., Hohjoh H., Inazumi T., Tsuchiya S., Sugimoto Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors.BBA-Mol. Cell Biol. L. 2015;1851:414-21.
CrossRef - Park M. G., Yoo J. D., Lee K. H. Current guidelines for non-steroidal anti-inflammatory drugs. J. Korean Orthop. Assoc. 2020;55:9-28.
CrossRef - Rensburg R. V., Reuter H. An overview of analgesics: NSAIDs, paracetamol, and topical analgesics Part 1.S. Afr. Fam.Pract. 2019;61:S4-S10.
CrossRef - Takeuchi K., Amagase K. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract.Curr. Pharm. Des.2018;24:2002-11.
CrossRef - Reddy K. K., Rajan V. K. V., Gupta A., Aparoy P., Reddanna P. Exploration of binding site pattern in arachidonic acid metabolizing enzymes, cyclooxygenases and lipoxygenases.BMC Res. Notes. 2015;8:152.
CrossRef - Shim Y. K., Kim N. Nonsteroidal anti-inflammatory drug and aspirin-induced peptic ulcer disease.Korean J. Gastroenterol. 2016;67:300-12.
CrossRef - Santos C. M. M., Ribeiro D., Silva A. M. S., Fernandes E. 2,3-Diarylxanthones as potential inhibitors of arachidonic acid metabolic pathways. Inflammation. 2017;40:956-64.
CrossRef - Siddiqui A. H., Farooq U., Siddiqui F. Curling Ulcer (Stress-induced Gastric) [Internet]. StatPearls [Internet]. U.S. National Library of Medicine; (2020) [cited 2020Mar26]. Available from: https://www.ncbi.nlm.nih.gov/ books/NBK482347/
- 36. Jung Y.-H., Jang J. H., Lee D., Choi Y., Choi S.-H., Kang D.-H. Relationships between catecholamine levels and stress or intelligence. Res.2019;44:1192-200.
CrossRef - 37. Ilkiv I., Lesyk R., Sklyarov O. Evaluation of novel 4-thiazolidinone-based derivatives as possible cytoprotective agents against stress model in rats. Appl. Pharm. Sci.2017;7:199-203.
CrossRef - 38. Allen M. J., Sharma S. Physiology, adrenocorticotropic hormone (ACTH) [Internet]. StatPearls [Internet]. U.S. National Library of Medicine; (2019) [cited 2020Mar26]. Available from: https://www.ncbi.nlm.nih.gov /books/NBK500031/
- 39. Samuel S., Nguyen T., Choi H. A. Pharmacologic characteristics of corticosteroids.Neurocrit. Care. 2017;10:53-9.
CrossRef - 40. Takagi K., Kasuya Y., Watanabe K. Studies on the drugs for peptic ulcer. A reliable method for producing stress ulcer in rats. Pharm. Bull.1964;12;465-72.
CrossRef - 41. Fomenko I. S., Yemelyanenko V. Y., Panasyuk N. B., Biletska L. P., Sklyarov O. Y. Action of nonsteroidal anti-inflammatory drugs on NO-synthase/arginase system in colon under the conditions of stress.Achievements of Clinical and Experimental Medicine.2013;2:207-9(in Ukrainian)
- 42. Ravayeva M. Y.,Chuyan Y. N.The change of nitrogen oxide synthesis under the action of low-intensity millimeter radiation.Scientific Notes of VI Vernadsky Taurida National University. 2011;24:201-10. (in Russian)
- Morrison A. C., Correll P. H. Activation of the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase by macrophage-stimulating protein results in the induction of arginase activity in murine peritoneal macrophages. Immunol.2002;168:853-60.
CrossRef - Abergas A. D., Aleria M. C., Elio A. E., De Lara A. F., Pillejera J. G. Comparative analysis of arginine content of three commercially available baby formula in the Philippines using multiple point standard addition method. IPCBEE. 2012;49:198-202.
- Kiselyk I. O., Lutsyk M. D., Shevchenko L. Y.Peculiarities of nitrites and nitratesdetermination in peripheral blood in patients with viral hepatitis and jaundice syndrome of other etiology. Laboratory Diagnostics. 2011;3:43-5. (in Ukrainian)
- Levashov P. A., Sutherland D. S., Besenbacher F., Shipovskov S. A robust method of determination of high concentrations of peptides and proteins.Biochem.2009;395:111-2.
CrossRef - Dai Z., Wu Z., Hang S., Zhu W., Wu G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Hum.Reprod. 2015;21:389-409.
CrossRef - Carbajal-Quintana D., Molina-Cuevas V., Ravelo-Calzado Y., Mas-Ferreiro R. Effects of D-002, a mixture of beeswax alcohols, on the acetic acid-induced writhing test in mice: a comparison with naproxen, aspirin, and paracetamol. Revista CENIC CienciasBiológicas. 2013;44.
- Becker F., Yi P., Al-Kofahi M., Ganta V. C., Morris J., Alexander J. S. Lymphatic dysregulation in intestinal inflammation: new insights into inflammatory bowel disease pathomechanisms. Lymphology. 2014;47:3-27
- Meriwether D., Sulaiman D., Volpe C., Dorfman A., Grijalva V., Dorreh N., Solorzano-Vargas R. S., Wang J., O`Connor J., Papesh J., Larauche M., Trost H., Palgunachari M. N., Anantharamaiah G. M., Herschman H. R., Martin M. G., Fogelman A. M., Reddy S. T.Apolipoprotein A-I mimetics mitigate intestinal inflammation in a COX2-dependent inflammatory disease model. Clin. Invest. 2019;129:3670-85.
CrossRef - Izumi T., Yokomizo T., Obinata H., Ogasawara H., Shimizu T. Leukotriene receptors: classification, gene expression, and signal transduction.Biochem.2002;132:1-6.
CrossRef - Grainger J. R., Konkel J. E., Zangerle-Murray T., Shaw T. N. Macrophages in gastrointestinal homeostasis and inflammation. PflügersArchiv – European Journal of Physiology. 2017;469:527-39.
CrossRef - Jiang Y., Xiao L., Fu W., Tang Y., Lertnimitphun P., Kim N., Zheng C., Tan H., Lu Y., Xu H.Gaudichaudione H inhibits inflammatory responses in macrophages and dextran sodium sulfate-induced colitis in mice.Pharmacol. 2019;10.
CrossRef - Josenhans C., Müthing J., Elling L., Bartfeld S., Schmidt H. How bacterial pathogens of the gastrointestinal tract use the mucosal glyco-code to harness mucus and microbiota: New ways to study an ancient bag of tricks. J. Med. Microbiol.2020;310(2):151392.
CrossRef - Hinz B., Cheremina O., Besz D., Zlotnick S., Brune K. Impact of naproxen sodium at over-the-counter doses on cyclooxygenase isoforms in human volunteers. J. Clin. Pharm. Th.2008;46:180-6.
CrossRef - Denysenko N. V.,Sklyarov O. Y.The action of COX/LOX inhibitors on antioxidant system and morphological state of rat’s colon mucosa under the conditions of stress. Medical and Clinical Chemistry. 2019;21:5-11
CrossRef - Fomenko I., Sklyarov A., Bondarchuk T., Biletska L., Panasyuk N.,Wallace J. L. Effects of conventional and hydrogen sulfide-releasing non-steroidal anti-inflammatory drugs in rats with stress-induced and epinephrine-induced gastric damage. Stress.2014;17:528-37.
CrossRef - Prabodh S. C., Sonia Y., Rakesh P., Archana S., Sandeep J. Naproxen: an update on physicochemical, analytical and pharmacological aspects. Anti-Infective Agents in Medicinal Chemistry.2011;10:339-50.
CrossRef - Cho K. H., Jee J.-P., Yang D. A., Kim S. T., Kang D., Kim D.-Y., Sim T., Park S. Y., Kim K., Jang D.-J. Improved dissolution and oral bioavailability of celecoxib by a dry elixir system.Nanosci.Nanotechno. 2018;18:1482-6.
CrossRef - Fomenko I. S., Korniychuk O. P., Hural A. R., Shykula R. H., Ilkiv I. I., Sklyarov O. Y. Role of cyclooxigenase in modification of intestinal microflora under the conditions of stress. Physiological Journal. 2015;61:42-9. (in Ukrainian)
CrossRef - Hong S.-K. S., Maltz B. E., Coburn L. A., Slaughter J. C., Chaturvedi R., Schwartz D. A., Wilson K. T.Increased serum levels of L-arginine in ulcerative colitis and correlation with disease severity. Bowel Dis.2010;16:105-11.
CrossRef - Lang Y., Fu F., Sun D., Xi C., Chen F. Labetalol prevents intestinal dysfunction induced by traumatic brain injury. Plos One. 2015;10:e0133215.
CrossRef - Braca A., Piaz F. D., Marzocco S., Autore G., Vassallo A., Tommasi N. D. Triterpene derivatives as inhibitors of protein involved in the inflammatory process: molecules interfering with phospholipase A2, cycloxygenase, and lipoxygenase. Drug Targets. 2011;12;302-21.
CrossRef - Rincel M., Darnaudéry M. Maternal separation in rodents: a journey from gut to brain and nutritional perspectives.Nutr. Soc. 2019;79:113-32.
CrossRef - Jerem P., Jenni-Eiermann S., Mckeegan D., Mccafferty D. J., Nager R. G. Eye region surface temperature dynamics during acute stress relate to baseline glucocorticoids independently of environmental conditions. Behav. 2019;210:112627.
CrossRef - Rodzian M. N. S., Ibrahim I. A. A., Fahami N. A. M., Ismail N. M. Pure tocotrienol concentrate protected rat gastric mucosa from acute stress-induced injury by a non-antioxidant mechanism. J.Pathol. 2013;1:52-8.
CrossRef - Lindemann D., Rack K. Glucocorticoid inhibition of interleukin-4 (IL-4) and interleukin-13 (IL-13) induced up-regulation of arginase in rat airway fibroblasts. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2003;368:546-50.
CrossRef - Mori M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. Nutr. 2007;137:1616S-20S.
CrossRef - Wink D. A., Hines H. B., Cheng R. Y. S., Switzer C. H., Flores-Santana W., Vitek M. P.,Ridnour L. A., Colton C. A.Nitric oxide and redox mechanisms in the immune response. Leukoc. Biol. 2011;89:873-91.
CrossRef - Fomenko I. S., Bondarchuk T. I., Biletska L. P., Panasyuk N. B., Sklyarov O. Y. The study of NO-synthase system role in gastric mucosa of rats under the conditions of combined action of nonsteroidal anti-inflammatory drugs and adrenaline-induced stress. Bulletin of Problems of Biology and Medicine. 2013;1:245-9. (in Ukrainian)
- Singh K., Coburn L. A., Barry D. P., Boucher J.-L., Chaturvedi R.,Wilson K. T. L-arginine uptake by cationic amino acid transporter 2 is essential for colonic epithelial cell restitution. J. Physiol.Gastrointest. Liver Physiol. 2012;302:G1061-73.
CrossRef - Mori M.,Gotoh T. Regulation of nitric oxide production by arginine metabolic enzymes. Biophys. Res.Commun. 2000;275:715-9.
CrossRef - Smith B. C., Fernhoff N. B.,Marletta M. A. Mechanism and kinetics of inducible nitric oxide synthase auto-S-nitrosation and inactivation. Biochemistry. 2012;51:1028-40.
CrossRef - Tommasi S., Elliot D. J., Boit M. D., Gray S. R., Lewis B. C., Mangoni A. A. Homoarginine and inhibition of human arginase activity: kinetic characterization and biological relevance. Rep. 2018;8:3697.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





