How to Cite | Publication History | PlumX Article Matrix
S. Venkatesan1*, P. Masilamani2, P. Janaki2, T. Eevera3, S. Sundareswaran3 and P. Rajkumar4
1Anbil Dharmalingam Agricultural College and Research Institute Tiruchirappalli - 27, Tamil Nadu, India
2Department of Soil Science and Agricultural Chemistry, TNAU, Coimbatore-3
3Seed Centre, Tamil Nadu Agricultural University, Coimbatore-3
4Agricultural Engineering College and Research Institute, TNAU, Coimbatore-3
Corresponding Author E-mail: svengat95@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2912
ABSTRACT:
In the process of sexual reproduction in angiosperms, making viable pollen grain to land on the surface of the receptive stigma of the same flower or other flower is the key process. Major factors like both biotic and abiotic play a crucial role in the process of making pollen to reach stigma during sexual mode of reproduction in angiosperms. In nature, some of the plants pollination take place without the support of any of the above said two factors, in some of the cases transfer of pollen aided by the biotic factors viz., insects, birds, animals etc., Particularly in the process of insect or bird aided pollen transfer, secretion of volatile compound in view of attracting pollinators have multiple role. In addition to above, flower with variety of colour also play a huge role in attraction of pollinators and its aid in timely occurrence of pollination. Further, due to climate change some of the pollinator population also become extinct, under that condition through evolutionary changes plant change their phenotypic expression by that way they attract other group of pollinator in the process of sexual mode of reproduction. Only the plant able to change their adaptation through the evolutionary process or through the event of mutation based on their requirement alone able to survive. This review discusses some of the important strategy adopted by the flowering plants in view of attraction of pollinators.
KEYWORDS: Floral Signals; Genes; Pollination; Pigments; Patterning; Volatiles
Download this article as:| Copy the following to cite this article: Venkatesan S, Masilamani P, Janaki P, Eevera T, Sundareswaran S, Rajkumar P. Genetic and Biochemical Characterization Underpinning the Development of Floral Features that Influence Pollination – A Review. Biosci Biotech Res Asia 2021;18(2). |
| Copy the following to cite this URL: Venkatesan S, Masilamani P, Janaki P, Eevera T, Sundareswaran S, Rajkumar P. Genetic and Biochemical Characterization Underpinning the Development of Floral Features that Influence Pollination – A Review. Biosci Biotech Res Asia 2021;18(2). Available from: https://bit.ly/38v1E6o |
Introduction
Flower contain both male and female organ or in some of the cases male or female organ alone is available in individual flower. Pollen grains are male reproductive cells and non-motile in nature. Pollen grains are normally transferred to stigma through insect or wind or water or animal etc., that is vary from plants to plants. Based on pollen received by the stigma through any mode plants are classified into two types like self –pollination (pollen grain formed in the plant transferred to the stigma of the same plant) and cross –pollination (reaching of pollen grain originated from one plant to stigma of the another plant of the same variety). In the case of gymnosperm, transfer of pollen from male cone to micropyle of female cone takes place during pollination.
In the gymnosperms, it is the transfer of pollen grains from a microsporophyll (in the male cone in the conifers) to the micropyle of an ovule (in the female cone in the conifers). Self-pollination is transfer of pollen grains from the anther of a flower to the stigma of either the same or genetically similar flower and cross pollination is the transfer of pollen grains from the anther of one flower to the stigma of a genetically different flower.
Occurrence of pollination and fertilization in angiosperm is very important and vital in the field of agriculture particularly seed production. The above was realized since from the era of plant cultivation for the purpose of food grain production. Now, in some of plant like sunflower, challenging part of the seed production is transfer of pollen grain between ear heads of two plants of sunflower. In order to attract pollinator most of the crops have the unique molecular mechanism to attract the pollinator during anthesis time. In angiosperm, the sequence of events between initiations of floral bud to ripening of fruits is under the control of gene regulatory network. Again sequence of gene expression is regulated by the environmental factors. Based on the review, it is inferred that occurrence of pollination, fertilization and post fertilization development process was totally under the control of combination of both genetic and environmental factors1-2.
In angiosperms, the volatile coming from the floral structure has different property3. In some of the floral exudates are making the floral parts unfit for consumption by the animals and other insects. Some of the plants exhibit, antimicrobial properties. Most of the cases, exudates are acting as a more potential attractant of pollinators. In this review, discussed important transcriptional factors that are involved in floral development, major floral volatiles emanated from flowers in pollinators attraction and defense mechanisms, floral signals, flower pigments and floral patterning.
Floral signals
Establishment of contact between the flower and pollinator is mainly decided by the production of pollinator specific floral traits/ volatiles and ability of the pollinator to sense the specific signals given by the plant through its floral traits. But these signals should deter or avoid antagonistic agents invasion in floral parts4-5. Normally, floral signals transmit variety of messages to the surrounding in view of making its presence known to its surrounding and particularly to the pollinators6.
The success of the pollinator aided pollination is mainly relying upon the effective means of communication between the flowering plants and pollinators even under the noisy environment. In the above process of communication between two, the plants mainly use flower and associated properties as a signal molecule to develop a communication channel. The signals may be like colorful display and visual attractions or through sending some vibration or through emitting particular compound and making pollinator to get attracted in response to above stimuli. Similarly, pollinators are also, mostly sense the flower and related characters to reach the specific plants in the event of pollination.
Many pollinator choices are visual and aesthesis stimuli for effective pollination. Aesthesis and visual cues are required to stimulate generalized nectar feeding responses in the hawk moth Manduca sexta feeding on Datura wrightii7. Attraction of mosquitoes to Tanacetum vulgare when plants emit visual and olfactory signals8.
Floral pigments
Colour of the floral structure is the important signal to the pollinators. Pigments like beatalains, carotenoids and anthocyanins are major chemical constituents responsible for making flower more attractive. Carotenoids are derivative of isoprenoids; betalains from tyrosine and anthocyanins from flavonoids. Among the above three, betalains are water soluble and nitrogenous compound derived by condensation of betalamic acid (Fig.1). For example Red beetroot, Portulaca, amaranth and prickly pear contain betalains9. Among the different color pigments, caryophyllales in which alone the compound betalains is reported to be occur. The transcriptional regulation of betalains remains largely unknown. But in Beta vulgaris10,betalin production related biochemical event was under the control of anthocyanin MYB –like protein.
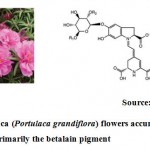 |
Figure 1: Portulaca (Portulaca grandiflora) flowers accumulating primarily the betalain pigment |
Carotenoids are plastid-synthesized and localized lipid-soluble C40 tetraterpenoids are found predominant in the plant kingdom.
Flower with different colours like blue, red, purple and orange are associated with accumulation of anthocyanin pigments (Fig 3). In the process of anthocyanin synthesis, various enzymes are involved. All those enzymes are need to be activated and for that purpose the role of R2R3 MYB – like protein, basic helix-loop-helix and WR – repeat proteins are very much needed. Without the above mentioned three protein factors involvement and co-operation the activation of enzymes involved in the anthocyanin production is practically not possible within a shortest span of time.
Carotenoids play a major role in the preserving the life of the plant by involving the following essential process viz., extending support to protect the plants from heavy light intensity during photosynthesis and acting as a source molecule for the synthesis of ABA. Carotenoids are responsible for most of the yellow to orange flower colours in ornamentals that include marigold (Tagetes eracta), daffodil (Narcissus), Freesia, Gerbera, Rosa, Lilium, and Calendula (Fig 2).Several hundreds of carotenoid structures are found to occur naturally, xanthophylls are very commonly known as an important pigment molecule of the flowers.
 |
Figure 2: Marigold (Tagetes patula) flowers accumulating the carotenoid pigment, lutein. |
Hummingbird is very much attracted by the flowers of Mimulus cardinalis. In Mimulus cardinalis production of yellow carotenoids were controlled by expressing the genes that are located in the locus YUP and making the plant to produce flower with red colour. In Mimulus lewisiiexpression of the YUP leads to production of flower with pink colour and it is mainly pollinated by honey bees11.
Almost all vascular plants contain anthocyanin and it’s a compound of water soluble nature. The flower with variety of colours ranging from orange, red, purple and blue is governed by the anthocyanin pigments that are available in the flowers (Fig. 3). Anthocyanins are derivative of flavonoid. In the production of anthocyanin, the role of R2R3 MYB, bHLH and WDR proteins are very vital.Without these three co-factors, activation of enzymes involved in the anthocyanin pigments production pathway is not possible. Hence, co-ordination and action among the three co-factors are conserved across wide variety of taxa 12.
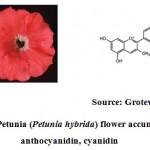 |
Figure 3: Petunia (Petunia hybrida) flower accumulating an anthocyanidin, cyanidin |
Floral patterning
In flowers, within the corolla and between petal cells lots of differences are being recorded with reference to pigmentation. This in turn gives different types of patterns for flowers, which includes stripes, spots and bicolors. The flower patterns are the key factor in the process of establishing communication between the flower and flower specific pollinators. For an example, Commelina communis has flower with very attractive blue colour petals and contrasting yellow colour anthers. This colour combination is considered to be a one of the ideal colour combination with reference to attraction of more number of pollinators. Furthermore, the flower has pucca orientation for landing of more number of pollinators at a time 13.
In Mimulus lewisii, due to prevalence of competition between the process of anthocyanain and flavonol synthesis flowers are expressing different combination of colour patterning under different environmental condition at which the plants are growing. Mimulus lewisii produces D-limonene, β-myrcene and E-β-ocimene compounds that will attract bumble bee upto 3010 m but in case of mimulus cardinalis produce D-limonene only so that it would attract humming bird upto 415 m only (Fig 4). Due to pink color flower with a section of white encircling at the throat of corolla in Mimulus lewisii, which attract more number of bumblebee in the process of pollination. The above pattern of flower color formation is indirectly under the control of R2R3 MYB transcription factor. This factor favor for expression of the gene related to flavonol synthase and make the condition not favorable for occurrence of anthocyanin pathway14-16.
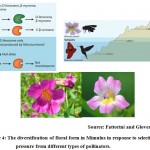 |
Figure 4: The diversification of floral form in Mimulus in response to selective pressure from different types of pollinators. |
Floral volatiles
Volatile organic compounds emitted by the flowers are the key component in most the angiosperm with reference to sending signals to the pollinators and making them to visit and land on the flowers 17 -20. Understanding the process of volatile organic compounds produced by the flowers of different plants is much complicated process. Because the volatile organic compounds emitted by the flowers are mixture of several compounds and are produced by the action of more number of pathways that are in operation in the plants 20. Till date, more than thousands of floral volatile have been reported in over 900 flowering plant species. Among the compounds, terpenoids, benzenoids, phenylpropanoids and derivatives of fatty acids are predominantly reported in more number of plants. The pathway of volatile organic compounds formation is reasonably established but genomics aspects such as identification of biosynthetic genes specific to attraction of particular pollinators are lacking.
The compound viz., isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP) act as a precursor molecule for the synthesis of largest class of floral volatile compound namely terpenoids22. Terpenoids consist of more than 500 number of scent compounds. In plants, the above mentioned C5 precursor molecules are produced by triggering two independent pathways viz., the mevalonic acid (MVA) and the methylerythritol phosphate (MEP). The above two pathways are very critical for production of terpenoid compounds in the plant system.
The MEP pathway operates in plastids play a key role in the production of mono – (C10) and diterpenes (C20) and terpenoids. Precursors for the production of sesquiternes (C15) are given raised by another pathway MVA. Activity of the MVA pathway is scattered among the cytosol, peroxisomes and endoplasmic reticulum23.
Floral volatiles – attraction
Floral volatiles depend on amount, composition and context of their emission. Normally the plants pollinated by moth produce and emit more amount of scent compounds than bee pollinated plants. Moth pollinated plants like Petunia axillaris and Silene latifolia is a perfect example for the above mentioned statement24-25. In the above two plants in addition to petals, pollen grain also contribute odour to attract pollinators. An attempt was made in antherless Rosa rugosa plants, to test verify the impact of exogenously added volatile compounds on pollinator attraction. In this experiment the researchers found that pollen collection behavior of bumblebees increased many fold.
In general, volatile compound not only giving signal for availability of food for foraging and it also giving signal for the availability of place for mating and oviposition26. Mainly to mimic oviposition sites, the plant species belongs to the families like Araceae, Rafflesiaceae, Annonaceae, Apocynaceae and Orchidaceae emit some sulphur containing volatile compounds. These sulphurs containing volatile compounds usually attract the pollinators like necrophagous, saprophagous and caprophagous insects27.
Chemical and rate of emission of chemical used for attacrtion of bumblebee and hummingbird is differ among the two different species of Mimulus. Mimulus lewisii flowers produce an array of volatiles dominated by D-limonene, β-myrcene and E-β-ocimene of these three monoterpenes, M. cardinalis flowers produce only D-limonene, released at just 0.9% the rate of Mimulus lewisii flowers28(Table 1).
Table 1: Mimulus lewisii(inbred line LF10) and Mimulus cardinalis (inbred line CE10) differ both qualitatively and quantitatively in their scent profiles.
| Volatile | Emission rate (ng h-1) | |
| Mimulus lewisii | Mimulus cardinalis | |
| Unk.MO | 0.34 | Absent |
| α- Pinene | 1.80 | 0.07 |
| Sabinene | 1.49 | Absent |
| (-)-β- Pinene | 1.23 | Absent |
| 1-Octen-3-ol | Absent | 0.17 |
| β- Myrcene | 3.31 | Absent |
| D- Limonene | 55.11 | 0.52 |
| E-β- Ocimene | 7.62 | Absent |
| y-Terpinene | 0.07 | Absent |
| Terpinolene | 0.26 | Absent |
| β-Farnesene | Absent | 0.19 |
| α-Farnesene | Absent | 0.14 |
Source: Byerset al., [2014]
Mimulus lewisiiexclusively produces monoterpenes of nine different volatile in the process of establishing communication with the pollinators. Mimulus cardinalis also produce five varieties of volatile compounds. But most of the floral scent compounds are produced by the Mimulus lewisiiwhen compared to other plants. Even though Mimulus cardinalis belongs to same genus, this is not able to produce seven of the scent compounds produced by the Mimulus lewisii. The missing compounds include two of the three most abundant compounds viz., β-myrcene and E-β-ocimene. D-Limonene is the most predominant compound found in both the plants, but production potential of this compound is 107 –fold higher in Mimulus lewisiiwhen compared to Mimulus cardinalis (Fig 5).
 |
Figure 5: Responses of Bombus vosnesenskiiantennal lobe neurons to gas chromatography-fractionated scent from Mimulus lewisiiflowers. |
The interaction between the pollinator and the flowers are very specific in few numbers of plants. This kind of association is one way useful for the isolation of that plant in the angle of sexual mode of reproduction. The specificity of the above relationship was known by studying the reproductive biology of Mimulus lewisii and its particular association with bumblebee for pollination. Likewise another specific pollinator and crop association was reported in Mimulus cardinalis. Mimulus cardinalis is associated with hummingbird for its pollination purpose. This kind of association isolates the crop in view of sexual mode of reproduction. Further scientist also confirmed the plant gene governing the above association follow the mendelian pattern of inheritance without any linkage with other hene that are available in those plants29.
In a crossing experiment, Mimulus lewisiiwas crossed with Mimulus cardinalis in order to know the heritable behavior of D-limonene producing gene. As an outcome of the above cross, F1 plants were subjected to verification of D-limonene production, it was found to produce 55.1ng. F1 yielded more quantity of D-limonene than Mimulus cardinalis parental inbred line (0.5 ng). This result clearly indicates that higher level of D-limonene production was inherited from the Mimulus lewisiias a dominant manner. In order to find out the nature of the gene involved in the D-limonene production, back cross was made between F1 and Mimulus cardinalis. In the back cross experiment, it was segregated with the ratio of 1:1 with reference to presence or absence of emission of two of the components of D-limonene (b-myrcene and E-b-ocimene). This clearly indicates that the gene involved in the D-limonene production follow the mendalian principle with reference to segregation (Fig 6).
 |
Figure 6: Bombus impatiens and Mimulus lewisiiwild-type and transgenic lines |
Fragaria virginiana (Rosaceae), the wild strawberry, is a gynodioecious perennial herb. Female flowers would have different scent profiles and emission rates than hermaphrodite flowers because they are smaller, allocate differently to floral organs, and do not produce pollen-bearing anthers. 2-phenylethanol was released in hermaphrodite flowers it attract many pollinators when compare to female flowers of Fragaria virginiana30(Fig 7).
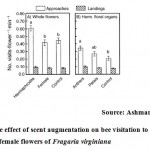 |
Figure 7: The effect of scent augmentation on bee visitation to female flowers of Fragaria virginiana |
Floral volatiles – defence
Flowers are generally deficient in physical barriers such as a highly lignified cell wall and impermeable cuticle, making them highly susceptible to pathogens.Flowers carry higher densities of microorganisms than other aerial plant surfaces due to their high moisture and nutrient content31. Flowers employ volatiles as an alternative mechanism that prevents damage to their reproductive structures.Many VOCs were shown to exhibit antimicrobial and antifungal activities32.Only few VOCs have been investigated for their role in defence against pathogens. (E)-β-caryophyllene emitted from stigmas of Arabidopsis flowers was shown to limit bacterial growth.VOCs emitted by Saponaria officinalis petals were shown to inhibit bacterial growth.Ants are very inefficient pollinators and occasionally feed on floral structures. It was shown that many common European ants are deterred by volatiles emitted from temperate fowers33.
Molecular mechanisms involved in floral volatiles
Petunia crossed with Inbred Petunia x hybrida cv ‘Mitchell Diploid’ (MD) plants were utilized as a ‘wild-type’ (ethylene sensitive). The ethylene insensitive CaMV 35S: etr1-1 line 44568, generated in the MD genetic background. In petunia seven genes responsible for production of major pollinator attracted volatiles released in 11 stages of flower development (Fig 8).Mitchell diploid (ethylene sensitive) line there was no expression of seven genes in 11th stages of flower development in case of 44568 (ethylene insensitive) line seven genes are expressed in 11th stages so that endogenousethylene affects floral volatile benzenoid/phenylpropanoid (FVBP) transcript accumulation34 (Fig9).
 |
Figure 8: Floral stages used for the developmental studies in MD and 44568 |
 |
Figure 9: Developmental transcript accumulation analysis of seven FVBP genes in MD (A) and 44568 (B). |
Bradshaw, (2003) revealed that the The monkeyflowers M. lewisiiand M. cardinalis are 99% reproductively isolated by the difference in their pollinator guilds. Near-isogenic lines were derived from two backcross (BC) populations: Mimulus lewisii x (Mimulus lewisii x Mimulus cardinalis) and Mimulus cardinalis x (Mimulus lewisiix Mimulus cardinalis). Artificial F2 hybrids between Mimulus lewisiiand Mimulus cardinalis, Showed that flower colour has marked effects on pollinator visitation, and that yellow pigment concentration is controlled in part by the major quantitative trait locus, alleles at a single locus, YELLOW UPPER5–7 (YUP). YUP controls the presence or absence of yellow carotenoid pigments in the petals of pink-flowered Mimulus lewisii, which is pollinated by bumblebees its red-flowered sister species M. cardinalis, which is pollinated by hummingbirds.
By substituting one allele for another using repeated backcrosses, NILs more closely mimic the effect of a single mutation likely to be part of an adaptive pollinator shift, that is from bumblebee pollinated to hummingbird-pollinated, or vice versa. The dominant Mimulus lewisiiYUP allele prevents carotenoid deposition, so the petals show only their pink anthocyanin pigments. The recessive Mimulus cardinalis yup allele allows carotenoid deposition in the petals and produces red flowers. A wild-type pink Mimulus lewisiiflower is 700 times more likely to be visited by a bumblebee than by a hummingbird, whereas the yellow-orange-flowered ‘mutant’ is only 1.8 times as likely to be visited by a bumblebee. In the Mimulus cardinalis background, a wild-type red flower is 1,200 times more likely to be visited by a hummingbird than by a bumblebee, but the pink-flowered ‘mutant’ is visited only 15 times as frequently by hummingbirds(Fig 10 and Table 2).
 |
Figure 10: Near-isogenic lines of Mimulus lewisiiand Mimulus cardinalis with alternate alleles at the YUP locus. |
Table 2: Pollinator visitation rates to NILs of Mimulus lewisiiand Mimulus cardinalis
| Bumblebees
(10-3 visits per flower per hour) |
Hummingbirds
(10-3 visits per flower per hour) |
|
| Mimulus lewisii
Wild-type (Pink) Mutant (Yellow) |
15.4 2.63 |
0.0212 1.44 |
| Mimulus cardinalis
Wild-type (red) Mutant (dark pink) |
0.148 10.9 |
189 168 |
Source: Bradshaw, (2003)
Conclusion
The process of establishing communication between the flower and pollinator is the deciding factor with reference to survival of certain plants and pollinators vice versa under changing climatic conditions. Due to threatening of climate change and over a period of evolutionary changes in plants and animals, certain pollinators are not able to sense their pollinating plants. Under this circumstance, identification and exploitation of genes responsible for key element production in the process of pollinator attraction should be done at the earliest. Now, based on technological advancement in the area of plant molecular biology, some of the study was initiated worldwide production of plant with appropriate floral traits to facilitate the process of pollination either by biotic or abiotic factors. But till date molecular understanding pertaining to adaptations of plants to wind pollination is very much lacking.
Conflict of Interest
We confirm that we do not have any conflict of interest.
Funding Source
There are No funding source
References
- Alvarez-Buylla E. R., Azpeitia E., Barrio R., Benítez M., Padilla-Longoria P. ABC genes to regulatory networks, epigenetic landscapes and flower morphogenesis: making biological sense of theoretical approaches.Seminars Cell Dev Biol. 2010;21:108–117.
CrossRef - Specht C. D., Howarth D. G. Adaptation in flower form: a comparative evodevo approach. New Phytol. 2015;206:74–90.
CrossRef - Dudareva N., Negre F., Nagegowda D., Orlova I. Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 2006;25:417–440.
CrossRef - Endler J. A. Signals, signal conditions, and the direction of evolution. Am. Nat.1992;139:125–S153.
CrossRef - Gumbert A. Color choices by bumble bees (Bombusterrestris): innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 2000;48:36–43.
CrossRef - Raguso R. A. Flowers as sensory billboards: progress towards an integrated understanding of floral advertisement. Curr. Opin. Plant Biol. 2004;7:434–440.
CrossRef - 7. Raguso R. A., Willis A. Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths, Manduca sexta. Anim. Behav. 2005;69(2):407–18.
CrossRef - Peach D. A. H., Gries R., Huimin Z., Young N., Gries G. Multimodal floral cues guide mosquitoes to tansy inflorescences. Sci. Rep. 2019;9:3908.
CrossRef - Grotewold E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006;57:761– 80.
CrossRef - HatlestadG. J., Akhavan N. A., Sunnadeniya R. M., Elam L., Cargile S. The beet Y locus encodes an anthocyanin MYB-like protein that activates the betalain red pigment pathway. Nat. Genet. 2015;47(1):92– 96.
CrossRef - Bradshaw H. D., Wilbert S. M., Otto K. G., Schemske D. W. Genetic mapping of floral traits associated with reproductive isolation in monkeyflowers (Mimulus). Nature.1995;376:762–65.
CrossRef - Davies K. M., Albert N. W., Schwinn K. E. From landing lights to mimicry: the molecular regulation of flower colouration and mechanisms for pigmentation patterning. Funct. Plant Biol. 2012;39(8):619–38.
CrossRef - Ushimaru A., Watanabe T., Nakata K. Colored floral organs influence pollinator behavior and pollen transfer in Commelina communis (Commelinaceae). Am. J. Bot. 2007;94(2):249-258.
CrossRef - Owen C. R., Bradshaw H. D. Induced mutations affecting pollinator choice in Mimulus lewisii(Phrymaceae). Arthropod Plant Interact. 2011;5(3):235–44.
CrossRef - Yuan Y. W., Rebocho A. B., Sagawa J. M., Stanley L. E., Bradshaw H. D. Competition between anthocyanin and flavonol biosynthesis produces spatial pattern variation of floral pigments between Mimulus species. PNAS. 2016;113(9):2448–53.
CrossRef - Fattorini R., Glover B. J. Molecular mechanisms of pollination biology. Annual review of plant biology. 2020;71:487-515.
CrossRef - 17. Dudareva N., Pichersky Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008;19(2):181–89.
CrossRef - Ashman A. T., Bradburn M., Cole D. H., Blaney B. H., Raguso R. A. The scent of a male: the role of floral volatiles in pollination of a gender dimorphic plant. Ecology. 2005;86(8):2099–105.
CrossRef - Cunningham J. P., Moore C. J., Zalucki M. P., West S. A. Learning, odour preference and flower foraging in moths. J. Exp. Biol. 2004;207(1):87–94.
CrossRef - Raguso R. A. Wake up and smell the roses: the ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst.2008;39:549–69.
CrossRef - Colquhoun T. A., Verdonk J. C., Schimmel B. C., Tieman D. M., Underwood B. A., Clark D. G. Petunia floral volatile benzenoid/phenylpropanoid genes are regulated in a similar manner. Phytochemistry, 2010;71(2-3):158-167.
CrossRef - Vranova E., Coman D., Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013;64:665–700.
CrossRef - Dudareva N., Klempien A., Muhlemann J. K., Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 12013;98(1):16–32.
CrossRef - Ando T., Nomura M., Tsukahara J., Watanabe H., Kokubun H., Tsukamoto T., Kitching I. J. Reproductive isolation in a native population of Petunia sensuJussieu (Solanaceae). Annals of Botany. 2001;88:403–413.
CrossRef - Waelti M. O., Muhlemann J. K., Widmer A., Schiestl F. P. Floral odour and reproductive isolation in two species of Silene. Journal of Evolutionary Biology2008;21:111–121.
CrossRef - Dobson H. E. M., Danielson E. M., Van Wesep I. D. Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae). Plant Species Biology1999;14:153–166.
CrossRef - Jurgens A., Wee S. L., Shuttleworth A., Johnson S. D. Chemical mimicry of insect oviposition sites: a global analysis of convergence in angiosperms. Ecology Letters2013;16:1157–1167.
CrossRef - Byers K. J., Bradshaw H. D., Riffell J. A. Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus). Journal of Experimental Biology. 2014;217(4):614-623.
CrossRef - Byers K. J., Vela J. P., Peng F., Riffell J. A., Bradshaw H. D. Floral volatile alleles can contribute to pollinator‐mediated reproductive isolation in monkeyflowers (Mimulus). The Plant Journal. 2014a;80(6):1031-1042.
CrossRef - Ashman T. L., Bradburn M., Cole D. H., Blaney B. H., Raguso R. A. The scent of a male: the role of floral volatiles in pollination of a gender dimorphic plant. Ecology. 2005;86(8):2099-2105.
CrossRef - 31. Johnson K. B., Stockwell O. Management of fire blight: a case study in microbial ecology. Annual Review of Phytopathology. 1998;36:227– 248.
CrossRef - BakkaliF., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils–a review. Food and chemical toxicology. 2008;46(2):446-475.
CrossRef - Huang M., Sanchez-Moreiras A. M., Abel C., Sohrabi R., Lee S., Gershenzon J., Tholl D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-beta-caryophyllene, is a defense against a bacterial pathogen. New Phytologist. 2012;193:997-1008.
CrossRef - Bradshaw H. D., Schemske D. W. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176-178.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





