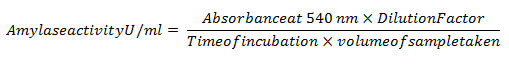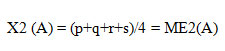How to Cite | Publication History | PlumX Article Matrix
Optimization of a-Amylase Production from Bacillus Amyloliquefaciens using Taguchi method
Pankhuri Sharma1 and Shilpa Chapadgaonkar2*
and Shilpa Chapadgaonkar2*
1ManavRachna International Institute of Research and Studies,Faridabad, Haryana
2MIT World Peace University, Pune, Maharashtra, India
Corresponding Author E-mail: shilpa.chapadgaonkar@mitwpu.edu.in,
DOI : http://dx.doi.org/10.13005/bbra/2920
ABSTRACT: α-amylase, an enzyme of industrial importance is used extensively in food, pharmaceutical, textile and detergent industries. Since, a substantial quantity of α-amylase isderived from microbial sources, manipulation of bacterial strain, fermentation conditions and media composition has a major effect on yield of enzyme. Bacillus amyloliqifaciens, obtained from MTCC culture collection was used to study the enhancement of α-amylase production using media concentration manipulation. Taguchi’s orthogonal array was designed for maximization of α-amylase output. The different media components selected as parameters to be optimized were calcium chloride, starch, tryptone, ammonium sulphate and glucose. The concentration of starch and tryptone demonstrated to have maximum effect on amylase production. The optimization strategy was successful in obtaining substantial increase in amylase production of about 2 folds as compared to the unoptimized medium.
KEYWORDS: α-amylase; Bacillus amyloliquefaciens; Enzyme Production; Fermentation; Optimization; Taguchi method
Download this article as:| Copy the following to cite this article: Sharma P, Chapadgaonka S. Optimization of a-Amylase Production from Bacillus Amyloliquefaciens using Taguchi method. Biosci Biotech Res Asia 2021;18(2). |
| Copy the following to cite this URL: Sharma P, Chapadgaonka S. Optimization of a-Amylase Production from Bacillus Amyloliquefaciens using Taguchi method. Biosci Biotech Res Asia 2021;18(2). Available from: https://bit.ly/3ymbS3B |
Introduction
α-amylase (E.C. 3.2.1.1.), derived from several sources, including plants, animals and microorganisms, accounts for approximately 30% of the world’s enzyme production.1-3 Microbial α-amylase is of immense significance due to its distinct application in food, textile, detergents, paper and pharmaceutical industries as it is more stable as compared to plant or animal based enzymes. Today, microbial amylases are preferred over chemical hydrolysis of starch in most of the starch processing industries4. α-amylases are endo- amylases that cleave 1, 4-α- D-glycosidic linkages of adjacent glucose units in linear chain of amylose thus yield products retaining alpha- anomeric configuration. Many studies have examined the process of submerged fermentation for α-amylase synthesis.5-8
Many species of Bacillus have been reported as excellent α-amylase producers. Because of their high-yielding capacity, wild-type strains of Bacillus licheniformis, Bacillus subtilis and Bacillus amyloliquefaciens are commercially utilized for the production of α-amylase 9-10. Concentration of media components influences the yield of the enzyme, whereas practicality and economic value of the process is influenced by the physical and chemical aspects of the process. Hence, for economic appeal, improved processes and media optimization can increase the yield of α-amylase multi fold.11 To design cost-effective and optimal fermentation method, it is imperative to select appropriate media components, concentrations and conditions as these variable entities interact amongst themselves to regulate the response12.
To efficiently optimize process parameters, several fractional factorial experimental designs like Placket-Burman design, central composite design1, and Box–Behnken design have been devised13. The traditional one factor- at-a-time methods or full factorial experiments, demand time, enormous labour and capital, as they deal with several variables at altered levels, leading to countless combinations thereby making it an unfeasible task. 1,13-15 Therefore, suitable experimental design, appropriate selection of components, optimal conditions of physical and chemical parameters is necessary for desired results.
Taguchi’s methodology with orthogonal array design and response surface methodology have been introduced for optimization of parameters, which considerably reduce number of experimental trials and gives reliable results14-15. The Orthogonal Array design (OA) provides a set (minimum) of well-balanced experiments and Taguchi’s Signal-to-Noise (S/N) ratios, which are log functions of desired output, serve as objective functions for optimization, help in data analysis and prediction of optimum results.
The Taguchi’s methodology is a robust design method for finding the significant effects and their contribution during optimization16. Bhakhtiari et al. (2006) used orthogonal array designs for medium optimization for urease production by Aspergillus niger17. Wei et al. (2007) used Taguchi designto optimize trace element composition for enhanced surfactin production by B. Subtilis18. Rao et al. (2008) reported Taguchi method as simple statistical tool involving a system of tabulated designs (arrays) that allows a maximum number of main effects to be estimated in an unbiased (orthogonal) fashion with a minimum experimental runs19. The modelling used signal-to-ratio to the control the variables. Taguchi principles and concepts have made extensive contributions to industry by focusing on robustness, noise and quality. The objective of the present work was to optimize amylase production by Bacillus amyloliquefaciens strain.
Material and Methods
Microbial cultures
Bacillus amyloliquefaciens MTCC 1207 was obtained from Institute of Microbial Technology (IMTECH), Microbial Type Culture Collection (MTCC), Chandigarh, India and used for this study.
Media and culture conditions
Bacillus amyloliquefaciens strain was maintained in slants of nutrient agar (Himedia, India) at 4°C. Routine sub-culturing was performed in 100 ml of nutrient media was prepared in a 250 ml Erlenmeyer flask. The media was autoclaved for 20 minutes at 15 lb/in2 pressure and 121°C temperature for sterilization. When media attained room temperature, it was inoculated with a loop full of bacterial culture in aseptic conditions. The flask was kept at 37°C at 200 rpm for 12-16 h.
Amylase activity
For determination of the amylase activity, a modified 3, 5- dinitrosalicylic acid (DNSA) method was adopted20. Maltose was used as the carbon source, and reducing sugars released,were estimated. The culture broth was centrifuged at 10,000 RPM for 10 minutes at 10°C. 1mL of this broth was incubated with 1 mL (1% w/v) starch solution at 37°C for 10 min. 1 mL DNS reagent (12.0 g of sodium potassiumtart rate tetra-hydrate, 8 mL of 2M NaOH and 96 mM 3, 5- dinitrosalicylic acid solution) was added. The contents were heated in a boiling water bath for 5 min. Two separate blanks were prepared, one without broth and another without the amylase enzyme, replaced by equal quantities of buffer (20 mM Sodium phosphate buffer with 6.7 mM Sodium chloride, pH 6.9 at 20°C). The absorbance was measured at 540 nm once the mixture reached room temperature. The reducing sugar released from starch was estimated as maltose equivalent from a standard graph.
Statistical Optimization
While optimising a particular production process, many factors are to be considered. Every factor which is utilized for creating the desired product has a role to play, and the process of optimization aims to identify the relevance of each factor. Traditional optimization methods are complicated, expensive and laborious specifically when number of process parameters increases. This leads to an increase in the number of experiments required for optimization. To overcome this challenge, Taguchi developed a well-defined method, commonly known as the Taguchi Experimental Design Method. This high quality designing system is effective and requires much less number of tests.
A specifically designed orthogonal array is used to study all the factors with limited number of experiments. The mean response for each run in the inner array is analysed. Signal- to- noise (S=N) ratio is used to study the variation. Quadratic loss of function derives the S/N ratios and the standards are with respect to nominal, lower and higher S/N ratios. To maximise response, as in the case of the present study where we aim to maximize amylase production, we rely on higher is better, which implies:
Where,
S/N = Signal to noise ratio
Y = Signal factor (amylase activity)
n = Number of repetitions in the experiment
Analysis of ranking of determines the significance of each factor. Main effects of each factor on the desired output are calculated and first order statistics are obtained. For optimization of output value (amylase production), the following equation is used:
Where:
X1 (A), is level 1 of factor A
X2 (A), is level 2 of factor A
a, b, c, d are output values (amylase production in different runs)
ME1 (A), is mean effect for X1 (A)
ME2 (A), is mean effect for X2 (A)
The difference in main effects Δ, is obtained as follows:
The optimal levels of each factor are obtained with graphical plots of means of means. The design array (Table 1) is arranged in the ascending order of the output value or amylase activity, for determining the relative importance of each factor.
To deduce the statistical significance of the results, Analysis of Variance or ANOVA was performed. Variation due to factors, their combination and/or noise is determined. Variance of the desired output is also calculated leading to the detection of factors having significant.
Results
Amylase Production
The production of amylase in the orthogonal experiment for Taguchi analysis was calculated by DNS method, as mentioned. Table 1 depicts the result of the production of amylase alongside the criteria used for specific runs of the orthogonal experimental design for the Taguchi analysis. The five factors studied were Calcium chloride, starch, tryptone, glucose and ammonium sulphate and different concentration were used to perform the optimization experiments as per Taguchi design.
Taguchi Optimization
To optimize the selected factors, eight runs were performed with a higher and lower concentration for each of them creating an L8 orthogonal array. Other factors related to the growth like pH, temperature, media components were kept constant for each run. Table 1 depicts the orthogonal design based experimental data with respect to amylase activity obtained during each run in duplicate (for statistical analysis). This data has been used to perform Taguchi optimization of amylase enzyme activity.
Table 1: L8 (25) orthogonal array of Taguchi experimental design and corresponding amylase production by Bacillus amyloliquefaciens.
| Run ID | Calcium Chloride | Starch | Tryptone | Glucose | Ammonium Sulphate | SET1 | SET2 |
| 1 | 0.0024 | 0.1 | 0.1 | 0.005 | 0.1 | 170 | 170 |
| 2 | 0.0024 | 0.1 | 0.1 | 0.2 | 0.4 | 213 | 170 |
| 3 | 0.0024 | 0.5 | 4 | 0.005 | 0.1 | 1000 | 1915 |
| 4 | 0.0024 | 0.5 | 4 | 0.2 | 0.4 | 1106 | 1404 |
| 5 | 0.01 | 0.1 | 4 | 0.005 | 0.4 | 936 | 489 |
| 6 | 0.01 | 0.1 | 4 | 0.2 | 0.1 | 957 | 957 |
| 7 | 0.01 | 0.5 | 0.1 | 0.005 | 0.4 | 936 | 1213 |
| 8 | 0.01 | 0.5 | 0.1 | 0.2 | 0.1 | 1191 | 617 |
The data obtained clearly depicts that highest amylase activity of 1915 U ml-1 was obtained during the third run with 0.0024% (w/v) calcium chloride, 0.5% (w/v) Starch, 4% Tryptone, 0.0005 % (w/v) Glucose and 0.1% (w/v) ammonium sulphate. However, the lowest amylase activity of 170 U ml-1 was observed in the first run, where a much lower concentration of starch (0.1% (w/v)) and tryptone (0.1%) were used as compared to the third run, while the concentration of calcium chloride, glucose and ammonium sulphate were the same in both runs.
 |
Figure 1: the lowest amylase activity of 170 U ml-1 |
The main effect plot of SN ratio (Figure 2) for each of the 5 factors, was plotted based on the amylase enzyme production during the 8-run orthogonal experiment. The plot indicated a significant positive effect of the concentration of calcium chloride, starch and tryptone on amylase production, whereas, no such experimental evidence could be seen for glucose and ammonium sulphate. Further, the main effect plot for means (Figure 3) depicts the role of Starch and tryptone only, as there is no considerable effect of calcium chloride. This indicated that the availability of starch and tryptone in an optimal amount could affect the production and activity of amylase enzyme.
 |
Figure 2: S/N ratio for main effect of calcium chloride, starch, tryptone, glucose and ammonium sulphate on the production of amylase enzyme. |
 |
Figure 3: Main effects plot of Means. |
It is noteworthy to study the response table for the SN ratios (Table 2), where Starch has the highest delta of 9.12 and is ranked 1 followed by tryptone, having delta of 7.71 and rank 2. Calcium chloride is ranked third, while glucose and ammonium sulphate are ranked fourth and fifth respectively, suggesting negligible role in amylase production. Similar result was observed in the response table for means (Table 3).
Table 2: Response Table for Signal to Noise Ratios
| Level | Calcium Chloride |
Starch | Tryptone | Glucose | Ammonium Sulphate |
| 1 | 53.46 | 51.37 | 52.07 | 55.69 | 56.00 |
| 2 | 58.39 | 60.49 | 59.78 | 56.17 | 55.86 |
| Delta | 4.93 | 9.12 | 7.71 | 0.48 | 0.14 |
| Rank | 3 | 1 | 2 | 4 | 5 |
Table 3: Response Table for Means
| Level | Calcium Chloride |
Starch | Tryptone | Glucose | Ammonium Sulphate |
| 1 | 768.6 | 508.0 | 585.1 | 853.7 | 872.3 |
| 2 | 912.2 | 1172.9 | 1095.7 | 827.1 | 808.5 |
| Delta | 143.6 | 664.9 | 510.6 | 26.6 | 63.8 |
| Rank | 3 | 1 | 2 | 5 | 4 |
Based on the above results and observations, starch and tryptone concentrations have substantial effect on the production of amylase enzyme. Calcium chloride may also have some effect, but the evidence to conclude its role based on this experimental data is weak. Glucose and ammonium sulphate concentrations have shown negligible effect on amylase production.
To understand the variation contributed by each factor of the orthogonal array experiment in Taguchi analysis, ANOVA is performed. This helps to determine the general trend for impact of each factor on the process to be optimised. Hence, ANOVA was performed to learn the statistical significance of each factor involved in the production of amylase. Linear model analysis and model coefficients can be seen in table 4. The results of ANOVA have been recorded in table 5. With confidence limit set to 95% and p<0.05, multiple correlation R2 co-efficient is 0.9633 i.e. the model can explain 99.69% response variation. A higher f- value and a lower p-value (<0.05) for starch and tryptone indicate the statistical significance of these factors.
Table 4: Linear model analysis with Estimated Model Coefficients for Means
| Term | Coef | SE Coef | T | P |
| Constant | 840.43 | 58.90 | 14.268 | 0.005 |
| Calcium 0.0024 | -71.81 | 58.90 | -1.219 | 0.347 |
| Starch 0.1 | -332.45 | 58.90 | -5.644 | 0.030 |
| Tryptone 0.1 | -255.32 | 58.90 | -4.335 | 0.049 |
| Glucose 0.005 | 13.30 | 58.90 | 0.226 | 0.842 |
| Ammonium 0.1 | 31.91 | 58.90 | 0.542 | 0.642 |
Table 5: Analysis of Variance for Means
| Source | DF | Seq SS | Adj SS | Adj MS | F | P |
| Calcium Chloride | 1 | 41252 | 41252 | 41252 | 1.49 | 0.347 |
| Starch | 1 | 884167 | 884167 | 884167 | 31.86 | 0.030 |
| Tryptone | 1 | 521503 | 521503 | 521503 | 18.79 | 0.049 |
| Glucose | 1 | 1415 | 1415 | 1415 | 0.05 | 0.842 |
| Ammonium Sulphate | 1 | 8148 | 8148 | 8148 | 0.29 | 0.642 |
| Residual Error | 2 | 55512 | 55512 | 27756 | ||
| Total | 7 | 1511996 |
Discussion
The presence of carbon source in the form of either monosaccharide or polysaccharides was reported to influence the amylase enzyme production. In our present study, influence of starch is found to be more prominent than glucose when investigated at different concentrations. In fact, glucose showed least effect on amylase activity.. As discussed in the results, amylase activity gets elevated with increase in concentration of starch from 0.1% (w/v) to 0.5% (w/v). No such effect of glucose was observed. This was in agreement with earlier reports which prove the enhancement of amylase activity in presence of starch is better than other sources that are investigated21. Khusro et al. (2017) and Vyas and Sharma (2015) examined the effect of starch and glucose on amylase activity and found that the influential potential of starch is much higher than glucose due to availability of carbon throughout the reaction22-23. In another study, effect of starch at different concentration was examined and it was reported that concentration of starch is directly proportional to the enzyme yield and activity24.
The nitrogen sources are secondary energy sources for the organism and play an important role in growth and production of valuable enzymes. Both nature and concentration of compound used influence the enzyme production. In this study, tryptone and ammonium sulphate were taken as nitrogen source and their effects are compared at different concentrations. Tryptone was found to be one of major parameter responsible for high amylase activity whereas negligible influential potential of ammonium sulphate was observed. This collaborated well with earlier reports where peptone or tryptone were taken as nitrogen source and increase in yield and amylase activity was observed whereas no such influence of ammonium sulphate was observed. Konsoula et al. (2006) reported that tryptone increases the amylase activity by providing essential amino acids in the media during reaction and work as enzyme inducer25.
The effects of metal ions have been well studied on several amylases from bacteria and literature studies reveals that the availability of ions increases enzyme production or activity. However, in change in concentration of calcium did not have any significant effect on amylase production in the present study. The results are similarto Kikani et al., 2012 who observed that alpha-amylase from B. Amyloliquefaciens BH1 was independent of Ca2+ ions25.
Conclusion
This study was aimed to optimize the production of amylase enzyme via the Taguchi Signal to Noise ratio method, using Bacillus amyloliquefaciens. We assessed five process parameters, which are known to affect the production of amylase, namely, calcium chloride, starch, tryptone, glucose and ammonium sulphate. In the orthogonal design experiment, starch and tryptone demonstrated to have maximum effect on amylase production and activity. At highest level of starch, 0.5% (w/v) and tryptone, 4% the amylase production was the highest i.e., 1915 Uml-1. Whereas, the lowest enzyme production of 170 Uml-1 was observed when the concentration of starch (0.1% w/v) and tryptone (0.1%) was lowest in the media. This was further validated with the Taguchi statistical experimental design, where the larger the better Signal to Noise ratios were highest for starch and tryptone. At level 2, which denotes higher concentration of starch and tryptone, the S/N ratio was 60.48 and 59.78 for starch and tryptone, respectively. However, at level 1, where in the concentration of starch and tryptone is the lowest, the S/N ratio was 51.37 for starch and 52.07 for tryptone. The mean response for S/N ratios depicted a similar pattern. Analogous result was observed when ANOVA was performed. The highest F-values of 31.86 and 18.79 were observed for starch and tryptone respectively. The optimization strategy was successful in increasing the amylase production more than 2 folds as compared to the un-optimized medium which had shown to produce about 750-800 U/ml of amylase.
Acknowledgement
We acknowledge the analytical inputs of our students Ms. Priyanka, Ms.Navneet Sandha and Dr. Girisha Malhotra for the study.
Funding source
The research funding and facilities were provided by Molecular Biosciences Research Cluster, Department of Biotechnology, Faculty of Engineering and Technology, Manav Rachna International Institute of Research and Studies, Faridabad, Haryana, India.
References
- Tanyildizi, M. S., Özer, D., & Elibol, M. (2005). Optimization of α-amylase production by Bacillus sp. using response surface methodology. Process biochemistry, 40(7), 2291-96.
CrossRef - Sivaramakrishnan, S., Gangadharan, D., Nampoothiri, K. M., Soccol, C. R., & Pandey, A. (2006). a-Amylases from microbial sources–an overview on recent developments. Food Technol Biotechnol, 44(2), 173-184.
- Gupta, R., Gigras, P., Mohapatra, H., Goswami, V. K., & Chauhan, B. (2003). Microbial α-amylases: a biotechnological perspective. Process biochemistry, 38(11), 1599-1616.
CrossRef - Pandey, A., Nigam, P., Soccol, C. R., Soccol, V. T., Singh, D., & Mohan, R. (2000). Advances in microbial amylases. Biotechnology and applied biochemistry, 31(2), 135-152.
CrossRef - Carlsen, M., Sphor, A. B., Nielsen, J., Villadesn, J. (1996) : “Morphology and physiology of an α-amylase producing strain of Aspergillus oryzae during batch cultivation”., Bioengg. 4, 266-76.
CrossRef - Francis, F., Sabu, A., Nampoothiri, K. M., Ramachandran, S., Ghosh, S., Szakacs, G., & Pandey, A. (2003). Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae. Biochemical Engineering Journal, 15(2), 107-115.
CrossRef - Pandey, A., Selvakumar, P., Soccol, C. R., Nigam, P. (1999) : “Solid-state fermentation for the production of industrial enzymes”, BioresourceTech., 77, 149-162.
- Kunamneni, A., Permaul, K., & Singh, S. (2005). Amylase production in solid state fermentation by the thermophilic fungus Thermomyces lanuginosus. Journal of bioscience and bioengineering, 100(2), 168-171..
CrossRef - Simair, A. A., Qureshi, A. S., Khushk, I., Ali, C. H., Lashari, S., Bhutto, M. A., … & Lu, C. (2017). Production and partial characterization of α-amylase enzyme from Bacillus sp. BCC 01-50 and potential applications. BioMed research international, 2017.
CrossRef - Du, R., Zhao, F., Qiao, X., Song, Q., Ye, G., Wang, Y.,,, & Zhou, Z. (2018). Optimization and partial characterization of ca-independent α-amylase from Bacillus amyloliquefaciens BH1. Preparative Biochemistry and Biotechnology, 48(8), 768-774.
CrossRef - Tanyildizi, M. S.; Elibol, M.; € Ozer, D. Optimization of Growth Medium for the Production of a-Amylase from Bacillus amyloliquefaciens Using Response Surface Methodology. J. Chem. Technol. Biotechnol. 2006, 81, 618–622.
CrossRef - Dhiman, S., & Chapadgaonkar, S. S. (2013). Optimization of lipase production medium for a bacterial isolate. Inter J Chem Technol Res, 5, 2837-43.
- Gangadharan, D., Sivaramakrishnan, S., Nampoothiri, K. M., Sukumaran, R. K., & Pandey, A. (2008). Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bioresource technology, 99(11), 4597-4602.
CrossRef - Mukherjee, A. K., Borah, M., & Rai, S. K. (2009). To study the influence of different components of fermentable substrates on induction of extracellular α-amylase synthesis by Bacillus subtilis DM-03 in solid-state fermentation and exploration of feasibility for inclusion of α-amylase in laundry detergent formulations. Biochemical Engineering Journal, 43(2), 149-156.
CrossRef - Gangadharan, D., Sivaramakrishnan, S., Nampoothiri, K. M., & Pandey, A. (2006). Solid culturing of Bacillus amyloliquefaciens for α-amylase production. Food Technology and Biotechnology, 44(2), 269-274.Kurosaki H, R. Radford,3J. Filliben, K. G. W. Inn (2008) : “An orthogonal design of experiment/exploratory data analysis for plutonium contamination.” Journal of Radioanalytical and Nuclear Chemistry, 276(2), 323–328.
CrossRef - Malhotra, G., Chapadgaonkar, S.S. (2020) : Taguchi optimization and scale up of xylanase from Bacillus licheniformis isolated from hot water geyser. J Genet Eng Biotechnol 18, https://doi.org/10.1186/s43141-020-00084-0
CrossRef - Bhakhtiari, M.R., Faezi M.G., Fallahpour, M., Noohi, A., Moazami, N., Amidi Z. (2006) : “Medium optimization by orthogonal array designs for urease production by Aspergillus niger PTCC5011”, Biochem., 41, 547–551.
CrossRef - Wei, Y. H., Lai, C. C., & Chang, J. S. (2007). Using Taguchi experimental design methods to optimize trace element composition for enhanced surfactin production by Bacillus subtilis ATCC 21332. Process Biochemistry, 42(1), 40-45.
CrossRef - Rao, R. S., Kumar, C. G., Prakasham, R. S., & Hobbs, P. J. (2008). The Taguchi methodology as a statistical tool for biotechnological applications: a critical appraisal. Biotechnology Journal: Healthcare Nutrition Technology, 3(4), 510-523.
CrossRef - Bailey, M. J. (1988). A note on the use of dinitrosalicylic acid for determining the products of enzymatic reactions. Applied Microbiology and Biotechnology, 29(5), 494-496.
CrossRef - Rajagopalan, G., & Krishnan, C. (2008). α-Amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresource technology, 99(8), 3044-3050.
CrossRef - Khusro, A., Barathikannan, K., Aarti, C., & Agastian, P. (2017). Optimization of thermo-alkali stable amylase production and biomass yield from Bacillus sp. under submerged cultivation. Fermentation, 3(1), 7.
CrossRef - Vyas, G. & Sharma, N. (2015). Production and optimization of α-amylase from a novel thermoalkalophilic Bacillus sonorensis GV2 isolated from mushroom compost. In Indian Natl. Sci. Acad(Vol. 81, pp. 1207-1221).
CrossRef - Shatta, A. M., El-Hamahmy, A. F., Ahmed, F. A., Ibrahim, M. M. K., & Arafa, M. A. I. (1990). The influence of certain nutritional and environmental factors on the production of amylase enzyme by Streptomyces aureofaciens 77. Islamic Acad. Sci, 3(2), 134-138.
- Konsoula, Z., & Liakopoulou-Kyriakides, M. (2006). Thermostable α-amylase production by Bacillus subtilis entrapped in calcium alginate gel capsules. Enzyme and Microbial Technology, 39(4), 690-696.
CrossRef - Kikani, B. A., & Singh, S. P. (2012). The stability and thermodynamic parameters of a very thermostable and calcium-independent α-amylase from a newly isolated bacterium, Anoxybacillus beppuensis TSSC-1. Process Biochemistry, 47(12), 1791-1798.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.