Manuscript accepted on : 27-07-2021
Published online on: 17-08-2021
Plagiarism Check: Yes
Reviewed by: Dr. Robert Susło
Second Review by: Dr. Naman Wadhwa
Final Approval by: Dr. Ghulam Md Ashraf
SARS-COV-2 and COVID-19: A Global Pandemic
Viswanath Vittaladevaram1* , Kranthi Kuruti2 and Sudheer Venkatesh Urity3
1Department of Chemistry, NUIG/CURAM, Galway, Ireland.
2Department of Biotechnology, Mahindra Group, Tirupathi, India.
3R and D,Sanofi , Hyderabad, India.
Corresponding Author E-mail: viswa.vittal@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2925
ABSTRACT:
The evolution of COVID-19 across the globe is rapid due to increased mobility which spreads and evolves continuously among human population. Based on phylogenetic analysis the virus is termed as SARS-COV-2 (Severe acute respiratory syndrome coronavirus 2) which spreads rapidly among human beings. The article focuses on aspects of virus structure, organization of genome, epidemiological characteristics, mode of transmission and global impact of Coronavirus. In addition to this, diagnosis and pharmacological approach, treatment, prevention procedures and vaccines that are currently in use were highlighted.
KEYWORDS: Diagnosis; Epidemiology; Genome organization; Prevention; Transmission
Download this article as:| Copy the following to cite this article: Vittaladevaram V, Kuruti K, Urity S. V. SARS-COV-2 and COVID-19: A Global Pandemic. Biosci Biotech Res Asia 2021;18(2). |
| Copy the following to cite this URL: Vittaladevaram V, Kuruti K, Urity S. V. SARS-COV-2 and COVID-19: A Global Pandemic. Biosci Biotech Res Asia 2021;18(2). Available from: https://bit.ly/3yQ1wKi |
Introduction
During the last two decades, several diseases such as H1N1 (Influenza A),Zika virus, Severe acute respiratory syndrome(SARS),Middle East respiratory syndrome(MERS) and Ebola caused outbreak throughout the world. Most recently the outbreak of COVID-19 disease caused by SARS-COV-2 virus led to massive impact around the world in terms of strain on public health resources, economic crisis and well-being of humans[1]. The initial outbreak of Corona virus was first noticed at Wuhan city , China ,where unknown source for the cause of respiratory infection was identified as Coronavirus i.e. SARS-C0V-2 which is quite closely related to outbreak of MERS in 2012 and SARS during the period of 2002 to 2004. The Coronavirus pandemic spread rapidly to 216 countries across the globe and World Health Organization(WHO) reported that 17.1 million people were infected so far, out of which 6,68,073 deaths were reported until July 20202.Different types of pandemics circulated viruses globally since 1918 and WHO made an assessment on 11th March,2020 that Coronavirus as pandemic(Fig.1).
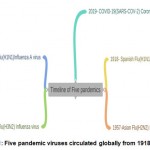 |
Figure 1: Five pandemic viruses circulated globally from 1918-2019[1] |
The process of evolution of Coronavirus to get transmitted into humans is still unclear but possible chances include either process of natural selection in human beings after zoonotic transmission or natural selection in animals before zoonotic transmission to human beings. The time period of incubation prior to onset of symptoms ranges from 1-14 days that include headache, myalgia, dry cough, loss of taste and smell, fever, rigor, shortness of breath, diarrhoea and sore throat 3. Risk factors of COVID-19 include ethnicity ,obesity, diabetes, cancer, and weak immune system.
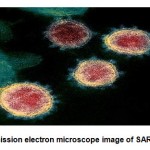 |
Figure 2: Transmission electron microscope image of SARS-COV-2 virus[3] |
The particles of SARS-COV-2 are spherical in shape and are comprised of protein spikes which protrudes from their surface. The protein spikes hold on to human cells and undergo changesin their structure which allow the membrane of virus to get fused with cell membrane. The spikes present on outer layer of virus provided the name Coronavirus which means Crown like(Fig.2). The protein spikes of viral genes enters the host cell and get copied resulting in more number of viruses and several research studies indicated that spikes of SARS-COV-2 binds to Angiotensin converting enzyme 2(ACE2) receptor present on cell surface of humans[4]. SARS-COV-2 consists of enveloped and single stranded RNA which shares 79% of genetic sequence with SARS-COV and 96% homology with coronavirus strain RATG13 in bats. The spike protein is found to bind more likely i.e. 10 to 20 times to ACE2 receptor present in human cells. The structure of SARS-COV-2 is comprised of four structural proteins include envelope, spike, membrane, single strand RNA and nucleocapsid(Fig.3). High affinity of spike protein associated with SARS-COV-2 will bind to ACE2 receptors and a functional cleavage site i.e. polybasic is present at the junction of spike protein subunits,S1 and S2 in humans 5. This will further enhance the cleavage of spike protein and increase the virus infection.
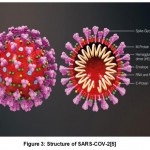 |
Figure 3: Structure of SARS-COV-2[5]. |
SARS-COV-2 genome organization
SARS-C0V-2 is comprised of RNA genome which is larger in size and comprised of 29,903 nucleotides. A total of 1/3rd of genome is comprised of genes that codes for structural proteins and accessory proteins were encoded by 8 genes which inhibit host defense mechanism 6. The remaining part of genome is comprised of replicase gene encodes for two polyproteins which are larger in size and further divided into a total of 16 non-structural proteins that contribute for proofreading on entire viral genome and replication process(Fig.4).
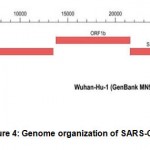 |
Figure 4: Genome organization of SARS-COV-2 [7] |
The virions of SARS-COV-2 bind to human cells with spike protein i.e. glycosylated in dense form and binds with greater affinity to ACE2 receptor present on human cells.S1 domain of spike protein manage the binding of receptor and S2 domain is associated with fusion of cell membrane. The receptor binding domain moderates the identification of ACE2 receptor which is generally present in different cell types all over the body which include liver, lungs, testes, blood vessels, heart and intestine7. All these cells have TMPRSS2 gene which encodes serine protease enzyme is used to divide the spike protein which facilitates the entry of cell by SARSCOV-2. This allows entry of virions and RNA gets released into the cells that are infected. Further replication and translation process is carried out to provide viral proteins. A total of 100-1000 virions are produced per day from infected cells 8
Global impact of COVID-19 pandemic
The spread of Corona virus infected millions of people across the world which halted the economic progress of countries due to restrictions that are imposed to prevent the spread of virus. As death toll is increasing which indicates world’s largest economic turmoil faced ever since past decades. Due to Corona virus pandemic it resulted in deep recession causing segmentation of trade and supply chain at global level. This resulted in economic crisis which is an indicator for immediate action to soften the pandemic’s economic and health consequences[9]. It is critical to protect susceptible populations due to pandemic and stage should be set for long lasting recovery. Many countries affected due to COVID-19 in terms of access to efficient healthcare systems, loss of tourism and trade, decreased remittances and capital flows. Different sectors have been impacted due to Coronavirus pandemic of which the sectors-Automotive, oil and gas, Tourism and hospitality, Airlines/Aviation, semi-conductors, consumer electronics and consumer products face severe consequences(Fig.5).
 |
Figure 5: Impact of COVID-19 on different industry sectors[9]. |
Epidemiology of SARS-COV-2
Research studies were conducted to address the emergence of Corona virus pandemic to identify potential therapeutic targets and vaccine development for management of Corona virus. The primary studies on COVID-19 included a cohort of 99 subjects in which the percentage of infection rate is observed mainly in older male population present with comorbidities in which symptoms appeared were respiratory pathologies that is either serious or fatal 10. The Chinese centre for disease control and prevention conducted epidemiological studies which showed increase in mortality rate from 0.2% to 14.8% in patients aged between 10-39 years to 80 years and also percentage of death rate is high in men than women11,12.
There are several COVID19 cases reported with pre-existing diseases such as diabetes, cardiovascular problems, hypertension and chronic respiratory failure. The mortality rate during the time of infection in an healthy individual is 0.9% and early 80% of the infections are present with no or mild symptoms,13% were present with severe symptoms and pathological related symptoms were exhibited among 4.7% of the patients which include multiple organ failure, septic shock or respiratory failure 13. The infection spread to 216 countries across the globe and number of cases is still increasing on daily basis(Fig.6)
 |
Figure 6: Coronavirus cases reported across the globe[13]. |
Transmission of COVID-19
World Health Organization reported the transmission route could be identical to earlier epidemics like MERS and SARS i.e. transmission occurs between human to human through direct contact, aerosols and droplets 14,15. More specifically the spread of infection is up to 1-2m through droplets that occur during sneezing, coughing and speaking by patients with symptoms16. There are possible chances of people getting infected without any symptoms and prior to onset of symptoms Other mode of transmission include the process of inhalation of aerosols which are referred as microparticles that are smaller in size with diameter around 5µm(Fig.7).
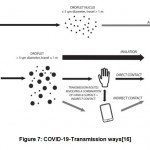 |
Figure 7: COVID-19-Transmission ways[16]. |
Diagnosis and pharmacological approach
Currently, PCR test is widely used diagnostic approach to detect the genetic material present within the virus[17]. In addition to this screening procedures were conducted worldwide using Nucleic acid amplification tests like rRT-PCR(real time reverse transcriptase polymerase chain reaction) is qualitative test used to identify the nucleic acid in lower and upper respiratory specimens which include sputum, nasopharyngeal swab and nasal aspirate etc[18,19]. Treatment procedures for Coronavirus infection is yet to be defined and development of vaccine is in progress. Different types of drugs are used for experimental purpose for treatment of patients suffering from COVID-19 and examples include Remdesivir, favipiravir and Chloroquine etc[20,21]. Further studies are required to evaluate the efficacy of drugs that are found to be suitable for treatment of COVID-19 infected patients. Currently, large scale efforts were carried out to develop vaccines against COVID-19 which include design, testing procedures and implementation procedures[22].There are several therapeutic agents currently in use to manage the infection caused due to COVID-19[Table 1].
Table 1: Therapeutic strategies for management of COVID-19.
| Therapeutic agents | Origin indication | Mechanism of action |
| Remdesivir | MERS-COV, Ebola virus | A nucleotide analogue inhibits RNA dependent RNA polymerase and prevent the process of viral replication |
| Azithromycin | Bacterial infections | Prevention of secondary bacterial infection and exhibits anti-viral activity |
| Interferon beta | Multiple sclerosis | Immunomodulatory and Anti-viral effects |
| Interferon alpha | Cancer and Viral infections | Innate anti-viral response of body is induced |
| Tocilizumab | Rheumatoid arthritis | Binding of monoclonal antibody specifically to membrane bound and soluble Interleukin 6 receptors to block the Interleukin 6 mediated responses. |
| Corticosteroids | Autoimmune and
inflammatory conditions |
Pain and immune suppression |
| Convalescent sera | Infection prevention | Antibodies present in plasma
from convalescent patients will supress viraemia |
| Zanamivir | Influenza | Inhibitor of Neuraminidase enzyme which prevents virus from entering host cell and
decrease infection and shedding of virus |
| Oseltamivir | Influenza | Inhibitor of Neuraminidase enzyme which prevents virus from entering host cell and decrease infection and
shedding of virus |
| Hydroxychloroquine | Autoimmune disease and Malaria | Changes in endosomal pH present in host cells and exhibit anti-inflammatory and immunomodulatory effects |
Prevention and protective measures
The objective of prevention and protection measures is to decrease the probability of exposure to SARS-COV-2 virus[23,24]. Some of the measures to avoid spread of infection or virus include organizational, environmental, personal and other safety protective measures(Fig.8).
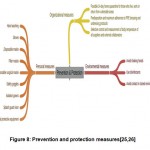 |
Figure 8: Prevention and protection measures[25,26] |
Vaccination
Researchers all over the world currently developing effective vaccines for COVID-19 which are designed to identify and block the virus by body’s immune system
There are different categories of vaccines currently in development phase, which include protein based,viral vector, RNA, DNA based and inactivated virus vaccines.This will protect body’s immune system and also from severe illness caused due to virus[26].On December 31, 2020, the Pfizer/BioNtech Comirnaty vaccine was added to the WHO’s Emergency Use List (EUL). On February 16, EUL was provided to the SII/Covishield and Astra Zeneca/AZD1222 vaccines (designed by AstraZeneca/Oxford and produced by the State Institute of India and SK Bio, respectively). Johnson & Johnson’s Janssen/Ad26.COV 2.S was approved for EUL on March 12, 2021. On 30 April 2021, the Moderna COVID-19 vaccine (mRNA 1273) was approved for EUL, and on 7 May 2021, the Sinopharm COVID-19 vaccine was approved for EUL. Beijing Bio-Institute of Biological Products Co Ltd, a subsidiary of China National Biotec Group, manufactures the Sinopharm vaccine (CNBG). Sinovac-CoronaVac was a joint venture between Sinovac and CoronaVac.
 |
Figure 9: Vaccination. |
Conclusion
The evolution of Coronavirus since December 2019 resulted in spread of virus and lead to cause 2,486,679deaths globally as on 25thFebruary,2021 reported by WHO(World Health Organization).There are further possibilities for spread of virus to humans as treatment procedures and thorough development of vaccines is still in progress. The geographical patterns of Coronavirus needs to be further investigated for development of vaccine for wide variety of populations. Preventive measures such as social distancing, quarantine should be followed to prevent the spread of virus. As the virus mutates and evolves constantly it is critical to consider studies on pathogenicity of virus, treatment modalities and vaccine development by taking into consideration the genetic features of virus. Vaccines for medical workers and high-risk persons should be given first priority. Vaccine ownership, large-scale manufacturing financing, and supply chain issues must all be addressed. The monopolisation of global supply by high-income nations must be stopped.
Acknowledgement
The authors have carried out the research on their own without any external support.
Conflicts of interest
There are no conflicts of interest.
Funding Source
None
References
- Abdullah, S. F., & Sharquie, I. K. (2020). SARS-CoV-2: A Piece of Bad News [SARSCoV-2: Bir Parça Kötü Haber]. Medeniyet Medical Journal, 35(2), 151–160. https://doi.org/10.5222/MMJ.2020.82584
CrossRef - WHO coronavirus disease (COVID-19) dashboard. Geneva: World Health Organization, 2020. Available online: https://covid19.who.int/ (last cited: [31-07- 2020]).
- Acter, T., Uddin, N., Das, J., Akhter, A., Choudhury, T. R., & Kim, S. (2020). Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. The Science of the total environment, 730, 138996. https://doi.org/10.1016/j.scitotenv.2020.138996
CrossRef - Amawi, H., Abu Deiab, G. I., A Aljabali, A. A., Dua, K., & Tambuwala, M. M. (2020). COVID-19 pandemic: an overview of epidemiology, pathogenesis, diagnostics and potential vaccines and therapeutics. Therapeutic delivery, 11(4), 245–268. https://doi.org/10.4155/tde-2020-0035
CrossRef - Chakraborty, S., & Basu, A. (2020). The COVID-19 pandemic: catching up with the cataclysm. F1000Research, 9, F1000 Faculty Rev-638. https://doi.org/10.12688/f1000research.24963.1
CrossRef - Feng, W., Zong, W., Wang, F., & Ju, S. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review. Molecular cancer, 19(1), 100. https://doi.org/10.1186/s12943-020-01218-1
CrossRef - Ge, H., Wang, X., Yuan, X., Xiao, G., Wang, C., Deng, T., Yuan, Q., & Xiao, X. (2020). The epidemiology and clinical information about COVID-19. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology, 39(6), 1011–1019. https://doi.org/10.1007/s10096- 020-03874-z
CrossRef - Helmy, Y. A., Fawzy, M., Elaswad, A., Sobieh, A., Kenney, S. P., & Shehata, A. A. (2020). The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. Journal of clinical medicine, 9(4), 1225. https://doi.org/10.3390/jcm9041225
CrossRef - Islam, M. S., Rahman, K. M., Sun, Y., Qureshi, M. O., Abdi, I., Chughtai, A. A., & Seale, H. (2020). Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: A global analysis. Infection control andhospital epidemiology, 1–11. Advance online publication. https://doi.org/10.1017/ice.2020.237
CrossRef - Khan, S., Liu, J., & Xue, M. (2020). Transmission of SARS-CoV-2, Required Developments in Research and Associated Public Health Concerns. Frontiers in medicine, 7, 310. https://doi.org/10.3389/fmed.2020.00310
CrossRef - Li, J., Shao, J., Wang, C., & Li, W. (2020). The epidemiology and therapeutic options for the COVID-19. Precision Clinical Medicine, pbaa017. https://doi.org/10.1093/pcmedi/pbaa017
CrossRef - Lisi, L., Lacal, P. M., Barbaccia, M. L., & Graziani, G. (2020). Approaching Coronavirus Disease 2019: mechanisms of action of repurposed drugs with potential activity against SARS-CoV-2. Biochemical pharmacology, 114169. Advance online publication. https://doi.org/10.1016/j.bcp.2020.114169
CrossRef - Lotfi, M., Hamblin, M. R., & Rezaei, N. (2020). COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clinica chimica acta; international journal of clinical chemistry, 508, 254–266. https://doi.org/10.1016/j.cca.2020.05.044
CrossRef - McCreary, E. K., & Pogue, J. M. (2020). Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options. Open forum infectious diseases, 7(4), ofaa105. https://doi.org/10.1093/ofid/ofaa105
CrossRef - Neerukonda, S. N., & Katneni, U. (2020). A Review on SARS-CoV-2 Virology, Pathophysiology, Animal Models, and Anti-Viral Interventions. Pathogens (Basel, Switzerland), 9(6), 426. https://doi.org/10.3390/pathogens9060426
CrossRef - Park S. H. (2020). Personal Protective Equipment for Healthcare Workers during the COVID-19 Pandemic. Infection & chemotherapy, 52(2), 165–182. https://doi.org/10.3947/ic.2020.52.2.165
CrossRef - Patel, K. P., Vunnam, S. R., Patel, P. A., Krill, K. L., Korbitz, P. M., Gallagher, J. P., Suh, J. E., & Vunnam, R. R. (2020). Transmission of SARS-CoV-2: an update of current literature. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology, 1–7. Advance online publication. https://doi.org/10.1007/s10096-020-03961-1
CrossRef - Peng M. (2020). Outbreak of COVID-19: An emerging global pandemic threat. Biomedicine & Pharmacotherapy, 129, 110499. https://doi.org/10.1016/j.biopha.2020.110499
CrossRef - Poduri, R., Joshi, G., & Jagadeesh, G. (2020). Drugs targeting various stages of the SARS-CoV-2 life cycle: Exploring promising drugs for the treatment of Covid-19. Cellular signalling, 109721. Advance online publication. https://doi.org/10.1016/j.cellsig.2020.109721
CrossRef - Sheervalilou, R., Shirvaliloo, M., Dadashzadeh, N., Shirvalilou, S., Shahraki, O., Pilehvar-Soltanahmadi, Y., Ghaznavi, H., Khoei, S., & Nazarlou, Z. (2020). COVID19 under spotlight: A close look at the origin, transmission, diagnosis, and treatment of the 2019-nCoV disease. Journal of cellular physiology, 10.1002/jcp.29735. Advance online publication. https://doi.org/10.1002/jcp.29735
CrossRef - Shetty, R., Ghosh, A., Honavar, S. G., Khamar, P., & Sethu, S. (2020). Therapeutic opportunities to manage COVID-19/SARS-CoV-2 infection: Present and future. Indian journal of ophthalmology, 68(5), 693–702. https://doi.org/10.4103/ijo.IJO_639_20
CrossRef - Singh, V. K., Mishra, A., Singh, S., Kumar, P., Singh, M., Jagannath, C., & Khan, A. (2020). Emerging Prevention and Treatment Strategies to Control COVID19. Pathogens (Basel, Switzerland), 9(6), 501. https://doi.org/10.3390/pathogens9060501
CrossRef - Tang, D., Comish, P., & Kang, R. (2020). The hallmarks of COVID-19 disease. PLoS pathogens, 16(5), e1008536. https://doi.org/10.1371/journal.ppat.1008536
CrossRef - Uddin, M., Mustafa, F., Rizvi, T. A., Loney, T., Suwaidi, H. A., Al-Marzouqi, A., Eldin, A. K., Alsabeeha, N., Adrian, T. E., Stefanini, C., Nowotny, N., Alsheikh-Ali, A., & Senok, A. C. (2020). SARS-CoV-2/COVID-19: Viral Genomics, Epidemiology, Vaccines, and Therapeutic Interventions. Viruses, 12(5), 526. https://doi.org/10.3390/v12050526
CrossRef - Zhang, L. P., Wang, M., Wang, Y., Zhu, J., & Zhang, N. (2020). Focus on a 2019-novel coronavirus (SARS-CoV-2). Future microbiology, 10.2217/fmb-2020-0063. Advance online publication. https://doi.org/10.2217/fmb-2020-0063
CrossRef - Zhai, P., Ding, Y., Wu, X., Long, J., Zhong, Y., & Li, Y. (2020). The epidemiology, diagnosis and treatment of COVID-19. International journal of antimicrobial agents, 55(5), 105955. https://doi.org/10.1016/j.ijantimicag.2020.105955
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





