How to Cite | Publication History | PlumX Article Matrix
Anamika Tripathi1 , N. S. Abbas2*
, N. S. Abbas2* , Amrita Nigam1
, Amrita Nigam1 , Sujata Bhardwaj2
, Sujata Bhardwaj2 , Babeeta C Kaula3
, Babeeta C Kaula3 and Alka B Vadakan4
and Alka B Vadakan4
1School of Sciences, Indira Gandhi National Open University, Maidan Garhi, New Delhi, India
2Department of Botany, Bhaskaracharya College of Applied Sciences, University of Delhi, New Delhi-110 075, India
3Department of Botany, Zakir Husain Delhi College, University of Delhi, J.L.N. Marg, New Delhi-110002, India.
4Department of Botany, Maitreyi College, University of Delhi, Bapudham complex, Chanakyapuri, New Delhi- 110021, India.
Corresponding Author E-mail: dr.nsabbas@bcas.du.ac.in
DOI : http://dx.doi.org/10.13005/bbra/2938
ABSTRACT: A novel cost-effective in vitro regeneration protocol has been evolved for the therapeutically important Ocimum citriodorum Vis. In the present study, table sugar (3%) and isabgol (Psyllium husk) (3.5%) were used as an alternate source of carbon and gelling agent respectively in Murashige and Skoog’s (MS) medium. The explant used in the current study was nodal segment. A significant observation revealed that all the cultures resulted in shoot induction and maximum number of shoots/ culture (6.04) and their average length (2.15 cm) was obtained on modified MS-medium supplemented with table sugar, isabgol and BAP. However, best root induction (95.83%) was obtained on ½ MS-medium augmented with table sugar (3%) , isabgol (3.5%) and NAA. An increase in average number of roots per shoot (6.91%) as well as average root length (2.73 cm) was also observed in the same modified medium. The in vitro regenerated plantlets were successfully transferred to the field and no notable variation was observed in their morphology. The overall cost of culture medium for in vitro propagation of O. citriodorum Vis. was reduced significantly by 92.69% when agar and sucrose were replaced by isabgol and table sugar, respectively.
KEYWORDS: Cost-effective medium; Isabgol; Ocimum plants; Regeneration; Table sugar
Download this article as:| Copy the following to cite this article: Tripathi A, Abbas N. S, Nigam A, Bhardwaj S, Kaula B. C, Vadakan A. B. An Alternate Cost-effective Medium for In Vitro Regeneration of Therapeutically Important Ocimum citriodorum Vis. Biosci Biotech Res Asia 2021;18(3). |
| Copy the following to cite this URL: Tripathi A, Abbas N. S, Nigam A, Bhardwaj S, Kaula B. C, Vadakan A. B. An Alternate Cost-effective Medium for In Vitro Regeneration of Therapeutically Important Ocimum citriodorum Vis. Biosci Biotech Res Asia 2021;18(3). Available from: https://bit.ly/2YYFxUr |
Introduction
Ocimum citriodorum Vis. (Lemon basil) is an important medicinal plant, abundant in volatile aromatic essential oils and is known to possess culinary properties1,2. It is an interspecific hybrid obtained between Ocimum basilicum and O. americanum. This herb is mainly cultivated in some parts of Asia and Africa not only for its essence but also as an important source of antioxidants. It is one of the most economically important spice used for flavor in many cuisines3. Due to its medicinal value, it has been used as anti-inflammatory agent4 and to treat people suffering from premature ejaculation, delayed menstruation cycle and stomach spasms5. The oil of Ocimum is valued for its therapeutic properties and possess antimicrobial, nematocidal, fungistatic and insecticidal activities6, 7. The essential oils of Ocimum species have been reported to elicit a greater escape response against Ades aegypti and Anopheles minimus, dengue and malaria vectors respectively8.
Ideally, the plants used in the preparation of medicines should have the same genetic make-up. However, Ocimum cultivars show a lot of variation due to out- crossing9, 10. In vitro propagation offers an alternative method to produce genetically uniform plants11. The technique of tissue culture has been practiced since long for fast multiplication and conservation of medicinal plants. Despite this, the in vitro regeneration technique has a major disadvantage, like high cost of production12. Therefore, the most challenging aspect of in vitro propagation is to reduce the cost of production12, 13; without compromising the quality14, 15 and quantity of the reproduce. As a result, several efforts have been made to reduce the cost of commercial micropropagation of plants12, 16, 17.
Recurring cost of micropropagation includes mainly the cost of chemicals used to prepare culture medium. These include sucrose, agar, major and minor elements inorganic elements and phytohormones. Out of these components of culture medium, sucrose (source of carbon) and agar (gelling agent) are the most expensive18. Therefore, it is imperative to have alternative cost-effective options for in vitro propagation of plants. The present study reports, successful cost-cutting of MS medium by substituting agar and sucrose with isabgol and table sugar, respectively for micropropagation of medicinally important O. citriodorum Vis.
Materials and Methods
Seeds of O.citriodorum procured from Central Institute of Medicinal & Aromatic Plants, Lucknow, India, were sown in Botanical Garden, University of Delhi, Delhi, India. Nodal segments (Fig. 1) obtained from one-year-old plants served as explant. They were surface sterilized with teepol (5%, v/v) (Rickett & Colman India Ltd.,) for 10 minutes, followed by a quick rinse with ethanol (70%, v/v). The explants were rinsed thrice with autoclaved water to wash off the sterilant.
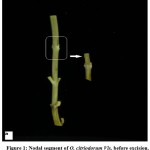 |
Figure 1: Nodal segment of O. citriodorum Vis. before excision. Arrow showing excised explant (x 1.2). |
Establishment of Cultures
Protocol for tissue culture raised plantlets of O.citriodorum Vis. through nodal segment has been standardized in the present study. MS medium augmented with optimal concentrations of chemicals was used for the in vitro regeneration of the plantlets19. To induce shoots, nodal segments were implanted on modified culture medium with table sugar (3%), isabgol (3.5%) and BAP (0.5 mg/l). Nodal segments inoculated on MS-medium having sucrose (3%) + agar (0.7%) + BAP (0.5 mg/l) served as control.
Similarly, for rooting of shoots modified culture medium with table sugar (3%) and isabgol (3.5%) was augmented with NAA (1 mg/l). MS-medium supplemented with sucrose (3%) + agar (0.7%) + NAA (1 mg/l) was used as control for root induction. To further reduce the cost of medium, lower concentrations of MS salts were tried for root induction. The tissue culture raised micro shoots were transferred to different strengths (1/2, 1/3 and 1/4) of modified MS medium with table sugar (3%), isabgol (3.5%) and NAA (1 mg/l).
Modified medium
T1– MS medium+ Table sugar +Isabgol + BAP (Shoot induction)
T2-Medium +Table sugar + Isabgol + NAA (Root induction)
Culture Conditions
The cultures were maintained in tissue culture laboratory with controlled conditions of temperature (250C± 20C) and relative humidity (50 ± 5%). The cultures were maintained under a regulated photoperiod for 16-hour light (fluorescent tubes, 40 W) followed by 8-hour dark period.
Acclimatization and Field Transfer of Plantlets
Tissue culture raised plantlets were gently cleaned with water to remove the gelling agent. Before transplantation, the plantlets were treated with fungicide (1% Bavistin) to avoid any contamination.
Further, to acclimatize the plantlets and to maintain humidity, the pots were covered with transparent polythene bags. Once in a week, MS basal solution (salt concentration one-tenth) without the addition of sucrose and inositol was added to the potted plantlets. A week after transplantation, holes were punched in polythene bags for aeration. For further acclimatization, the plants were kept in green house for a duration of 02-weeks and then successfully transferred to the field.
Cost Analysis of the Medium with Isabgol -gelling agent and Table Sugar-carbon source
Existing cost of sucrose, agar, table sugar and isabgol was estimated and compared in Indian and US currency. The cost of sucrose and agar per kg in Indian currency is Rs. 11,404.20 and Rs. 33,620.00 and in USD 570.10 and 310.00 respectively for the current year. All the chemicals used for the preparation of the culture media were from Sigma-Aldrich. The cost was calculated by taking the weight of each component utilized for preparing 10 liter medium.
Data record and statistical analysis
Morphogenetic responses of nodal explant were recorded based on average values 06 weeks post culture for (i) shoot induction frequency, (ii) shoots/culture and (iii) shoot length/culture. The data for rhizogenesis, was recorded on the given criteria: (i) number of shoots producing roots (ii) roots/shoot and (iii) root-length. Efficacy of the explants for in vitro regeneration has been evaluated by calculating shoots/ culture, shoot-length, roots/shoot, and root-length. The scored data was represented as the mean-values with standard-error based on twelve replicates. For reproducibility of the results, each experiment was repeated. ANOVA (Analysis of Variance) through SPSS (Statistical Package for Social Sciences) version 16.0 was used for statistical analyses. Duncan’s Multiple Range Test (DMRT) at p=0.05 was used to find out the difference in the mean values to test the significance.
Results
Shoot induction and elongation
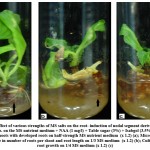
Table -1 presents the effect of various gelling agents and carbon sources on shoot regeneration responses of the explants. The nodal segments were implanted on MS medium containing table sugar (3%) and isabgol (3.5%) supplemented with BAP (0.5 mg/l). For control, MS medium with sucrose (3%) + agar (0.7%) + BAP (0.5 mg/l) was used. It was observed that sugar quality and gelling agent type did not influence the morphogenetic response of the explant (Fig. 2 a- d). No significant difference was observed in percent cultures in the morphogenic response on control MS medium (Fig. 2c) and modified MS medium containing table sugar and isabgol (Fig. 2d). On modified MS medium (table sugar and isabgol), shoot buds emerged a week after inoculation which eventually differentiated into shoots (Fig. 3 a- d). Maximum number of shoots/ culture (6.04) and shoot length (2.15 cm) was observed on modified MS medium containing table sugar 3% and isabgol 3.5% + BAP 0.5 mg/l (T1 medium). However, shoot inducing frequency (5.86) and average shoot length (2.02 cm) declined when table sugar (3%) was used in combination with agar (0.7%) (Fig. 2a). Similarly, reduced shoot inducing frequency (5.27) and shoot length (1.68 cm) was observed when isabgol (3.5%) was used in combination with sucrose (3%) (Fig. 2b).
Table 1: Effect of different gelling agents and carbon sources with optimal concentration of BAP (0.5 mg/l) on the shoot regeneration frequency of the nodal segment of O. citriodorum Vis.
| PGR | Cultures producing shoots
(Percent) |
Shoots /culture | Shoot-length (cm) |
| Table sugar (3%) + Agar (0.7%) | 70.83a | 5.86 ± 0.36a | 2.02 ± 0.04a |
| Sucrose (3%) + Isabgol (3.5%) | 75.00a | 5.27 ± 0.27a | 1.68 ± 0.04a |
| Sucrose (3%) + Agar (0.7%) | 100.00b | 5.83 ± 0.25a | 2.08 ± 0.23b |
| Table sugar (3%) + Isabgol (3.5%) | 100.00b | 6.04 ± 0.21a | 2.15 ± 0.06b |
 |
Figure 2: (a-d) Morphogenic responses of nodal explant of O. citriodorum Vis. on MS nutrient medium with optimal level of BAP ( 0.5 mg/l ) incorporated with different gelling agents and carbon source after 3 weeks of culture |
 |
Figure 3: (a-d) Developmental stages of in vitro shooting of the nodal segment of O. citriodorum Vis.on the MS nutrient medium + BAP ( 0.5 mg/l ) + table sugar (3%) + Isabgol (3.5%); |
Rooting
Table -2 presents effect of various strengths of MS medium on root induction. Rooting was induced on all the media compositions. However, maximum root regeneration (95.83%) was obtained on modified MS-nutrient medium with table sugar and isabgol supplemented with NAA 1 mg/l (T2 medium).
Flow Chart
Table 2: Rooting of shoots from the nodal segment of O. citriodorum Vis. on the table sugar containing isabgol gelled MS medium of different strengths containing NAA (1 mg/l)
| PGR | Cultures producing root
(Percent) |
Roots/shoot | Root-length
(cm) |
| 1/2 MS | 95.83b | 6.91± 0.08b | 2.73 ± 0.07b |
| 1/3 MS | 91.66b | 6.72 ± 0.00ab | 2.51 ± 0.01ab |
| 1/4 MS | 58.33a | 6.41 ± 0.08a | 2.42 ± 0.07a |
To further reduce the cost of medium, lower concentrations of MS salts were tested. The in vitro regenerated micro shoots were also sub-cultured on various strengths (1/2, 1/3 and 1/4) of MS medium augmented with table sugar (3%), isabgol (3.5%) and NAA (1 mg/l). There was no notable difference observed in rooting of the micro shoots at full strength MS (95.83%) medium as well as 1/2 strength MS medium (95.83%, Fig. 4a). However, there was an increase in the length of roots (2.73 cm) on 1/2 MS medium. It was observed that there was an overall decline in morphogenetic response of rooting on 1/3 MS medium (Fig. 4b, Table 2). On further decreasing the concentration of MS salts, a drastic reduction in the root inducing frequency (58.33%) of the micro shoots was observed (1/4 MS medium) (Fig. 4c). Table 3, presents results of various gelling agents and carbon sources on the induction of roots from shoots. The maximum number of shoots inducing roots (6.52) as well as root length (2 cm) was higher in the modified MS medium (table sugar and isabgol) in comparison to control MS -medium containing sucrose and agar (6.49 roots per shoot and 1.98 cm).
Table 3: Effect of different gelling agents & carbon sources with optimal concentration of NAA( 1 mg/l) on the root induction of micro shoots obtained from nodal segment of O. citriodorum Vis.
| PGR | Cultures producing root
(Percent) |
Roots/shoot | Root-length
(cm) |
| Table sugar (3%) + Agar (0.7%) | 58.33 a | 4.91±0.08 a | 1.53±0.01 a |
| Surose (3%) + Isabgol (3.5%) | 70.83 a | 5.40±0.03 b | 1.49±0.03 a |
| Sucrose (3%) + Agar (0.7%) | 100 b | 6.49±0.08 c | 1.98±0.03 b |
| Table sugar (3%) + Isabgol (3.5%) | 95.83 b | 6.52±0.02 c | 2.00±0.03 b |
Acclimatization and Field Transfer of Plantlets
Tissue culture raised plantlets with fully-grown roots (Fig. 5a) were washed gently to avoid any contamination. Subsequently for hardening, the plantlets were transplanted into small containers filled with autoclaved soil and humus (Fig. 5b). To maintain humidity and allow light to pass through, the plantlets were protected with transparent polythene bags. Three weeks after hardening, the polythene bags were removed. The potted plantlets were kept in green house and thereafter, transferred to the field that showed high survival rate (85%). Thus, complete plantlets were obtained successfully from the nodal segments of O. citriodorum Vis. on cost-effective medium (Fig. 6). The plantlets produced through tissue culture were identical to in vivo plants.
 |
Figure 5: (a- b) Hardening of in vitro raised nodal explant derived plantlets of O. citriodorum Vis. regenerated on cost-effective medium. |
 |
Figure 6: Schematic representation of various stages of plantlet formation from the nodal explant of O. citriodorum Vis. |
Cost Analysis of MS-Medium with Isabgol and Table Sugar
The estimated cost per kg of sucrose vs table sugar and agar vs isabgol in USD and Indian currency has been summarized in Table 4. There is a stark difference in the price of both sucrose (USD 570.10) vs table sugar (USD 8.05) and agar (USD 310.00) vs isabgol (USD 30.00).
Table 4: Cost of media used for the in vitro regeneration of O. citriodorum Vis.
| Item | USD | INR | Brand |
| Sucrose- 1 kg
|
570.10 | 11,404.20 | Sigma-Aldrich |
| Table Sugar -1 kg | 8.05 | 60.00 | Domino pure granulated white sugar (USA)
Madhur Pure and Hygienic Sugar (India), |
| Agar Powder – 1 kg
|
310.00 | 33,620.00 | Sigma-Aldrich |
| Isabgol – 1 kg
|
30.00 | 1025.00 | Frontier Psyllium Husk Powder (USA)
Sat-Isabgol Psyllium Husk |
Table 5, presents comparative cost analysis of sucrose vs table sugar and agar vs isabgol. It was estimated that the final cost of 10 liter modified-medium (MM) containing table sugar and isabgol was less expensive in comparison to control medium (CM) augmented with sucrose and agar. Total cost of the control medium (CM) is USD 193.99 while that of modified medium (MM) (table sugar and isabgol) is USD 14.17. Thus, 92.69% cost reduction of the medium has been achieved successfully by replacing sucrose and agar in MS medium with table sugar and isabgol respectively for in vitro regeneration of O. citriodorum Vis.
Table 5: Comparative cost analysis of media used for the in vitro regeneration of O. citriodorum Vis.
| Medium Codea | Cost of mediumb 10 Liters (USD) | Percent decrease in cost in compared to CM |
| CM | 193.99 | 92.69 |
Note: aCM= Control Medium, MM= Modified Medium supplemented with table sugar and isabgol
bCost (/US$) were calculated as follows: sucrose = 570.10/kg; agar = 310.00/kg; isabgol = 30.00/kg;
table sugar = 8.05/kg; other components = 1.26/10 l of medium
Discussion
Sucrose is widely used carbon source in plant tissue culture medium. Murashige &Skoog used sucrose (3%) in tissue culture medium preparation as the staple source of carbon 19. It acts as a fuel during the plant tissue culture for sustaining photomixotrophic metabolism and thus ensures optimal development. It also helps in maintaining the osmotic potential of cells20. However, it is very costly and contributes about 34% of the total production cost14. There are many reports which suggest a remarkable reduction in the in vitro regeneration cost by using table sugar instead of sucrose14, 21-24.
Agar used as a gelling agent imparts viscosity to tissue culture medium, due to which explants remain on surface of the nutrient medium. It is most frequently utilized gelling agent in tissue culture but is highly priced. It accounts for about seventy percent of the total cost of production25. Its wide use is mainly attributed to its quality for transparency, stability, and resistance to metabolism. However, some reports Agar used as a gelling agent imparts viscosity to tissue culture medium, due to which explants remain on surface of the nutrient medium. It is most frequently utilized gelling agent in tissue culture but is highly priced. It accounts for about seventy percent of the total cost of production25. Its wide use is mainly attributed to its quality for transparency, stability, and resistance to metabolism. However, some reports from other sources like potato, rice, wheat, barley, tapioca26, cassava and sago25 have been used as gelling agents, with varying degrees of success. Isabgol or psyllium husk derived from Plantago ovate Forsk seeds has been used successfully for the solidification of nutrient medium. The value addition of isabgol as a thickening factor is due to large amount of mucilage in its husk. The mucilage swells into a jelly like mass and provides mechanical support to the explant. Isabgol mucilage is a polysaccharide composed of xylose, arabinose, rhamnose, galactose and galacturonic acid26 and therefore it is a safe gelling agent.
In the present study, isabgol and table sugar were used as an alternative gelling agent and carbon source respectively. They did not show any adverse effect on the tissue culture raised plantlets of O. citriodorum Vis. The results were in conformity with an earlier study27. They successfully used market sugar (2%) as a low-cost alternative to sucrose in the multiplication of Chrysanthemum morifolium. There was an increase in the number of shoots/explant (6.04) and length of shoots (2.15 cm) on nutrient medium with table sugar (3%) and isabgol (3.5%) than in comparison to MS medium with sucrose and agar (number of shoots/explants, 5.83 and length of shoots, 2.08 cm) (Table1). Such response may be due to high levels of sucrose in the medium which affect photosynthetic efficiency of tissue cultured plants20. Results of the present study were supported by earlier research studies which reported favourable effect of isabgol on shoot multiplication27, 28, 29. The findings of present study agreed with the reports of Demo et al for micropropagation of Solanum tuberosum L22. They also used that table sugar (0.3%, w/v) in culture media that resulted in more shoot proliferation as compared to sucrose. They concluded that the difference in the response of shoot multiplication to different sources of carbon may be due their easy translocation and assimilation by the explants leading to enhanced growth. Gelling agents also affect the numbers of shoots/explant. This may be due to the difference in hardness of the gelling agents used for the preparation of culture medium. The gelling agents differ in their chemical composition and structure that eventually results in the variable diffusion rates of different elements and hormones in the nutrient medium30.
In the current study, maximum root induction (95.83%) was obtained on medium containing table sugar as carbon source and isabgol as gelling agent supplemented with NAA (1 mg/l). These results were in consonance with the study conducted by Agrawal et.al21. They evolved a protocol for in vitro conservation at low- cost for banana utilizing isabgol and market sugar. Several researchers have reported that isabgol serves as thickening factor in culture medium used for the micropropagation of different plants like Chrysanthemum31, turmeric18, tobacco32 and banana21, 30. However, it was observed that maximum number of shoots inducing roots (6.62) as well as root length (2 cm) was higher in the table sugar containing isabgol gelled medium in comparison to conventional sucrose containing agar gelled medium (6.49 roots per shoot and 1.98 cm) (Table 2). Similar results were reported in earlier studies by Dhanlakshmi and Stephan 14 who obtained higher number of roots for the Monthan variety of banana on the low-cost medium in comparison to conventional medium.
The in vitro regenerated micro shoots were transferred to lower concentrations of MS salts for rooting to further reduce the cost of the medium. No significant difference in the morphogenic response in terms of rooting of micro shoots at full strength MS (95.83%) medium as well as 1/2 strength MS medium (95.83%, Fig. 4a) was observed. However, average root length (2.73 cm) increased in the 1/2 MS medium. Such response might be due to the high salt concentration of culture medium that affects the osmotic potential, limits water absorption and low ionic strengths suitable for rooting 33 (Table 3, Fig. 4 a-c).
Acclimatization and high rate of survival of in vitro regenerated plantlets is impacted by their ability to resist the transplanting stress as well as how much readily they can adapt to autotrophic mode of nutrition 34. In the present study, the rate of survival of tissue culture raised plantlets was 85%. The high rate of success might be due to the formation of well-developed root system of plantlets during acclimatization.
The positive role of isabgol and table sugar as substitute for agar and sucrose respectively has not been reported previously during in vitro culture of O. citriodorum Viz. In the present study, cost reduction was achieved by 92.69% in the in vitro regeneration of O. citriodorum Viz. These results were in accordance with the results of Zapata35, who successfully reduced 90% cost of the tissue culture raised banana by substituting sucrose with commercial sugar. Similarly, Dhanlakshmi and Stephan 14 achieved 61.4% cost reduction in the medium during tissue culture of banana by substituting sucrose and agar with table sugar and isabgol respectively. Likewise, an earlier study observed 34% cost reduction by using table sugar instead of sucrose in agar gelled medium22. Pant et al reported that there was a cost reduction by more than six-times when isabogol and market sugar was used in the culture medium for micropropagation of Chrysanthemum morifolium. Likewise, Agrawal et al. reported 59% reduction in in vitro regeneration cost of banana by utilizing market sugar as carbon source and isabgol as thickening agent 21.
Conclusion
The current research work emphasized that the major obstacle in the application of in vitro regeneration technology in terms of high cost of production has been overcome. A novel cost-effective protocol has been established for the mass multiplication of medicinally important plant Ocimum citriodorum Vis. Sucrose and agar being the major components of the medium incur heavy expenditure and thus pose an economic challenge in full exploitation of tissue culture of therapeutically important plants. In the present study, cost of culture medium has been significantly reduced by 92.69 % by utilizing table sugar and isabgol as alternate carbon source and gelling agent, respectively. Findings of the present study have helped in formulating standard culture medium and opened up possibilities for increasing the production of O.citriodorum Vis. many folds at comparatively low cost. There is still enough scope for further reduction in the cost of tissue-culture medium for micropropagation of medicinally important plants in healthcare system.
Acknowledgement
The authors are grateful to Professor V. P. Singh, Head (former), Botany Department, University of Delhi, for extending facility to conduct the current research work. Authors are thankful to the Principals, BCAS, ZHDC, MC, University of Delhi for their consistent encouragement towards socio-academic activity. The fellowship awarded to Anamika Tripathi by IGNOU, Maidan Garhi, New Delhi is gratefully acknowledged.
Conflict of interest
No conflict of interest among authors
Funding Source
The authors received no financial support for this research and publication of this article.
References
- Venugopal L.G., Rao A.P. In vitro propagation and callus induction of endangered medicinal herb Ocimum citriodorus. World Congress on Biotechnology, Biotechnology.
- Tripathi A., Abbas N.S., Nigam A. Micropropagation of an Endangered Medicinal Herb Ocimum citriodorum, Journal of Plant Development Sciences. 2014; 6(3): 365- 374.
- Janarthanam B., Ethiraj S. Plantlet regeneration from nodal explants of Ocimum citriodorum Bangladesh Journal of Scientific and Industrial Research. 2012; 47(4):433-436.
CrossRef - Majdi C., Pereira C., Dias M.I., Calhelha R.C., Alves M.J., Rhourri-Frih B., Charrouf Z., Barros L., Amaral J.S., Ferreira I.C. Phytochemical characterization and bioactive properties of cinnamon basil (Ocimum basilicum‘Cinnamon’) and lemon basil (Ocimum× citriodorum). Antioxidants. 2020; 9(5):1-17.
CrossRef - Epriliati I., Ginjom I.R. Bioavailability of phytochemicals, Phytochemicals – A Global Perspective of Their Role in Nutrition and Health, Dr Venketeshwer Rao (Ed.). (InTech.). 2012.
CrossRef - Mittal R., Kumar R., Chahal H.S. Antimicrobial activity of Ocimum sanctum leaves extracts and oil. J Drug Deli Therap. 2018; 8(6):201-204.
CrossRef - Tripathi A., Abbas N.S., Nigam A. In Vitro Culture- An Alternative Method for the Enhanced Production of Some Volatile Components of Ocimum citriodorum, J Sci Tech Res. 2016; 6(1): 21-31.
CrossRef - Sathantriphop S., Achee N., Sanguanpong U., Chareonviriyaphap T. The effects of plant essential oils on escape response and mortality rate of Aedes aegypti and Anopheles Journal of Vector Ecology. 2015; 40(2), 318–326, 2015 doi:10.1111/jvec.12170
CrossRef - Darrah H.H. Investigation of the cultivars of the Basils (Ocimum). Bot. 1974; 28:63-67.
CrossRef - Nation R.G., Janick J., Simon J.E. Estimation of outcrossing in basil. Hort Science. 1992; 27(11):1221-1222.
CrossRef - Vieira R.F., Grayer R.J., Paton A., Simon J.E. Genetic diversity of Ocimum gratissimum based on volatile oil constituents, flavonoids and RAPD markers. Biochem. Syst. Ecol. 2001. 29(3):287-304.
CrossRef - Datta S.K., Chakraborty D., Janakiram T. Low cost tissue culture: an overview. Plant Res. 2017; 33(2):181-199.
- Ahloowalia B.S., Prakash J. Incorporation of low-cost options, In: Low cost option for tissue culture technology in developing countries, Proceedings of a Technical Meeting Organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food And Agriculture and held in Vienna, 26-30 August 2002, IAEA- TECDOC- 1384: 2004; 886, 17-28.
- Dhanalakshmi S., Stephan R. Low cost media options for the production of banana (Musa paradisiaca) through plant tissue culture. J Acad Ind Res. 2014; 2(9):509-512.
- Sahay S, Braganza V. J. Low Cost Multiplication of Curculigo Orchioides in Shake Flask Culture. Biosci Biotech Res Asia 2017; 14(3).
CrossRef - Jain R., Babbar S.B. Guar gum and isubgol as cost-effective alternative gelling agents for in vitro multiplication of an orchid, Dendrobium chrysotoxum. Sci. 2005; 88:292–295.
CrossRef - Sharifi A, Moshtaghi N, Bagheri A (2010) Agar alternatives for micropropagation of African violet (Saintpaulia ionantha). J. Biotechnol. 9(54):9199-9203.
CrossRef - Tyagi R.K., Agrawal A., Mahalakshmi C., Hussain Z., Tyagi H. Low-cost media for in vitro conservation of turmeric (Curcuma longa) and genetic stability assessment using RAPD markers. In Vitro Cell. Dev. Biol. – Plant. 2007; 43(1):51-58.
CrossRef - Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant. 1962; 15(3):473-497.
CrossRef - Gago J., Martínez-Núñez L., Landín M., Flexas J., Gallego P.P. Modeling the effects of light and sucrose on in vitro propagated plants: a multiscale system analysis using artificial intelligence technology. PLoS One. 2014; 9(1):1-11, e85989.
CrossRef - Agrawal A., Sanayaima R., Tandon R., Tyagi R.K. Cost-effective in vitro conservation of banana using alternatives of gelling agent (isabgol) and carbon source (market sugar). Acta Physiol. Plant. 2010; 32(4):703-711.
CrossRef - Demo P., Kuria P., Nyende A.B., Kahangi E.M. Table sugar as an alternative low cost medium component for in vitro micro-propagation of potato (Solanum tuberosum). Afr. J. Biotechnol. 2008; 7(15): 2578-2584.
CrossRef - Kaur R., Gautam H., Sharma D.R. A low cost strategy for micropropagation of strawberry (Fragaria x Ananassa) cv. Chandler. Acta Hortic. 2005; 696:129-133.
CrossRef - Prakash S., Haque M.I., Brinks T. Culture media and containers, In: Low cost option for tissue culture technology in developing countries, Proceedings of a Technical Meeting Organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food And Agriculture and held in Vienna, 26-30 August 2002, IAEA- TECDOC- 1384: 886, 29-40.
- Lalitha N., Devi L.M., Banerjee R., Chattopadhyay S., Saha A.K., Bindroo B.B. Effect of plant derived gelling agents as agar substitute in micropropagation of mulberry (Morus indica cv. S-1635). Int J Advan Res. 2014; 2(2):683-690.
- Babbar S.B., Jain N. Isubgol’as an alternative gelling agent in plant tissue culture media. Plant Cell Rep. 1998;17(4):318-322.
CrossRef - Pant M., Lal A., Jain R. A simple cost-effective method for mass propagation of Chrysanthemum morifolium and antibacterial activity assessment of in vitro raised plantlets. Appl. Pharm. Sci. 2015; 5(07):103-111.
CrossRef - Fischer M.H., Yu N., Gray G.R., Ralph J., Anderson L., Marlett J.A. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Res. 2004; 339(11):2009-2017.
CrossRef - Yusuf A., Kumar R.T., Nikhilesh S., Rao P.S. Effects of antioxidants and gelling agents on regeneration, in vitro conservation and genetic stability of Bacopa monnieri (L.) Pennell. International Journal of Ayurvedic and Herbal Medicine. 2011; 1(03).
- Saraswathi M.S., Uma S., Kannan G., Selvasumathi M., Mustaffa M.M., Backiyarani S. Cost-effective tissue culture media for large-scale propagation of three commercial banana (Musa) varieties. J. Hortic. Sci. Biotechnol. 2016; 91(1):23-29.
CrossRef - Bhattacharya P., Dey S., Bhattacharyya B.C. Use of low-cost gelling agents and support matrices for industrial scale plant tissue culture. Plant Cell Tiss Organ Cult. 1994; 37(1):15-23.
CrossRef - Ozel C.A., Khawar K.M., Arslan O. A comparison of the gelling of isubgol, agar and gelrite on in vitro shoot regeneration and rooting of variety Samsun of tobacco (Nicotiana tabacum). Sci. Hortic. 2008; 117(2):174-181.
CrossRef - Etsè K.D., Aïdam A.V., de Souza C., Crèche J., Lanoue A. In vitro propagation of Zanthoxylum zanthoxyloides, an endangered African medicinal plant. Acta Bot. Gall. 2011; 158(1):47-55.
CrossRef - Ziv M. In Vitro hardening and acclimatisation of tissue cultured plants. In: Plant tissue culture and its agricultural applications. 1986. Withers LA, Alderson PG.
CrossRef - Zapata A. Cost reduction in tissue culture of banana (Special leaflet), Int. Atom Energy Labs. Agric and Biotech. Lab. Austria. 2001.

This work is licensed under a Creative Commons Attribution 4.0 International License.