How to Cite | Publication History | PlumX Article Matrix
Myong-Hun Han1,3* and Kwang Yong Kim 2
and Kwang Yong Kim 2
1Department of Genetics, Faculty of Life Science, KIM IL SUNG University, Pyongyang 999093, Democratic People's Republic of Korea
2Institute of Analytical Chemistry, Han Dock Su Pyongyang University of Light Industry, Pyongyang 999093, Democratic People's Republic of Korea
3State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai 200237, P. R. China,
Corresponding Author E-mail: hanmx111@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2939
ABSTRACT:
Rhododendron is one of the plants with the broadest spectrum species, the most extended history of traditional medicine use, and the wide range of pharmacological properties. In 2013, a report was published to summarize the studies reported from 1898 to 2012. Many phytochemical compounds and their various treatment effects of over 40 Rhododendron species were mentioned in the present review. This review aims to evaluate the newly discovered and observed phytochemical compounds in recent years and their activities in some Rhododendron species.
KEYWORDS: Compounds; Pharmaceutical Activity; Rhododendron species
Download this article as:| Copy the following to cite this article: Han M. H, Kim K. Y. Research Progress on the Identification and Pharmacological Activity of the Active Compo-nents of the Rhododendron Species.Biosci Biotech Res Asia 2021;18(3). |
| Copy the following to cite this URL: Han M. H, Kim K. Y. Research Progress on the Identification and Pharmacological Activity of the Active Compo-nents of the Rhododendron Species.Biosci Biotech Res Asia 2021;18(3). Available from: https://bit.ly/3E54XPl |
Introduction
Traditional natural chemicals rely primarily on selection based on ethnic pharmacological or epidemiological knowledge, followed by plant extract screening and activity-induced fractions. The genus of Rhododendron has been chosen as a case study because rhododendron extracts are still used in large numbers of ethnic medical applications 1. Rhododendron is found worldwide; at present, there are about 1024 species of this genus (excluding subspecies classification) worldwide, spanning multiple climatic zones from south to north, and mainly distributed in latitudes between 20 ° S and 65 ° N 2.
Rhododendrons are mainly distributed in Asia, North America, and Europe, especially in the eastern and western Himalayas 3. Popescu et al. 1 and Cai et al. 4 published a comprehensive report on Rhododendron species, including anti-inflammatory, anti-injury, antimicrobial, and antiprotozoal, antiviral activity, immunomodulatory activity, antioxidant activity, anti-diabetic activity, and cytotoxicity. In addition, Pandita et al. 5 presented various phytochemical compounds, including the Rhododendron plant, which has significant medicinal value. Rhododendron micranthum tastes bitter. It has the effect of ventilating, regulating pain, and coughing of sputum. For example, the branches are drugged for chronic tracheitis, rheumatism, lumbar pain, menorrhea, postpartum joint pain 6. The dry leaves of Rhododendron dauricum are scented with hardness, hardness, and cold with cough and sputum, and are of ten used to treat acute, chronic bronchitis, asthma, and other conditions 7. This review aims to comprehensively discuss the newly discovered and observed effects of compounds and drugs in Rhododendron species after 2013 and the further development prospects for producing potent and effective drugs.
Individual Rhododendron Species
Rhododendron aganniphum
Polysaccharides have various biological activities such as antioxidants, immunomodulators, antipruritic agents, hypoglycemic properties, skin penetration enhancers, and thickener properties. Because of these ideal characteristics, they have been used in many industrial fields. However, to extract polysaccha rides involved in plants, some problems are due to hydrolysis, ionization, or oxidation due to heating or longer extraction time. Moreover, it leads to a reduction in the added value of polysaccharides. Guo et al. 8 used Ultrasonic Assisted Extraction (UAE), cheap, environmentally friendly, less time-consuming, effective, and efficient. The optimization method they used was a Box-Behnken design (BBD)-like optimization procedure, which in turn investigated the antioxidant and rheological properties of polysaccharides. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, hydroxyl radical scavenging activity, and superoxide free radical scavenging activity were used to analyze polysaccharides, and stable shear behavior and frequency scanning measurement were used to obtain a rheological characterization of polysaccharides. The optimized ultrasonic-assisted polysaccharide extraction method is ex traction temperature 25℃, liquid-solid ratio 25:1, extraction time 2.2 h, ultrasonic treatment power 20w. The experimental yield of polysaccharides was 9.428%. In comparison, the traditional method was 8.951%, which was higher than the control based on in vitro antioxidant activity: The free radical scavenging activity of DPPH was about four times higher than the control, similar to the vitamin E (VE) at a concentration of 0.2 mg/ml. Under the concentration of 0.2 mg/ml, ultrasonic extraction, traditional hot water extraction, and VE scavenging rates were 84.139%, 52.671%, and 94.176%, respectively. The EC50 and VE were 0.041, 0.194 and 0.02 mg/ml, respectively. The hydroxyl radical scavenging activities are as follows: when the concentration is 0.2 mg/ml, methods 1, 2, and ascorbic acid (Vc) are 39.773%, 16.581%, and 61.283%, respectively. The EC50 values and Vc were 0.529, 0.783 and 0.092 mg/ml, respectively. The superoxide radical scavenging activities are 84.871%, 80.296%, and 96.033%, respectively. The measurement results show that the ultrasonic-assisted extraction method affects the apparent viscosity and dynamic viscoelasticity.
Rhododendron album Blume
Rhododendron album Blume (R.A.) is mainly distributed in smaller and smaller high-altitude forests in West Java and Central Java, Indonesia. By Park et al. 9 and Park et al. 10, it was found that the methanol extract of R.A. showed anti-inflammatory activity, especially R. A. statistically inhibited the iNOS and COX-2 expression in lipopolysaccharide (LPS)-stimulated RAW267 cells. They proved that R.A. is a natural and powerful anti-inflammatory compound that can block the activation of NF-κB and mitogen-activated protein kinase (MAPKs) pathways induced by LPS. The molecular mechanism is that the methanol extract of R.A. inhibits LPS-induced NF-κB activation in RAW 264.7 macrophages by inhibiting the phosphorylation of NF-κB and its subsequent inhibition of NF-κB nuclear transfer. The methanol extract of R.A. significantly inhibited the phosphorylation of p38, extracellular signal- regulated kinase (ERK) 1/2, and c-Jun N-terminal kinase (JNK) in RAW 264.7 cells stimulated by LPS.
Rhododendron alutaceum
Rhododendron alutaceum only grows in the alpine forests of southwest China. According to Li et al. 11, it is used in traditional Chinese medicine for relieving cough and resolving phlegm. In this regard, Li et al. 11 tried to find compounds with pharmacological activity from the leaves of this Rhodo dendron alutaceum. Extraction was performed by using a 75% Me2CO/H2O solution and at room temperature. In addition, the filtrate was concentrated in a vacuum oven, and the resulting residue was extracted with petroleum ether and ethyl acetate, respectively. In this study, a new phenolic glycoside, 3′- keto rhododendrin (Fig. 1. 1), and a new sesquilignan, alutaceuol (Fig. 1. 2), together with twelve known phenolic compounds like rhododendrin.
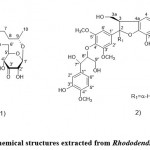 |
Figure 1: Chemical structures extracted from Rhododendron alutaceum |
Rhododendron ambiguum
Rhododendron ambiguum and Rhododendron cinnabarinum belong to the subsection Triflora and Cinnabaria, respectively. Shrestha et al. 12 studied the polyphenolic properties of these two plants of different ages and used 80% methanol solution to extract polyphenol. They identified 59 polyphenol compounds from two Rhododendron species. However, 46 of these compounds have been established in these plants. It was found that there was no significant difference in polyphenols in different years, but the variety and concentration of these compounds were the highest.
Furthermore, they used the radius of the inhibition zone to measure the inhibitory activity of these compounds against Gram-positive bacteria. This measurement found that the compounds in flowers have lower biological activity than leaves and fruits. The main biologically active compounds are as follows: Methyl gallate hexoside, Salicylic acid- O -hexoside, 4- O -Caffeoylquinic acid, Myricetin- O -rhamnoside, Myricetin- O -pentoxide, Quercetin- O -Pentoside, Quercetin- O -rhamnoside, (Epi)gallocatechin-(epi) Gallocatechin, Procyanidin Trimer C, (Epi) Gallocatechin-(epi)catechin and (Epi)cate catechin- O -D-glucopyranoside.
Rhododendron anthopogonoides Maxim
Rhododendron anthopogonoides Maxim grows on high-altitude mountain slopes, especially in Tibet, China. It has been used as traditional medicine. Its therapeutic effects include clearing heat and warming the body, clearing heat and detoxification, relieving cough and phlegm, relieving swelling and pain, relieving phlegm and relieving spasm, relieving inflammation, and relieving pain 13. The correlation was also analyzed between the phenolic and flavonoid contents of the extracts and their antioxidant properties. In vitro antioxidant study 14, the effect of the crude extract and solvent fractions on to tal antioxidant activity, reducing power, DPPH radical scavenging, 2,2’-azino-bis-(3-ethyl benzothiazoline-6-sulfonic acid) disodium salt (ABTS) radical scavenging, superoxide radical scavenging, hydroxyl radical scavenging, and nitric oxide radical scavenging was examined. In addition, Jing et al.14 studied how the extract affects the hypoxia-induced damage in PC12 cells through cell viability, lactate dehydrogenase release, malondialdehyde production, and antioxidant enzyme activity. According to the results of this study, among the extracts of hexane, ethyl acetate, n-butanol, and water, the highest yield was found in the ethyl acetate part, while the n-hexane part showed the lowest yield. In addition, the number of phenols and flavonoids in the above solvent extracts was found to be the highest in n-butanol and ethyl acetate. The hydroxyl scavenging activities of the above solvent extracts were n-butanol, crude extract, water, ethyl acetate, and hexane in turn. When the EC50 of ascorbic acid was 50.73 ± 1.83 μg/ml, the EC50 values were 145.87 ± 0.83 μg/ml, 204.65 ± 4.26 μg/ml, 232.95 ± 2.87 μg/ml, 240.39 ± 2.50 μg/ml, and 468.93 ± 16.17 μg/ml, respectively. Various solvent extracts’ DPPH free radical scavenging activities are n-butanol, ethyl acetate, crude extract, hexane, and water. When the EC50 of the ascorbic acid control was 30.39 ± 0.23 μg/ml, the EC50 values were 53.11 ± 0.12 μg/ml, 61.78 ± 0.11 μg/ml, 63.73 ± 0.95 μg/ml, 259.65 ± 13.04 μg/ml, and 273.35 ± 1.4 μg/ml, respectively. When the EC50 value of ascorbic acid was 18.53 ± 10.13 mm/ml, these extracts’ ABTS radical scavenging activity from various solvents decreased in the following order: ethyl acetate, n-butanol, crude extract, hexane, water. The different solvent extracts showed superoxide radical scavenging activity in n-butanol, ethyl acetate, crude extract, n-hexane, and water. When the EC50 value of ascorbic acid is 450.32 ± 6.28 μg/ml, the EC50 value is 270.67 ± 0.58 μg/ml, 433.33 ± 7.68 μg/ml, 483.00 ± 2.65μg/ml, >1,000 μg/ml and >1,000 μg/ml. Various solvent extracts showed nitric oxide radical scavenging activity, followed by n-butanol, ethyl acetate, crude extracts, hexane, and water. When the EC50 value of ascorbic acid is 126.72 ± 7.24 μg/ml, the EC50 value is respectively 151.34 ± 3.67 μg/ml, 183.83 ± 2.34 μg/ml, 197.38 ± 4.25 μg/ml, >1,000 μg/ml and >1,000 μg/ml.
Rhododendron arboreum
Rhododendron arboreum is growing in the Seram Valley in the Hazara branch of Jammu and Kashmir, Pakistan, and it has a variety of pharmacological effects, such as spasmolysis and convergence diuresis, and sialagogic 15. In order to study related compounds with the above effects, Nisar et al. 16 first obtained methanol extracts of Rhododendron arboreum bark and then conducted activity-guided fractionation of n-hexane, n-butanol, and chloroform. The identified compounds were ursolic acid, -sitosterol, pineal, dandelion, betulinol, acetic acid-induced torsion, carrageenan-induced mouse foot edema, and the possibility of anti-inflammatory drugs in Rhododendron was investigated. The lipoxygenase activity associated with inhibiting the above two models was also measured. The results are as follows: The ethyl acetate fraction (200 mg/kg i.p.) indicated the maximum analgesic effect (82%) on acetic acid-induced writhing, followed by the crude extract and chloroform fractions, both at a dose of 200 mg/kg i.p. In addition, the n-butanol and water-containing fractions showed a reduction in pain irritation (33.77% and 48.88%, respectively), while the n-hexane fraction (200 mg/kgi.p.) showed any analgesic effect. The lipoxygenase inhibitory activity of the crude methanol extract in vitro and solvent extract of Rhododendron are: while the standard value is 98.78 ± 0.35%, the inhibitory rates of crude extract, n-hexane, chloroform, ethyl acetate, butanol, and water are 50.44 ± 0.92%, 8.23 ± 0.30%, 68.47 ± 1.45%, 90.58 ± 1.02%, 22.11 ± 0.85%, and 48.35 ± 1.22%, respectively. In addition, the various results of the extracts as mentioned earlier/fractions on carrageenan-induced paw edema in mice are as follows: each sample shows a dose-dependent manner (1-5 hours) for all time courses (1-5 hour) anti-inflammatory activity 200 mg/kgi.p.), except for n-butanol.
Ali et al. 17 studied the effects of plant compounds in Rhododendron arboreum bark on renal cell carcinoma (A498), non-small cell lung cancer (NCl-H226), squamous cell carcinoma (H157), and human ovarian cancer (MDR). -2780AD). For MDR2780AD, HepG2, H157, and NCl-H226, the isolated compound 15-tallow acid showed potential anticancer activity with IC50 values of 2.3 ± 0.1μM, 4.9 ± 0.2μM, 9.2 ± 0.2μM, and 10.3 ± 0.1μM, respectively. The value of A498 is 32.8 ± 1.2μM.
Rhododendron brachycarpum G. Don
Rhododendron brachycarpum G. Don mainly grows in the central and northern regions of PRK, the Kuril Islands, and Japan’s northern and central regions. These days, due to radical climate changes, the number of short-fruited Rhododendrons continues to decrease, so it is considered a rare endangered species 18. This plant, especially the leaves, has been used to treat diabetes (D.M.), high blood pressure, headaches, and rheumatoid arthritis. In addition, phytochemical compounds in these treatments have been studied, such as flavonoids, diterpenoids (grey toxin I), and triterpenoids. Choi et al. 19 purified seven potential PTP1B inhibitors and a new triterpene compound (Fig. 2.1) and determined the potential inhibitory effects of these compounds on PTP1B. According to the results of the present study, the isolated compounds of neo triterpene and haptenic acid A (Fig. 2.1) showed significant inhibitory effects with IC50 values of 3.1 ± 0.3 μM and 6.3 ± 0.6 μM, respectively, compared with 4.0 ± 0.3 μM using RK- 682 as a positive control. Jeon et al. 20 isolated a new phytochemical component (Fig. 2.2) from the Rhododendron leaves and determined that its chemical structure contains phenylpropanol and glucopyranoside. In addition, they investigated the effect of this Rhododendron against inflammation. They proved that it has intracellular ROS (reactive oxygen species) scavenging activity and inhibits nuclear factor-κB (NF-κB) nuclear transcription. The mechanism is as follows: the process is through Inhibit the phosphorylation of NF-κB, inhibit NF-κB (IkBα) and IkBα kinase (IKKα/β).
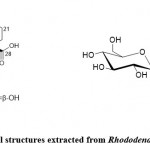 |
Figure 2: Chemical structures extracted from Rhododendron brachycarpum |
Tuan et al.22 isolated the derivative (Fig. 3.2) of Candida griseo-9(11)-ene and five known Grifola frondosa toxins (Grinidae B, rhodomoside A, piersformoside, grayanotoxin III, and grayanotoxin I) for the first time. Topical treatment of Rhododendron isolated from Rhododendron shows potential skin inflammation. According to their results, the reasons are as follows: first, rhododendron treatment reduces the signs and symptoms of imiquimod-induced psoriasis-like skin inflammation. In addition, Rhododendron reduces the levels of IMQ-induced TLR-7 downstream targets and MAP kinase signaling in normal human epidermal keratinocytes. Finally, Rhododendron may inhibit IMQ-induced interaction between IRAK1 and TRAF6 in cultured primary keratinocytes and retain the expression of dimple-1 in IMQ-treated keratinocytes. Liquid chromatography-quadrupole time Mass spectrometry (LC-QTOF-MS) combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS) to identify grayanotoxins from rhododendrons. The grayanotoxins I and grayanotoxins III in dietary supplements and homemade wine.According to their results, Rhododendron reduced the expression of pro-inflammatory mediators in TNFα/TFN-γ-stimulated keratinocytes, involving cyclooxygenase-2, intracellular adhesion molecule-1, interleukin ligand 1, and Chemokine (CC motif) ligand 17. In addition, they observed that Rhododendron has a promising candidate molecule for the treatment of inflammatory skin diseases such as psoriasis. Zhou et al. 21 discovered 22 compounds in Rhododendron azalea for the first time, such as highly oxidized diterpenoids. They emphasized that the compounds contained in the plant have good resources due to their remarkable homogeneity in chemical molecules. They isolated a new compound, rhododendron A (Fig. 3.1), from Rhododendron breviflora and determined its structure (3S, 5S, 6R)-1,1,5,9-tetramethyl-3-β-D-pyridine Glucopyranoxy-2,3,4,5-tetrahydro-7H-chromene-6-ol. This study demonstrated that compounds with megaglucosides, including Rhododendron A migrate HMGB1-in duced pro-inflammatory vascular stimulation, ultimately alleviate the lethality of sepsis.
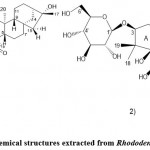 |
Figure 3: Chemical structures extracted from Rhododendron brachycarpum |
Rhododendron Capitatum Maxim.
Rhododendron capitatum is a small deciduous shrub in China’s rich resources. Liao et al.23 separated two enantiomeric carotenoids, (-)- and (+)-carotenoids A (Fig 4. 1a and 2a) and B (Fig. 4. 2a and ab). They identified these compounds by using spectral data, X-ray crystallography, and electronic circular dichroism systems. Carotenoid A (Fig. 4. 1a and 1b) has a unique 6/6/6/4 ring. In addition, determine whether these compounds (Fig. 4. 1a, 1b, 2a, and 2b) also inhibit PTP1B. According to their research results, compounds 2a and 2b showed inhibitory effects, and the IC50 values were 43.56 ±8.53μM and 30.38 ± 13.41μM, respectively, when oleanolic acid (a potent natural PTP1B inhibitor) was used in the assay. The positive control is 2.46 ± 0.18μM. They also isolated five new enantiomers (+)-/ (-)-carotenoids C (Fig. 4. 1a and 1b), E (Fig. 4. 3a and 3b), F (Fig. 4. 4a and 4b), (-)-/ (+)-carotenoids D (Fig. 4. 2a and 2b) and G (Fig. 4. 5a and 5b). Therefore, 1a and 1b are the first pair of mero monoterpenes containing an unprecedented 6/6/6/5 ring system among these compounds. 1a has antiviral activity against herpes simplex virus type 1 (HSV1) in vitro, with an IC50 value of 80.6 ± 4.7μM. However, none of these compounds 1a to 5a showed an inhibitory effect on PTP1B.
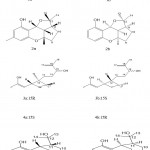 |
Figure 4: Chemical structures extracted from Rhododendron capitatum Maxim. |
Rhododendron collettianum
Said et al. 24 used a 90% methanol aqueous solution to extract the crude leaf extract. Using liquid chromatography-electrospray ionization-time-of-flight mass spectrometry (LC-ESI-micro TOF-MS) and NMR, cannabis dichromatic tannin was characterized. They determined the antimicrobial effect of the compound against Gram-positive (Staphylococcus epidermidis, Micrococcus leuteus, Bacillus aquimarius, Bacillus subtilis, Bacillus thioparus, Bacillus thuringiensis, Rhodococcus corynebacterioides, and Clavibacter michiganensis) and Gram-negative bacterium, and Escherichia coli strains DH5α and TG1. Compared with the other thirteen Rhododendron species, the crude extract of Rhododendron showed the most potent inhibitory effect. Therefore, they studied the effect of the diploid isochromic acid of Rhododendron on the inhibition of the above bacteria. According to their measurement, canna bis chromogenic acid shows a substantial inhibitory value for all Gram-positive bacteria, and the MIC (Minimum Inhibitory Concentration) value is in the range of 0.9 to 15.6 mm/ml. MIC is for Gram-negative bacterium Escherichia coli (TG1) reaches 1,000 μg/ml.
Rhododendron dauricum L.
Studies have found that farrerol, a known flavonoid compound isolated from Rhododendron dauricum L., can inhibit the apoptosis of cancer cells 25. This experiment studied the cytotoxic and apoptosis effects of farrerol on human gastric cancer SGC-7901 cells through a mitochondrial-mediated pathway. According to MTT analysis and Annexin-V FTTC/PI double staining method, farrerol’s IC50 value for SGC-7901 cells is 40.4 μmol/l for 24 h.
Lou et al.26 discovered two new C-methyl flavanones, 5, 7, 3′, 5′ -tetrahydroxy-6, 8-di-C-methyl flavanone, and 5, 4’-dihydroxy-8-C-methylflavanone-7-O-β-D-glucopyranoside and one new flavonoid glycoside, quercetin-3-O-β-D-(6″-O-cinnamoyl)-galactoside. (See Fig. 5.) Therefore, seven known compounds were isolated, syzalterin, poriolin, farrerol-7-O-β-D-glucopyranoside, myrciacetin, quercetin-3-O-β-D-(6-p-hydroxy-benzoyl)-galactoside, quercetin-3-O-β-D-(6-p-coumaroyl)-galactoside, and 5, 7, 3′, 5’-tetrahydroxyl flavanones.
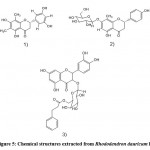 |
Figure 5: Chemical structures extracted from Rhododendron dauricum L. |
Using high-performance liquid chromatography and quadrupole time of flight tandem mass spectrometry, Wang et al. 7 identified 24 types of flavonoids, six types of phenolic acids, two coumarins, and 1 type of terpene. From their perspective, among these compounds, poriolin, farrerol-7-O-β-D-(6-p-hydroxy benzoyl)-galactoside, quercetin-3-O-β-D-(6-p-coumaroyl)-galactoside and myrciacetin were identified for the first time from this genus, respectively. In addition to these compounds, seven biologically active ingredients were also identified, gallic acid, scopoletin, dihydroquercetin, quercetin, kaempferol, 8‐desmethyl farrerol, and farrerol. Daurichromenic acid (DSA) is isolated from the leaves of Rhododendron dauricum and has anti-HIV solid activity. However, the designation of this plant as a protected species makes this compound unrestricted to find new resource applications for drugs. For this reason, 20 heterologous expressions from Stachybotrys bisbyi and grifolic acid synthase and daurichromeric acid synthase from Rhododendron dauricum in Aspergillus oryzae through enantioselective 6-endo-tricyclization-mediated three-step combinatorial biosynthesis (+)-lauric acid. Moreover, it introduces a halogenase derived from Fusarium. Enter the DCA production strain and complete the synthesis of new (+)-5-chloromeroterpenoids. In addition, it was studied that two chlorinated derivatives showed significant antibacterial activity compared to non-chlorinated compounds. There are several studies on this daurichromenic acid. 27-29
Rhododendron ferrugineum
Rhododendron ferrugineum, a subalpine shrub, is known to be found throughout the Pyrenees and European Alps. Various phytochemical compounds have also been identified in the leaves of this species: triterpenoids, furans A to C, short-chain organic acids, volatile oils, flavonoids, flavan-3-ols, oli gomeric proanthocyanidins, cinnamin B1, Original green acid, monosaccharide, oligosaccharide, fructan, arbutin. However, there are no reports of toxic diterpenoid maleimide derivatives. They first discovered the GT-1 quantitative detection method to determine the puerarin methylmercury compounds using Gas chromatography-mass spectrometry (GC-MS) to determine the rhododendron gray mold virus 30. Porphyromonas gingivalis is known to cause periodontitis and systemic chronic diseases, such as rheumatoid arthritis, cardiovascular disease, diabetes, and different oral tumors. Lohr et al. 31 inves tigated the effects of azalea water extract and water-soluble raw polysaccharides on the adhesion, virulence, and biofilm formation of Porphyromonas gingivalis. According to their research results, this aqueous extract involves 2R, 3R-taxifolin-3-O-β-L-arabinopyranoside, hyperoside, isoquercitrin, and chlorogenic acid, respectively. They are 12.1%, 1.6%, 0.9% and 1.6%. In addition, the tannin content value is 8.7% (w/w), and the tannin is mainly oligomeric proanthocyanidins. Moreover, in this study, the following ideal mechanism was observed: the original polysaccharides from the alpine rose Rhodo dendron and interact in a dose-dependent manner (each 1 mg/ml drop by up to 25%) and the adhesion of Porphyromonas gingivalis Attached by affecting Arg, this plant inhibits the gene expression of arginine-specific gingipainrgp A and IL-1β, IL-6, IL-8 and TNFα in KB cells.
Rhododendron formosanum Hemsl
This plant is an endemic species distributed in the mountains of central Taiwan. It is previously known to have various biological activities, such as antimicrobial activity, anti-proliferation, enzyme inhibition, free radical scavenging properties, separate hydrophobic compounds, and two isomeric epoxy sterols. A study using 2D-NMR, CD spectrum, and MS separation and identification of 5 kinds of proanthocyanidins in Rhododendron leaf extract studied the biological effects of these compounds. According to their research results, all five compounds showed potential antioxidant activity, and when the MIC value was 4 μg/ml, the new compound 3 showed intense antibacterial activity against Staphylococcus aureus. Other studies 32 have found that type A proanthocyanidin trimer ursolic acid extracted from rhododendron leaves has potential antitumor activity, especially the cytoprotective function of cinnatanninD1 treated non-small cell lung cancer (NSCLC) cell autophagy. In addition, they observed that the compound inhibits the Akt/mammalian target of the rapamycin (mTOR) pathway and activates the extracellular signal-regulated kinase 1/2 pathway, leading to the induction of autophagy.
Rhododendron groenlandicum
Rhododendron groenlandicum is known as Labrador tea, and it is also one of the most promising anti-diabetic plants traditionally used by James Bay Cree in the east. This plant extract has shown significant effects in restoring the mechanism of glucose homeostasis in high-fat diet (HFD) mice. Li et al. 33 studied the ability of this plant extract to significantly increase blood sugar, insulinemia, and glucose tolerance in vivo, and the test was used in HFD-induced obesity, immunohistochemistry (IHC), and biochemical parameters were used to evaluate the renal protective potential of Rhododendron treatment for another eight weeks. After all, this plant extract reduced renal steatosis by about half, while the expression of Bcl-2 modifiers decreased from 13.96 AU to 9.43 AU. Ouchfoun et al. 34 conducted a similar investigation on Rhododendron. It was emphasized that Labrador tea showed promising anti-diabetic effects through research results to improve insulin sensitivity.
Rhododendron hainanense
Studies 35 have shown that the compound isolated from the aerial part of Rhododendron has good pharmacological activity on human leukemia cells (HL-60) and human liver cancer cells (SMMC-7721), and the preliminary structure-effect of the sulforhodamine B method relationship. According to these results, the compound isolated and identified here shows the solid inhibitory activity as 2,3 dihydro-5-hydroxy-7-methoxy-2-(4-methoxyphenyl)-6,8-di Methyl-4H-benzopyran-4-one and 5-hydroxy-7,4′-dimethoxy-6,8-dimethylflavan have IC50 values of 15.2μM and 13.2μM, respectively. To HL-60.
Rhododendron japonicum
Koda et al. 36 investigated a 61-year-old man who developed dizziness, severe nausea, and abdominal discomfort after taking 50 grams of azalea. In addition, there is significant hypotension and sinus bradycardia. The results show that this symptom is caused by the toxicity of some azalea species, namely the action of grayanotoxin.
Rhododendron latoucheae
The plant is distributed in mainland China’s southern and southwestern parts and has historically been used for expectorating, relieving cough, promoting blood circulation, and removing stasis. Liu et al. 37 identified four red terpenoids (Fig. 6) by high-performance liquid chromatography-mass spectrometry-solid phase extraction-nuclear magnetic resonance separation, among which 3 are newly rearranged triterpenoids (A, B, and C) and rhododendron Red steroid D resonance (HPLC-SPE-NMR) and extensive spectroscopic methods and electronic circular dichroism (ECD) analysis in flowers. In addition, they found that these compounds A and D showed potential activity against herpes simplex virus 1 (HSV-1) with IC50 values of 8.62 and 6.87 μM, respectively.
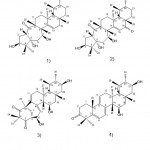 |
Figure 6: Chemical structures extracted from Rhododendron latoucheae |
Rhododendron liqueur
Choi et al. 38 used liquid chromatography-tandem mass spectrometry to study the levels of grayanotoxin (Ⅰ and Ⅲ) in the blood of patients with grayanotoxin poisoning after taking cuckoo alcohol. According to their findings, the initial blood concentration of these patients ranged from 2.9 to 58.0 ng/ml, and the patients’ mean arterial pressure was 36.7 to 76.7 mmHg. However, after 20 hours, the content of grayanotoxin in the blood dropped from the lower limit of quantification (LLOQ) to 17.8 and 2.52 ng/ml, while the average arterial pressure was 76.7 mmHg and 93.3 mmHg. More specifically, toxin III in the blood causes hypotension when the minimum value of the blood is 17.83-27.3 ng/ml, and the symptom is caused when the minimum value of the grayanotoxin I am 2.52 to 4.55 ng/ml.
Rhododendron luteum
Demir et al. 39 had quantified the contents of total polyphenols and flavonoids in the extract of dimethyl sulfoxide from Rhododendron and evaluated their effects on three kinds of cancer and human prepuce fibroblasts. According to their findings, polyphenols and flavonoids showed potent cytotoxicity to colon and liver cancer cells but no significant cytotoxicity to breast cancer.
Rhododendron micranthum
Zhang et al. 40used high-performance liquid chromatography, proton nuclear magnetic resonance spectroscopy, and electron paramagnetic resonance to separate and identify some new compounds from Rhododendron leaves, including new diterpenes, mica ketone A (Fig. 7. 1), and two new ash Neem diterpenoids, red melanin A (Fig. 7. 2) and B (Fig. 7. 3) and three known diterpenoids of fennel. According to their results, micrathanone A exhibited a new tetracyclic diterpene carbon skeleton, and rhodomicranols A and B have a 5,6-(3,4-dihydroxybenzylidene acetal) theme. In addition, 11 new diterpenoids of gray fennel and one new diterpenoid of resveratrol were identified, including 1-epifenneltoxin XⅦ, 6-deoxymagnolin XⅦ, 16-acetyl gray-blue Toxin II, 3-Oxypicline Toxin IX, 14-Deoxymagnolia Toxin VIII, 14-Acetyl Isosugar Toxin II, rhodomicrands C, D, E, and F. 41 In addition, known diterpenoids have been isolated. However, according to these researchers, these compounds have been identified from rhododendrons for the first time.
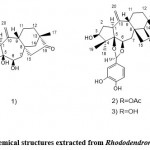 |
Figure 7: Chemical structures extracted from Rhododendron micranthum |
Rhododendron molle
Rhododendron molle is widely distributed throughout China and is a poisonous plant, especially flowers with anesthetic and insecticidal activities.
Zhou et al. 42 isolated and identified eight compounds. Namely, cleaved myristicone (1,5-opening Kalman-type skeleton), taxol XXI, 6-O-acetylgrubin XXI, 6,14- Di-O used high-performance liquid chromatography (HPLC), infrared spectroscopy (IR), nuclear magnetic resonance (NMR), and single-crystal X-ray diffraction analysis methods to study the acetyl progestin XXI, rutin XXII, 2- O-methyl Glycoside XI, Oryzaol XXII and Eucalyptus XXIV. Among these compounds, ionaldehyde XXI, 6-O-acetylerythromycin XXI, 6,14-di-O-acetylerythromycin XXI, viburnum XXII, and 2-O-methylerythromycin XⅠ are respectively named New gray alkane diterpenes. In addition, they also identified seven known diterpenoids, such as Rhodomolleins XI and XI and Rhodojaponins I, II, III, VI, and VII.
Li et al. 43 isolated two new ash descending bodies from Rhododendron fruit, namely, mollanol A (Fig. 8. 1) and rhodomolleinn XXⅤ (Fig. 8. 2). In addition, they confirmed that in different cell types (such as IEC-6,293T and RAW264.7 cells), mollanolA exhibited transcriptional activation of the xbp1 upstream promoter and activation at 10μM was 112,148, and 207%, respectively. Handle comparison of photos. They also identified nine new ash drops from the roots of Rhododedron molle by using spectral analysis, high-resolution electrospray ionization mass spectroscopy (HRESIMS), and 1D and 2D NMR data. (See Fig. 9) The acetic acid-induced writhing test, hot plate test, formalin test, diabetic neuropathological model, and acute cerebral ischemia model found Jerusalem artichoke saponins Ⅲ, Ⅵ, and grayanotoxin Ⅲ showed high anti-inflammatory and anti-diabetic activities compared with morphine and gabapentin, respectively.
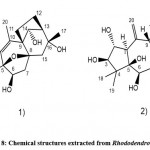 |
Figure 8: Chemical structures extracted from Rhododendron molle |
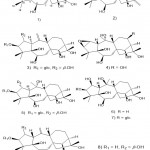 |
Figure 9: Chemical structures extracted from the root of Rhododedron molle |
As a result of continued efforts, Li et al. 44 identified two new gray matter subclasses, rhodomollin A (Fig. 10. 1) and B (Fig. 10. 2) from rhododendron fruits. Furthermore, they found that using HPLC, spectroscopy, and X-ray crystallography to study these gray tartaric acids have a new skeleton (characterized by a 5-hand-7-pound-6-pound dense ring system). In addition, by using MDCK cells infected with influenza A/95-359 virus in vitro, rhodomollin B showed moderate activity against influenza virus A/95-359, and the measured IC50 value was 19.24 μM.
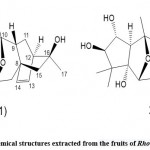 |
Figure 10: Chemical structures extracted from the fruits of Rhododedron molle |
Zhou et al. 45 used high-performance liquid chromatography, spectroscopy, and single-crystal X-ray diffraction technology to separate and identify three new diterpenoids from Rhododendron leaves Rhodiola A, B, and C.(See Fig. 11). They found that red bean alcohol acetal A revealed a novel cis/cis/cis/cis 6/6/6/6/5 pentacyclic ring system (features 11,13,18troxa-pentacy-clo [8.7.1.15,8. O2,8. O12,17] nonadecane scaffold) and red bean alcohol acetal B and C show a pair of C-6 epimers with a rare 4-oxa Tricyclic [7.2.1.01,6] dodecane moiety and 2,3-dihydro-4H-pyran-4-one unit. This study confirmed that these taxol compounds (A, B, and C) showed PTP1B inhibitory activity, and the IC50 values were 42.42 ± 1.40μM, respectively. According to these researchers, 4-oxatricyclo [7.2.1.01,6] dodecane and 2-methoxy-2,3-dihydro-4H-pyran-4-one units may inhibit the activity of PTP1B related. This opinion is estimated through a preliminary structure-activity relationship study.
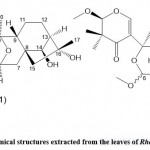 |
Figure 11: Chemical structures extracted from the leaves of Rhododedron mole |
Zhou et al. 46 used spectroscopy and single-crystal X-ray diffraction techniques to isolate and identify rhodomollanol A (Fig. 12. 1) and a new grayanane diterpenoid, rhodomollein XXXI (Fig. 12. 2) from Rhododendron leaves. rhodomollacetal A shows a unique cis/trans/trans/cis/cis fused 3/5/7/5/5/5 six-ring system, consisting of the rare 7-oxa two Ring [4.2.1] nonane core and three cyclopentane units. Rhodomollein XXXI is estimated to be 2β,3β-epoxy-5β,6β,10α,14β-tetrahydroxy gray yellow-15(16)-ene. Among these compounds, rhodomollanol A showed potent PTP 1B inhibitory activity in vitro, and when oleanolic acid was used as a control, IC 50 value was 24.32 ± 0.56 μM, IC50 value was 4.54 ± 0.25 μM, and Rhodomollein XXXI showed Weak PTP 1B inhibitory activity (IC50 value > 200 μM).
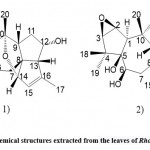 |
Figure 12: Chemical structures extracted from the leaves of Rhododedron mole |
Three new dimer diterpenes, birhodomollein A, B, and C (Fig. 13), were isolated and identified from rhododendron flowers by HPLC, 1D, and 2D NMR, IR, and ESIMS. 47 As a result, it is estimated that dihydroanthraphenols A, B, and C are α-chloro-14’β-hydroxy-14β, 2’α-oxo-bis-(6β-acetoxy-3β, 5β, 10α, 16α- Tetrahydroxyglarane), 2α,14’β-dihydroxy-14β, 2’α-oxo-di- (6β-acetoxy-3β, 5β, 10α, 16α-tetrahydroxypueraran) and 2β, 3β-epoxy-3’β, 14’β-dihydroxy-14β, 2’α-oxo-bis (6β-acetoxy-5β, 10α, 16α-trihydroxyglalan).
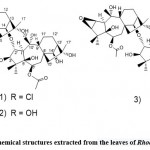 |
Figure 13: Chemical structures extracted from the leaves of Rhododedron mole |
Zhou et al. 48 used high-performance liquid chromatography (HRESIMS) to separate 13 new fennel diterpenoids from rhododendron leaves and discovered a fennel diterpene compound with anti-inflammatory effects, NMR, and single-crystal X-ray diffraction. These compounds are shown in Figure 14. In addition, they used mouse macrophages and cell viability assays to study the anti-inflammatory activity of 29 such compounds against LPS-induced NO production in RAW 264.7.
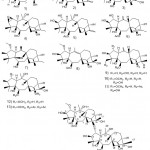 |
Figure 14: Chemical structures extracted from the leaves of Rhododedron mole |
Li et al. 49 isolated and identified 12 new diterpenoids (Fig. 15) from the fruits of Rhododendron. In addition, they studied the anti-nociceptive activity of these compounds through a writhing test induced by acetic acid. Among them, 2, 3, 12, 13, and 15 showed significant analgesic effects at shallow doses (0.4 mg/kg), while 1, 4, 9, 16, and 18 at high doses (2 mg/kg) showed significant analgesic effect) Moreover, others are inactive at a dose of 2mg/kg.
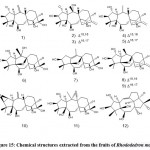 |
Figure 15: Chemical structures extracted from the fruits of Rhododedron mole |
Rhododendron mucronulatum for. albiflorum
Rhododendron mucronulatum for. albiflorum is distributed in mountainous areas and belongs to sunny areas in DPR Korea, China, and Japan. It is traditionally called a drug with high efficacy, such as tonic, diuretic, and stomachic. Flavonoids were isolated and identified from Rhododendron by Mok et al. 50. The compounds isolated and purified from the flowers of Rhododendron mucronulatum for. albiflorum are as follows: kaempferol, afzelin, quercetin, quercetin, myricetin, and myricetin. These compounds have also been studied for AR inhibitory activity. The results showed that amaranth, quercetin, and quercetin had higher AR inhibitory activities, with IC50 values of 0.31, 0.4, and 0.13μg/ml, respectively, and stronger than the control (tetramethyleneglutaric acid, IC50 value of 0.96 μg/ml)/ml). They emphasized that the flavonoid rhamnosides (quercetin and quercetin) had a more substantial inhibitory effect on AR than flavonoid aglycones (afzelin and kaemferol) the dihydroxyflavonoids in the B ring showed better than trihydroxyflavonoids Compounds have higher inhibition of AR activity.
Rhododendron nivale Hook. f.
Guo et al. 51 focused on the leaves and essential oils of Rhododendron and studied the oil composition and acaricidal activity of essential oils compared with the extracts of 70% ethanol, ethyl acetate, chloroform, and petroleum ether. As a result, in vivo and in vitro, the main compound of essential oil, δ-cadinene, showed the most vigorous acaricidal activity against P. moth.
Rhododendron oldhamii Maxim
Tung et al. 52 studied the antioxidant phytochemical components of Rhododendron. The leaf was measured by the HPLC-DPPH method, and the anti-hyperuricemia effects were tested using acute hyperuricemia induced by potassium oxazine (PO). Among these ingredients ((2R,3R)-epicatechin, (2R,3R)-taxifolin, (2R,3R)-astilbin, hyposide, guaijaverin, and quercitrin) ((2R, 3R)-astilbin, hyposide, guaijaverin, and quercitrin) showed pronounced reduction in renal damage. In addition, these significant phytochemicals significantly inhibited the concentration of serum uric acid, which was 5.41, 35.1, 56.3, 56.3, and 53.2% compared with the PO group. They found that rhododendron leaf extract (ethyl acetate part) improved fatty liver syndrome for the first time. 53 The results showed that this leaf extract could inhibit free fatty acid (FFA)-induced fat accumulation in HepG2 cells and improve fatty liver syndrome in nonalcoholic fatty liver disease (NAFLD) mice induced by HFD. Among these phytochemicals in the ethyl acetate portion of rhododendron leaf extract ((2R)-Pasteurella Vulgaris, Pasteurella guaijavin and quercitrin), (2R-Pasteur 3R)-Pasteurium and Pasteurella showed vigorous fat accumulation inhibitory activity.
Rhododendron ponticum nectar
Kurtoglu et al. 54 tried to find changes in grayanotoxins I and III concentration during the 6-month storage period. However, there is no significant difference. In addition, various effects of azalea money on human health were investigated. 55-57
Rhododendron pulchrum
Chai et al. 58 used matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS) mass spectrometry to study the structure anti-tyrosinase activity and mechanism of proanthocyanidins obtained from Rhododendron and high-performance liquid chromatography -Electrospray ionization mass spectrometry (HPLC-ESI-MS). Procyanidins are a complex mixture of procyanidins, prodelphinidins, protoveratrine, and their derivatives, and procyanidins are the main compound. Their research results on the anti-tyrosinase analysis of the above compounds show reversible and mixed competitive tyrosinase inhibitors. Observe these results through their molecular docking analysis.
Rhododendron pumilum Hook. f.
Zhang et al. 59 used HR-EIMS, NMR, IR, and other methods to isolate and identify two new dimers of gray alkane diterpenoids D (Fig. 16. 1) from azalea fruits with 3-O-2′ linkages. And E (Fig. 16. 2) and ultraviolet light. The name birhodomollein D reveals that the dimer grayanane-type diterpene processes these two compounds’ (C (3)-O-C (2′) linkage.
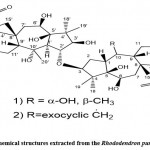 |
Figure 16: Chemical structures extracted from the Rhododendron pumilum Hook. f. |
Rhododendron seniavinii Maxim
Wang et al. 60 identified phytochemical components from Eucommia ulmoides leaves. Among them, the compound revealed a new flavonoid glycoside 5,7,3′-trimethoxy-quercetin 3-O-β-D-glucopyranoside (Fig. 17). It was confirmed by UV, FTIR, and NMR analysis.
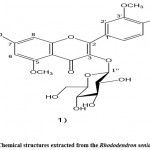 |
Figure 17: Chemical structures extracted from the Rhododendron seniavinii Maxim. |
Rhododendron simsii Planch
Rhododendron simsii Planch. is well known for treating bronchitis, pain, and coronary heart disease.
Cheng et al. 61 studied whether the total flavonoids of Rhododendron contain total flavonoids. The flower comprises hyperoside, rutin, quercetin, and other flavonoids, inhibiting ventricular remodeling after coronary artery ligation-induced myocardial infarction. It indicates that the urotensin-II receptor (UTR)-RhoA-Rho-kinase (ROCK) pathway may involve the use of echocardiography, Morphological, histological and immunochemical analysis, Picrosirius red staining, Western blot analysis.
Rhododendron tomentosum
Koudelkova et al. 62 had identified the most common leaf endophytes in Rhododendron leaves. They found that essential oils selected from Rhododendrons have different effects on oils isolated from Rhododendrons, which are different from those isolated from other substrates. Compared to corresponding strains of the same fungal species. This study investigated fungal species such as Apiosporamontagnei, Chaetomium globosum, Desmazierella acicula, Hypoxylon howeanum, and Khuskiaoryzae. They used headspace solid-phase microextraction (HP-SPME) and GC-MS to separate the volatile compounds in the essential oil of Rhododendron. They confirmed that the three volatile compounds mentioned above, p-cymene, myrene alcohol, and Alpha-terpinene, potentially affect endophytic bacteria growth.
Synthesis and synchronization of Rhododendron species
Recently, many researchers have conducted comprehensive and simultaneous studies on Rhododendron species. Jaiswal et al. 63 studied whether proanthocyanidins were found in 16 species of Rhododendrons and confirmed that these compounds were based on the proanthocyanidin of (epi) catechin and (epi) Gallocatechin and used the negative ion mode tandem mass spectrometry. Lin et al.64 studied the antioxidant activity of methanol extract from 10 leaves of Rhododendron in Taiwan: Rhododendron breviperulatum Hayata, Rhododendron ellipticum Maxim., Rhododendron. formosanum Hemsl., Rhododendron Kanehirai Wilson, Rhododendron mariesii Hemsl. And Wilson, Rhododendron oldhamii Maxim., Rhododendron pseudochrysanthum Hayata, Rhododendron rubropilosum Hayata var. rubropilosum, Rhododendron rubropilosum Hayata var. taiwanalpinum and Rhododendron simsii Planch. Four antioxidant activity methods were used, involving DPPH free radical scavenging activity, super oxide free radical scavenging determination, ferrous ion chelation determination, and reducing power determination. According to their research results, the n-butanol part of the Rhododendron leaf extract of the Rhododendron species showed the most potent antioxidant activity. (2R, 3S)-Catechin, (2R, 3R)-Epicatechin, (2R, 3R)-Dihydromyricetin 3-O-β-L-arabinopyranoside (1), (2S, (2R, 3R)-paclitaxel 3-O-β-1-arabinofuranoside (2), myricetin 3-O-β-D-glucopyranoside, rutin, hyperoside and quercetin, and It was confirmed that among these compounds, 1 and 2 were the main compounds.
Rezk et al. 65 conducted a phylogenetic study on the antibacterial effects of 120 leaf extracts of Rhododendron plants against 26 bacteria (Gram-positive and Gram-negative bacteria). They conducted an agar diffusion test on 80% methanol extracts, Rhododendron species and confirmed that 17 species showed potential antibacterial activity against Gram-positive bacteria. However, they showed a small amount of leaf extract against Gram-negative bacteria. These species are as follows: Rhododendron ferrugineum L., Rhododendron ambiguum Hemsley, Rhododendron anthopogon Don ssp. anthopogon Betty Graham, Rhododendron hirsutum L., Rhododendron anthopogon ssp. Hypanthium Bale. F. & Cullen, Rhododendron concinnum Hemsley, Rhododendron sichotense Pojarkova, Rhododendron cinnabarinum Hooker, Rhododendron racemosum Franchet, Rhododendron ledebourii Pojarkova, Rhododendron rubiginosum Franchet, Rhododendron xanthostephanum Merrill, Rhododendron myrtifolium Schott & Kotschy, Rhododendron minus Michaux, Rhododendron polycladum Franchet, Rhododendron spinuliferum Franchet and Rhododendron hippophaeoides var. hippophaeoides Hutchinson. According to these results, common genetic characteristics are responsible for producing biologically active secondary metabolites that mainly act on Gram-positive organisms, which may affect Gram-negative bacteria, depending on the activity of the multidrug efflux pump in the outer cell membrane.
Someone 66 has studied the cytotoxicity of 12 leaf extracts with potential antibacterial activity against epidermal keratinocytes (human HaCaT cell line) and intestinal epithelial cells (rat IEC6 cell line). To this end, five quantitative methods are used, such as plasma membrane integrity, cell viability, proliferation rate, cell metabolism, cytoskeletal structure, and determining the initiation of the cell death pathway through morphological and biochemical means. According to the results of total cell number and dead cell percentage of IEC6 and HaCaT cell cultures treated with azalea leaf extract, azalea extract is non-toxic to IEC6 cells, and azalea and azalea extracts did not affect HaCaT cells. Thus, this research provides robust data to produce effective and promising antibacterial agents suitable for human medicine. Furthermore, low cytotoxicity is significant for safe human antibiotic application.
Grimbs et al.67 studied the relationship between the antibacterial activity and cytotoxic activity of 87 Rhododendron species. To this end, they first assessed the abnormal diversity of Rhododendron based on DNA regions, trnK, trnLF, and ITS. In addition, they determined the antimicrobial (against Bacillus subtilis) and cytotoxicity (against IEC-6 and HaCaT cells) of Rhododendron species. They detected more than 48,000 LC-MS peaks in their phytochemical fingerprints, and for each peak from the mass-to-charge ratio (m/z), statistical evaluation methods such as principal component analysis and classification analysis are used. Based on these data, they explored the relationship between their respective biological activity classifications and phytochemical maps, identified secondary metabolites, and detected LC-MS peaks. Finally, they discovered and confirmed seven non-cytotoxic compounds (caffeoylquinic acid) from Rhododendron subgenus with potential antimicrobial activity.
Shrestha et al. 68 used a high-performance liquid chromatography-ion trap and time-of-flight mass spectrometer to separate and identify 69 kinds of hydroxycinnamic acid and its chlorogenic acid derivatives in the leaves of 69 species of Rhododendron. Among these compounds, the five most abundant hydroxycinnamates are p-coumarin-O-hexosidic isomer, 3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 3 -Op-coumarinic acid, and cis-5-Op-coumarinic acid. They emphasized that NMR studies must further analyze the more precise and perfect derivatives obtained from this study.
Conclusion
From ancient times, plants represent the primary source of drugs and alternative medicine to combat disease: Plants are a rich source of valuable 69sites such as alkaloids, quinones, tannins, terpenoids, flavonoids, and polyphenols 69. Rhododendron species have long been used in traditional medicine and animal studies, and in vitro studies have identified anti-inflammatory and liver-protective activities due to antioxidant effects of flavonoids or other phenolic compounds and saponins contained by plants 70,71. This review involves an investigation of 29 individual Rhododendron species and a comprehensive and simultaneous study of Rhododendron. In the study of antibacterial activity 72, it was found that the study on gram-positive bacteria and its activity against gram-positive bacteria were significant, but no visible components were found for gram-negative bacteria. The highly effective substances mentioned in this review will continue to increase in value as pioneers in drug development. In the future, research on substances that have a strong inhibitory effect on gram-negative bacteria should be strengthened, and specific research on the relationship between the structure and pharmacological properties of the compounds of the genus Rhododendron is needed.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China(21372049 ).
Conflict of Interest
The author declares no conflict of Interest
Funding source
National Natural Science Foundation of China (NSFC) 21372049
References
- Popescu R, Kopp B. The genus Rhododendron: An ethnopharmacological and toxicological review. Journal of Ethnopharmacology. May 2 2013;147(1):42-62. doi:10.1016/j.jep.2013.02.022
CrossRef - Shi Q, Li TT, Wu YM, et al. Meroterpenoids with diverse structures and anti-inflammatory activities from Rhododendron anthopogonoides. Phytochemistry. Dec 2020;180. doi:10.1016/j.phytochem.2020.112524
CrossRef - Bhattacharyya D. Rhododendron species and their uses with special reference to Himalayas–a review. Assam University Journal of Science and Technology. 2011;7(1):161-167.
- Cai YQ, Hu JH, Qin J, Sun T, Li XL. Rhododendron Molle (Ericaceae): phytochemistry, pharmacology, and toxicology. Chin J Nat Med. Jun 2018;16(6):401-410. doi:10.1016/S1875-5364(18)30073-6
CrossRef - Pandita D, Pandita A. Secondary Metabolites in Medicinal and Aromatic Plants (MAPs): Potent Molecules in Nature’s Arsenal to Fight Human Diseases. Medicinal and Aromatic Plants: Healthcare and Industrial Applications. 2021:41.
CrossRef - Sun N, Zhu Y, Zhou H, et al. Grayanane Diterpenoid Glucosides from the Leaves of Rhododendron micranthum and Their Bioactivities Evaluation. J Nat Prod. Dec 28 2018;81(12):2673-2681. doi:10.1021/acs.jnatprod.8b00490
CrossRef - Wang L, Zhu X, Lou X, et al. Systematic characterization and simultaneous quantification of the multiple components of Rhododendron dauricum based on high-performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J Sep Sci. Sep 2015;38(18):3161-3169. doi:10.1002/jssc.201500553
CrossRef - Guo X, Shang XF, Zhou XZ, Zhao BT, Zhang JY. Ultrasound-assisted extraction of polysaccharides from Rhododendron aganniphum: Antioxidant activity and rheological properties. Ultrasonics Sonochemistry. Sep 2017;38:246-255. doi:10.1016/j.ultsonch.2017.03.021
CrossRef - Park JW, Kwon OK, Kim JH, et al. Rhododendron album Blume inhibits iNOS and COX-2 expression in LPS-stimulated RAW264.7 cells through the downregulation of NF-kappaB signaling. Int J Mol Med. Apr 2015;35(4):987-94. doi:10.3892/ijmm.2015.2107
CrossRef - Park JW, Lee HS, Lim Y, et al. Rhododendron album Blume extract inhibits TNF-α/IFN-γ-induced chemokine production via blockade of NF-κB and JAK/STAT activation in human epidermal keratinocytes. International journal of molecular medicine. 2018;41(6):3642-3652.
CrossRef - Li HZ, Song HJ, Li HM, Pan YY, Li RT. Characterization of phenolic compounds from Rhododendron alutaceum. Arch Pharm Res. Nov 2012;35(11):1887-93. doi:10.1007/s12272-012-1104-9
CrossRef - Shrestha A, Rezk A, Said IH, et al. Comparison of the polyphenolic profile and antibacterial activity of the leaves, fruits and flowers of Rhododendron ambiguum and Rhododendron cinnabarinum. BMC Res Notes. Jul 20 2017;10(1):297. doi:10.1186/s13104-017-2601-1
CrossRef - Shi Q, Li T-T, Wu Y-M, et al. Meroterpenoids with diverse structures and anti-inflammatory activities from Rhododendron anthopogonoides. Phytochemistry. 2020;180:112524.
CrossRef - Jing L, Ma H, Fan P, Gao R, Jia Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement Altern Med. Aug 18 2015;15:287. doi:10.1186/s12906-015-0820-3
CrossRef - Sharma H, Kumar P, Singh A, et al. Development of polymorphic EST-SSR markers and their applicability in genetic diversity evaluation in Rhododendron arboreum. Molecular biology reports. 2020;47(4):2447-2457.
CrossRef - Nisar M, Ali S, Muhammad N, et al. Antinociceptive and anti-inflammatory potential of Rhododendron arboreum bark. Toxicol Ind Health. Jul 2016;32(7):1254-1259. doi:10.1177/0748233714555391
CrossRef - Ali S, Nisar M, Qaisar M, Khan A, Khan AA. Evaluation of the cytotoxic potential of a new pentacyclic triterpene from Rhododendron arboreum stem bark. Pharmaceutical Biology. Jun 29 2017;55(1):1927-1930. doi:10.1080/13880209.2017.1343359
CrossRef - Zhou W, Oh J, Li W, Kim DW, Lee SH, Na M. Phytochemical studies of Korean endangered plants: a new flavone from Rhododendron brachycarpum G. Don. Bulletin of the Korean Chemical Society. 2013;34(8):2535-2538.
CrossRef - Choi YH, Zhou W, Oh J, et al. Rhododendric acid A, a new ursane-type PTP1B inhibitor from the endangered plant Rhododendron brachycarpum G. Don. Bioorg Med Chem Lett. Oct 1 2012;22(19):6116-6119. doi:10.1016/j.bmcl.2012.08.029
CrossRef - Jeon YJ, Kim BH, Kim S, et al. Rhododendrin ameliorates skin inflammation through inhibition of NF-kappa B, MAPK, and PI3K/Akt signaling. European Journal of Pharmacology. Aug 15 2013;714(1-3):7-14. doi:10.1016/j.ejphar.2013.05.041
CrossRef - Zhou W, Oh J, Li W, et al. Chemical constituents of the Korean endangered species Rhododendron brachycarpum. Biochem Syst Ecol. Oct 2014;56:231-236. doi:10.1016/j.bse.2014.06.003
CrossRef - Tuan NQ, Oh J, Park HB, et al. A grayanotox-9(11)-ene derivative from Rhododendron brachycarpum and its structural assignment via a protocol combining NMR and DP4 plus application. Phytochemistry. Jan 2017;133:45-50. doi:10.1016/j.phytochem.2016.10.010
CrossRef - Liao HB, Huang GH, Yu MH, Lei C, Hou AJ. Five Pairs of Meroterpenoid Enantiomers from Rhododendron capitatum. Journal of Organic Chemistry. Feb 3 2017;82(3):1632-1637. doi:10.1021/acs.joc.6b02800
CrossRef - Said IH, Rezk A, Hussain I, et al. Metabolome Comparison of Bioactive and Inactive Rhododendron Extracts and Identification of an Antibacterial Cannabinoid(s) from Rhododendron collettianum. Phytochem Analysis. Sep-Oct 2017;28(5):454-464. doi:10.1002/pca.2694
CrossRef - Liu EL, Liang TG, Wang XJ, Ban SR, Han LG, Li QS. Apoptosis induced by farrerol in human gastric cancer SGC-7901 cells through the mitochondrial-mediated pathway. Eur J Cancer Prev. Sep 2015;24(5):365-372. doi:10.1097/Cej.0000000000000104
CrossRef - Lou XW, Lin QH, Zhang GY, Liu WY, Qu W. Identification and characterization of three new flavonoids from Rhododendron dauricum. Chin J Nat Medicines. 2015;13(8)
CrossRef - Iijima M, Munakata R, Takahashi H, et al. Identification and Characterization of Daurichromenic Acid Synthase Active in Anti-HIV Biosynthesis. Plant Physiol. Aug 2017;174(4):2213-2230. doi:10.1104/pp.17.00586
CrossRef - Taura F, Iijima M, Yamanaka E, et al. A Novel Class of Plant Type III Polyketide Synthase Involved in Orsellinic Acid Biosynthesis from Rhododendron dauricum. Front Plant Sci. 2016;7:1452. doi:10.3389/fpls.2016.01452
CrossRef - Taura F, Iijima M, Kurosaki F. Daurichromenic acid and grifolic acid: Phytotoxic meroterpenoids that induce cell death in cell culture of their producer Rhododendron dauricum. Plant Signal Behav. 2018;13(1). doi:10.1080/15592324.2017.1422463
CrossRef - Lechtenberg M, Dierks F, Sendker J, Louis A, Schepker H, Hensel A. Extracts from Rhododendron ferrugineum Do Not Exhibit Grayanotoxin I: An Analytical Survey on Grayanotoxin I within the Genus Rhododendron. Planta Medica. Oct 2014;80(15):1321-1328. doi:10.1055/s-0034-1383039
CrossRef - Lohr G, Beikler T, Hensel A. Inhibition of in vitro adhesion and virulence of Porphyromonas gingivalis by aqueous extract and polysaccharides from Rhododendron ferrugineum L. A new way for prophylaxis of periodontitis? Fitoterapia. Dec 2015;107:105-113. doi:10.1016/j.fitote.2015.10.010
CrossRef - Wang CM, Hsu YM, Jhan YL, et al. Structure Elucidation of Procyanidins Isolated from Rhododendron formosanum and Their Anti-Oxidative and Anti-Bacterial Activities. Molecules. Jul 15 2015;20(7):12787-803. doi:10.3390/molecules200712787
CrossRef - Li SL, Brault A, Villavicencio MS, Haddad PS. Rhododendron groenlandicum (Labrador tea), an antidiabetic plant from the traditional pharmacopoeia of the Canadian Eastern James Bay Cree, improves renal integrity in the diet-induced obese mouse model. Pharmaceutical Biology. 2016;54(10):1998-2006. doi:10.3109/13880209.2015.1137953
CrossRef - Ouchfoun M, Eid HM, Musallam L, et al. Labrador tea (Rhododendron groenlandicum) attenuates insulin resistance in a diet-induced obesity mouse model. European journal of nutrition. Apr 2016;55(3):941-54. doi:10.1007/s00394-015-0908-z
CrossRef - Zhao J, Ding HX, Zhao DG, Wang CM, Gao K. Isolation, modification and cytotoxic evaluation of flavonoids from Rhododendron hainanense. Journal of Pharmacy and Pharmacology. Dec 2012;64(12):1785-1792. doi:10.1111/j.2042-7158.2012.01560.x
CrossRef - Koda R, Honma M, Suzuki K, et al. Hypotension and Bradycardia Caused by the Inadvertent Ingestion of Rhododendron japonicum. Internal Med. 2016;55(7):839-842. doi:10.2169/internalmedicine.55.6144
CrossRef - Liu F, Wang YN, Li Y, et al. Rhodoterpenoids A-C, Three New Rearranged Triterpenoids from Rhododendron latoucheae by HPLC-MS-SPE-NMR. Scientific Reports. Aug 11 2017;7. doi:10.1038/s41598-017-06320-x
CrossRef - Choi HL, Park KH, Park JS, Choi HY, Kim H, Kim SM. Relationship between blood toxin level and clinical features in patients with grayanotoxin poisoning – six clinical cases. Clin Toxicol. 2017;55(9):991-995. doi:10.1080/15563650.2017.1331448
CrossRef - Demir S, Turan I, Aliyazicioglu Y. Selective cytotoxic effect of Rhododendron luteum extract on human colon and liver cancer cells. J Buon. Jul-Aug 2016;21(4):883-888.
CrossRef - Zhang MK, Zhu Y, Zhan GQ, et al. Micranthanone A, a New Diterpene with an Unprecedented Carbon Skeleton from Rhododendron micranthum. Organic Letters. Jun 21 2013;15(12):3094-3097. doi:10.1021/ol401292y
CrossRef - Zhang MK, Xie YY, Zhan GQ, et al. Grayanane and leucothane diterpenoids from the leaves of Rhododendron micranthum. Phytochemistry. Sep 2015;117:107-115. doi:10.1016/j.phytochem.2015.06.007
CrossRef - Zhou SZ, Yao S, Tang C, et al. Diterpenoids from the flowers of Rhododendron molle. J Nat Prod. May 23 2014;77(5):1185-92. doi:10.1021/np500074q
CrossRef - Li Y, Liu YB, Liu YL, et al. Mollanol A, a Diterpenoid with a New C-Nor-D-homograyanane Skeleton from the Fruits of Rhododendron molle. Organic Letters. Aug 15 2014;16(16):4320-4323. doi:10.1021/ol5020653
CrossRef - Li Y, Liu YB, Yan HM, et al. Rhodomollins A and B, two Diterpenoids with an Unprecedented Backbone from the Fruits of Rhododendron molle. Scientific Reports. Nov 14 2016;6. doi:10.1038/srep36752
CrossRef - Zhou JF, Sun N, Zhang HQ, Zheng GJ, Liu JJ, Yao GM. Rhodomollacetals A-C, PTP1B Inhibitory Diterpenoids with a 2,3:5,6-Di-seco-grayanane Skeleton from the Leaves of Rhododendron molle. Organic Letters. Oct 6 2017;19(19):5352-5355. doi:10.1021/acs.orglett.7b02633
CrossRef - Zhou J, Zhan G, Zhang H, et al. Rhodomollanol A, a Highly Oxygenated Diterpenoid with a 5/7/5/5 Tetracyclic Carbon Skeleton from the Leaves of Rhododendron molle. Org Lett. Jul 21 2017;19(14):3935-3938. doi:10.1021/acs.orglett.7b01863
CrossRef - Zhou SZ, Tang CP, Ke CQ, Yao S, Lin G, Ye Y. Three new dimeric diterpenes from Rhododendi-on molle. Chinese Chem Lett. Jun 2017;28(6):1205-1209. doi:DOI 10.1016/j.cclet.2017.02.020
CrossRef - Zhou J, Liu T, Zhang H, et al. Anti-inflammatory Grayanane Diterpenoids from the Leaves of Rhododendron molle. J Nat Prod. Jan 26 2018;81(1):151-161. doi:10.1021/acs.jnatprod.7b00799
CrossRef - Li Y, Zhu YX, Zhang ZX, et al. Diterpenoids from the fruits of Rhododendron molle, potent analgesics for acute pain. Tetrahedron. Feb 15 2018;74(7):693-699. doi:10.1016/j.tet.2017.12.017
CrossRef - Mok SY, Lee S. Identification of flavonoids and flavonoid rhamnosides from Rhododendron mucronulatum for. albiflorum and their inhibitory activities against aldose reductase. Food chemistry. Jan 15 2013;136(2):969-74. doi:10.1016/j.foodchem.2012.08.091
CrossRef - Guo X, Shang X, Li B, Zhou XZ, Wen H, Zhang J. Acaricidal activities of the essential oil from Rhododendron nivale Hook. f. and its main compund, delta-cadinene against Psoroptes cuniculi. Vet Parasitol. Mar 15 2017;236:51-54. doi:10.1016/j.vetpar.2017.01.028
CrossRef - Tung YT, Lin LC, Liu YL, et al. Antioxidative phytochemicals from Rhododendron oldhamii Maxim. leaf extracts reduce serum uric acid levels in potassium oxonate-induced hyperuricemic mice. Bmc Complem Altern M. Dec 1 2015;15. doi:10.1186/s12906-015-0950-7
CrossRef - Liu YL, Lin LC, Tung YT, et al. Rhododendron oldhamii leaf extract improves fatty liver syndrome by increasing lipid oxidation and decreasing the lipogenesis pathway in mice. Int J Med Sci. 2017;14(9):862-870. doi:10.7150/ijms.19553
CrossRef - Kurtoglu AB, Yavuz R, Evrendilek GA. Characterisation and fate of grayanatoxins in mad honey produced from Rhododendron ponticum nectar. Food chemistry. Oct 15 2014;161:47-52. doi:10.1016/j.foodchem.2014.03.127
CrossRef - Erenler AK. Cardiac Effects of Mad Honey Poisoning and Its Management in Emergency Department: A Review from Turkey. Cardiovasc Toxicol. Jan 2016;16(1):1-4. doi:10.1007/s12012-015-9310-6
CrossRef - Silici S, Dogan Z, Sahin H, Atayoglu T, Yakan B. Acute effects of grayanotoxin in rhododendron honey on kidney functions in rats. Environ Sci Pollut R. Jan 2016;23(4):3300-3309. doi:10.1007/s11356-015-5534-z
CrossRef - Sibel S, Enis YM, Huseyin S, Timucin AA, Duran O. Analysis of grayanatoxin in Rhododendron honey and effect on antioxidant parameters in rats. Journal of Ethnopharmacology. Oct 28 2014;156:155-161. doi:10.1016/j.jep.2014.08.027
CrossRef - Chai WM, Wang R, Wei MK, et al. Proanthocyanidins Extracted from Rhododendron pulchrum Leaves as Source of Tyrosinase Inhibitors: Structure, Activity, and Mechanism. PloS one. Dec 29 2015;10(12). doi:10.1371/journal.pone.0145483
CrossRef - Zhang R, Tang CP, Ke CQ, Yao S, Lin G, Ye Y. Birhodomolleins D and E, two new dimeric grayanane diterpenes with a 3-O-2 ‘ linkage from the fruits of Rhododendron pumilum. Chinese Chem Lett. Jan 2018;29(1):123-126. doi:10.1016/j.cclet.2017.07.009
CrossRef - Wang QQ, Wu C, Zhang Y, Liu BL, Zhou GX. A new flavonoid glucoside from Rhododendron seniavinii. Journal of Asian Natural Products Research. Jul 3 2015;17(7):778-782. doi:10.1080/10286020.2014.989222
CrossRef - Cheng XQ, Zhang J, Chen ZW. Effects of Total Flavone from Rhododendron simsii Planch. Flower on Postischemic Cardiac Dysfunction and Cardiac Remodeling in Rats. Evid-Based Compl Alt. 2017;2017. doi:10.1155/2017/5389272
CrossRef - Koudelkova B, Jarosova R, Koukol O. Are endophytic fungi from Rhododendron tomentosum preadapted for its essential oil? Biochem Syst Ecol. Dec 2017;75:21-26. doi:10.1016/j.bse.2017.11.001
CrossRef - Jaiswal R, Jayasinghe L, Kuhnert N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J Mass Spectrom. Apr 2012;47(4):502-515. doi:10.1002/jms.2954
CrossRef - Lin CY, Lin LC, Ho ST, Tung YT, Tseng YH, Wu JH. Antioxidant Activities and Phytochemicals of Leaf Extracts from 10 Native Rhododendron Species in Taiwan. Evid-Based Compl Alt. 2014;2014. doi:10.1155/2014/283938
CrossRef - Rezk A, Nolzen J, Schepker H, Albach DC, Brix K, Ullrich MS. Phylogenetic spectrum and analysis of antibacterial activities of leaf extracts from plants of the genus Rhododendron. Bmc Complem Altern M. Mar 18 2015;15. doi:10.1186/s12906-015-0596-5
CrossRef - Cooper R, Morré DJ, Morré DM. Medicinal benefits of green tea: part II. Review of anticancer properties. Journal of Alternative & Complementary Medicine. 2005;11(4):639-652.
CrossRef - Grimbs A, Shrestha A, Rezk ASD, et al. Bioactivity in Rhododendron: A Systemic Analysis of Antimicrobial and Cytotoxic Activities and Their Phylogenetic and Phytochemical Origins. Front Plant Sci. Apr 13 2017;8. doi:10.3389/fpls.2017.00551
CrossRef - Shrestha A, Said IH, Grimbs A, et al. Determination of hydroxycinnamic acids present in Rhododendron species. Phytochemistry. Dec 2017;144:216-225. doi:10.1016/j.phytochem.2017.09.018
CrossRef - Jubair N, Rajagopal M, Chinnappan S, Abdullah NB, Fatima A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid-Based Compl Alt. 2021/08/17 2021;2021:3663315. doi:10.1155/2021/3663315
CrossRef - Liu Y-L, Lin L-C, Tung Y-T, et al. Rhododendron oldhamii leaf extract improves fatty liver syndrome by increasing lipid oxidation and decreasing the lipogenesis pathway in mice. Int J Med Sci. 2017;14(9):862-870. doi:10.7150/ijms.19553
CrossRef - McKinnon R, Kopp B. The genus Rhododendron: An ethnopharmacological and toxicological review. Journal of ethnopharmacology. 02/27 2013;doi:10.1016/j.jep.2013.02.022
CrossRef - Yuan G, Guan Y, Yi H, Lai S, Sun Y, Cao S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Scientific Reports. 2021/05/18 2021;11(1):10471. doi:10.1038/s41598-021-90035-7
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





