Manuscript accepted on : 29-11-2021
Published online on: 09-12-2021
Plagiarism Check: Yes
Reviewed by: Dr. Bishop Julius Soyinka
Second Review by: Dr. Daya Shankar Gautam
Final Approval by: Dr. Hifzur R. Siddique
Paroma Arefin1,2* , Md Shehan Habib1
, Md Shehan Habib1 , Mohammad Mostafa1
, Mohammad Mostafa1 , Dipankar Chakraborty1
, Dipankar Chakraborty1 , Sreebash Chandra Bhattacharjee1
, Sreebash Chandra Bhattacharjee1 , Md Saidul Arefin3
, Md Saidul Arefin3 and Debabrata Karmakar4
and Debabrata Karmakar4
1BCSIR Chattogram Laboratories, Bangladesh Council of Scientific and Industrial Research, Bangladesh
2Institute of Food Science and Technology, Bangladesh Council of Scientific and Industrial Research, Bangladesh
3Institute of Nutrition and Food Science, University of Dhaka, Dhaka-1000, Bangladesh
4Institute of Technology Transfer and Innovation, Bangladesh Council of Scientific and Industrial Research, Bangladesh
Corresponding Author E-mail: paroma.arefin@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2955
ABSTRACT:
Microspheres, a potential drug delivery approach, has opened a new era for attaining versatile release patterns needed. By optimizing the formulation variables, they can be prepared to obtain targeted release, immediate release, sustained release patterns. The release of the active drug material depends upon a number of formulation parameters such as polymers, stirring speed (rpm), methodology, surfactants, etc. Fexofenadine hydrochloride (HCl) is a second generation antihistamine. Our present research has explored the effects of using different rpm (600- 1000 rpm) in preparing fexofenadine hydrochloride (HCl) microspheres by emulsion solvent evaporation method. The formulation is aimed to provide sustained release for the required long period with a high margin of safety. We used a blended mixture of Hydroxy Propyl Methyl Cellulose (HPMC) K 100 MCR and Eudragit RL100 polymers to have sustained-release microspheres. The impact of different rpm on Yield, drug encapsulation efficiency, flow properties, and dissolution pattern were appraised. We observed the release of the drug for 10 hours in phosphate buffer (pH 6.8) and evaluated the drug release spectrophotometrically. Our study finds that the release of fexofenadine HCl from the microspheres was significantly increased with drug loading. We found the dosage forms to follow Higuchi release kinetics and Hixson-Crowell release kinetics the most, indicating successful achievement of sustained-release pattern in the dosage form. The change in drug release rate was statistically significant for variation in the stirring rate. We found that 600 rpm was the most optimized stirring rate for preparing microspheres in the emulsion solvent evaporation method.
KEYWORDS: Dissolution; Fexofenadine; HCl; Microsphere; RPM; Solvent Evaporation Method
Download this article as:| Copy the following to cite this article: Arefin P, Habib M. D. S, Mostafa M, Chakraborty D, Bhattacharjee S. C, Arefin M. D. S, Karmakar D. Evaluation of the Influence of Stirring Speed on the Release Kinetics of Fexofenadine HCl Polymeric Microspheres. Biosci Biotech Res Asia 2021;18(4). |
| Copy the following to cite this URL: Arefin P, Habib M. D. S, Mostafa M, Chakraborty D, Bhattacharjee S. C, Arefin M. D. S, Karmakar D. Evaluation of the Influence of Stirring Speed on the Release Kinetics of Fexofenadine HCl Polymeric Microspheres. Biosci Biotech Res Asia 2021;18(4). Available from: https://bit.ly/3Gp8MjG |
Introduction
The microsphere dosage form is one of the most potential novel drug delivery systems, and it has opened a new era for individualized drug delivery options minimizing the side effects and maximizing the convenience. 1–7 Microspheres formulations can be designed in a variety of approaches depending on the purpose, such as targeted release, immediate release, sustained release, and so on. 8–10 The diameter of the microspheres ranges from 1 micrometer to 1000 micrometers. 11–13 Microspheres can contain 10-90% w/w active or core ingredient in general. The solvent-evaporation is the most frequently used method for microsphere dosage form preparation 14–16. In this method, a solution of drug and a combination of polymers is emulsified with another solvent with the help of an emulsifying agent, and the formulations are prepared with different stirring speed (RPM) in an overhead stirrer. 17–19 The stirring rate of 400-1200 rpm is usually used for microsphere preparation in the solvent evaporation method 1,16,18,20,21. The stirring rate (RPM) determines the shape, morphology, surface structure, drug entrapment, and drug release4,22,23. Therefore, it is a crucial formulation variable to be optimized to obtain the best quality microspheres. In our present study, we have explored the effects of using different rpm (600 -1000 rpm) on the characteristics of microspheres of Fexofenadine HCl. It is a second-generation H1 receptor antagonist. 24–26 The non-sedating antihistamine drug is extensively prescribed in seasonal allergic rhinitis, chronic urticaria, and other allergic diseases.26,27 It belongs to biopharmaceutical classification system (BCS) class III, i.e., it has low absorption and high solubility profile. 13,27 Arefin et al. used polymers HPMC K 100 MCR and Eudragit RL 100 to sustain the drug to have better absorption and increased bioavailability. They found the polymers compatible with this drug by Fourier Transform Infrared (FTIR) Spectroscopy and Differential Scanning Calorimetry (DSC). 4,13 In this research, we have used polymeric blends of HPMC K100M CR and Eudragit RL100 to prepare Fexofenadine HCl sustained-release microspheres at different rpm to optimize the rpm for this dosage form. Due to the long half-life (14.4 hours) of the drug, the low polymer contents have been used to balance the sustaining effect and long half-life properties.
Materials and methods
Materials
HPMC K100 M CR and Eudragit RL 100 were used for sustaining the drug release which were collected from Evonik Industries, Germany. Other formulation ingredients like ethanol, n-hexane, sorbitan monooleate, light liquid paraffin, potassium dihydrogen phosphate and dipotassium hydrogen phosphate were collected from MERCK, Germany.
Methods
Formulation of microspheres
Fexofenadine HCl sustained-release microspheres were prepared using the “emulsion-solvent evaporation technique”. Ethanol and Span80 were used as solvent and emulsifying agent respectively.13
Formulation design
Five batches of fexofenadine HCl microspheres were formulated in total with a batch size of 900 mg, designated as DF1 to DF5. The detailed design is presented in Table 1.
Table 1: Formulation Design of microspheres prepared at different RPM.
| Formulation | Drug to Polymer ratio | Drug (mg) | HPMC K 100 M CR (mg) | Eudragit RL 100 (mg) | RPM |
| DF1 | 1:1 | 450 | 300 | 150 | 600 |
| DF2 | 1:1 | 450 | 300 | 150 | 700 |
| DF3 | 1:1 | 450 | 300 | 150 | 800 |
| DF4 | 1:1 | 450 | 300 | 150 | 900 |
| DF5 | 1:1 | 450 | 300 | 150 | 1000 |
Characterization of fexofenadine HCl microspheres
Production yield
The Yield (%) was determined by was calculated by the formula 28
Determination of drug entrapment efficiency
A glass mortar-pestle was used for crushing a precise amount of 10mg microsphere. The crushed amount was taken in 10ml phosphate buffer with pH 6.8. The solution remained in rest for 24 hours period. Then it was filtered, and after that, the filtrate was examined in UV spectrophotometer for active drug content. The drug entrapment efficiency was determined by the formula 29,30:
Micromeritics study
Carr’s compressibility index and Hausner ratio were used as the determining tool for flow properties. Bulk density and tapped density were calculated using a volumetric cylinder. In the range of 5-10, 1.00-1.11, and 25-30, respectively, the Carr’s Compressibility Index, Hausner Ratio, and Angle of Repose value suggest the particles have excellent flow properties. 13,29
Assessment of surface structure
For the proper perception of the surface structure, morphology, or shape, the scanning electron microscope (s-3400N, Hitachi) was used under different levels of magnification. The impact of the concentration of polymer and the shape, integrity, active agent release from the microsphere was interpreted by this study.31,32
In vitro dissolution study of microspheres containing Fexofenadine HCl
In vitro release pattern of the active agent from the microspheres was evaluated using Type II dissolution apparatus (Copley DIS 800i) .9,13,31 The dissolution was performed in pH 6.8 to feign intestinal fluid with Phosphate Buffer Solution (PBS) at a controlled temperature of 37°C. The release pattern was observed for 10 hours. 10 ml dissolution sample was collected with a one-hour interval up to 10 hours, but the first one was withdrawn after 30 minutes.4,31,33 The absorbance of the solution was measured at 259nm in a UV spectrophotometer (UV-2700i-Shimadzu).13,34
Data analysis
The in vitro release pattern and kinetics were determined by various kinetic models. Whereas the zero-order rate indicates that release rate does not depend on its concentration, the first order designates the concentration-dependent release. 13 Higuchi (1963) defined the release as a square root of the time-dependent method based on Fickian diffusion. The Hixson-Crowell law has shown the release pattern where there is an alteration in the surface area and radius of drug particle . 17–19
Successive fractional dissolution time
The dissolution time, T25%, T50%,T80% and MDT were evaluated.
Statistical analysis
One-way analysis of variance (ANOVA) followed was done.4
Results and discussion
Yield and Drug Entrapment Efficiency (DEE)
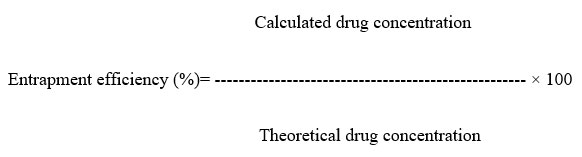
As the RPM increased, the yield value for the formulations decreased accordingly. The 100% yield (%) values were not achieved probably due to materials loss or evaporation of the solvent. A yield value greater than 100% was achieved, perhaps due to incomplete washing and drying of the microspheres. Figure 1 indicates that, for microspheres (formulations DF1-DF5) prepared with HPMC K100 M CR and Eudragit RL 100 at different rpm, the Yield (%) decreases with an increase in the rpm. Figure 1 indicates an opposite relationship between the DEE and the RPM. The bar diagram displays that the DEE decreases as RPM increases. The maximum DEE is 97.57% for DF1 (600 RPM), and the minimum DEE is 79.84% for DF5 (1000 RPM).
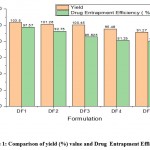 |
Figure 1: Comparison of yield (%) value and Drug Entrapment Efficiency (%) of formulation DF1-DF5 |
Table 2: Impact of RPM on the Yield and Drug Entrapment Efficiency (DEE)
| Variable used | Regression coefficient
|
P-value
|
| Yield (%) | -0.030260 | 0.00708 |
| DEE (%) | -0.046960 | 0.00369 |
Impact of RPM change on Yield (%) and Drug entrapment efficiency (%) using Simple Linear Regression Model (Level of significance, 0.05) was analyzed. Table 2 statistically shows that, with an increase in the stirring rate (rpm) by 1 rpm, drug entrapment efficiency decreases by 0.030260%. P-value has been observed to be 0.00708 for formulations DF1-DF5, which is less than 0.05. So, the effect of the change in stirring rate (rpm) on Yield (%) is statistically significant.
Table 2 also indicates that, for DF1-DF5, the drug entrapment efficiency (%) decreases with an increase in the rpm. With an increase in the stirring rate (rpm) by1 rpm, drug entrapment efficiency decreases by 0.046960 %. P-value is 0.00369 (level of significance 0.05) for formulations DF1-DF5 which is less than 0.05. Therefore, the change in stirring rate (rpm) on drug entrapment efficiency is statistically significant.
Results of Micromeritics Study
The flow property of the microspheres was evaluated based on bulk density, tapped density, Carr’s compressibility measure, Hausner ratio.
Table 3: Micromeritics study of DF1-DF5
| Formulation | Bulk Density (gm/ml) | Tapped Density (gm/ml) | Carr’s Compressibility Index | Hausner Ratio |
| DF1 | 0.10 | 0.11 | 11.76 | 1.13 |
| DF2 | 0.10 | 0.12 | 12.24 | 1.14 |
| DF3 | 0.11 | 0.13 | 15.56 | 1.18 |
| DF4 | 0.11 | 0.13 | 15.79 | 1.19 |
| DF5 | 0.10 | 0.12 | 16.98 | 1.20 |
Surface Morphology Study
Table 3 indicates that the formulations at different rpm exhibit good to fair flow properties. DF1(600 rpm) showed the best texture and flowability, whereas the most unexpected flow properties were indicated by DF5 (1000 rpm).
Figure 2 reveals the combined effect of HPMC K100 M CR and Eudragit RL100 at 600 rpm on the texture of formulations. The particles were spherical, and the surface was not rough. Figure 3 reveals the combined effect of HPMC K100 M CR and Eudragit RL100 at 1000 rpm on the morphology of the microsphere. The shape of the microspheres is not well-defined, and the surface of the particles is highly rough. As the rpm increases, the surface becomes rougher and more porous.
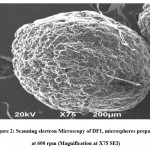 |
Figure 2: Scanning electron Microscopy of DF1, microspheres prepared at 600 rpm (Magnification at X75 SEI) |
 |
Figure 3: Scanning electron Microscopy of DF5 (1000 rpm) (Magnification at X100 SEI) |
Dissolution and Kinetic Studies
The dissolution study of microspheres of Fexofenadine HCl was done in USP Type II dissolution apparatus (Copley Scientific DIS600i). The data of dissolution studies are described in five different segments. Microspheres prepared at different rpm (600 rpm,700 rpm,800 rpm,900 rpm,1000 rpm) and various kinetics suitability were examined for the data for investigating their release pattern.
 |
Figure 4: In vitro release kinetics of five formulations (DF1-DF5) of microspheres A. Zero Order Release. B. First Order Release C. Higuchi plot D. Korsmeyer-Peppas plot E. Hixson-Crowell plot. |
Table 4: Drug release kinetics of formulations DF1-DF5
| Formulation | Zero Order
|
First Order
|
Higuchi
|
Korsmeyer Peppas
|
Hixson Crowell
|
||||||
| K0 | R2 | K1 | R2 | KH | R2 | n | KKP | R2 | KHC | R2 | |
| DF1 | 7.725 | 0.953 | -0.196 | 0.960 | 27.17 | 0.985 | 0.450 | 0.292 | 0.996 | 0.216 | 0.980 |
| DF2 | 7.904 | 0.946 | -0.218 | 0.957 | 27.9 | 0.985 | 0.434 | 0.313 | 0.963 | 0.232 | 0.979 |
| DF3 | 8.102 | 0.945 | -0.251 | 0.927 | 28.6 | 0.983 | 0.423 | 0.329 | 0.956 | 0.253 | 0.970 |
| DF4 | 7.961 | 0.916 | -0.289 | 0.899 | 28.49 | 0.980 | 0.369 | 0.382 | 0.951 | 0.272 | 0.960 |
| DF5 | 7.874 | 0.898 | -0.346 | 0.764 | 28.46 | 0.981 | 0.353 | 0.405 | 0.963 | 0.290 | 0.926 |
Successive fractional dissolution time of DF1- DF5
Successive fractional dissolution times of five formulations of Fexofenadine HCl microspheres were calculated and presented in Figure 5. T25%, T50%, T80% and MDT values were evaluated to interpret the release pattern of drug from the formulations and the retentive efficiency of the polymers.
 |
Figure 5: Successive fractional dissolution time of DF1- DF5. |
DF1 was the batch of Fexofenadine HCl microspheres prepared at 600 rpm. The rate of drug release ( %) after 1 hour, 5 hours, and 10 hours was 29.81%, 57.75%, and 89.06%, respectively. Higuchi(R2=0.985), Korsmeyer- Peppas(R2=0.996), and Hixson-Crowell (R2=0.980) were the best-fitted model for DF1, and the release mechanism was non-Fickian type (. Table 4 shows that, for DF2 (prepared at 700 rpm) and DF3 (prepared at 800 rpm), R2 value shows that the best-fitted model was Higuchi and Hixson-Crowell and the n value indicates the release mechanism following non-Fickian transport. For DF4 (prepared at 900 rpm) and DF5 (prepared at 1000 rpm), the most suitable model was Higuchi (R2=0.981), and they also followed the Quasi-Fickian transport mechanism. So, it can be inferred that all of these formulations have a sustained release effect, but with an increase in rpm, the sustaining effects decrease. Analysis of the impact of different rpm on the duration for 50% of drug release (T50%) at 95% confidence level indicates that the impact is statistically significant. AS in all ca
The duration expected for 50 % of drug release (T50 percent) for microspheres DF1-DF5 decreases with a rise in rpm. Statistically, it indicates that T50 % falls by 0.003853 hours, increasing the stirring speed (rpm) by 1 rpm. Given the significance level of 0.05 for DF1-DF5 formulations, the P-value was calculated to be 0.001133. This shows that the value of P is < 0.05. Therefore, it can be inferred that there is a statistically significant influence of the stirring speed (rpm) on T50 %.
Conclusion
The findings of our study indicate that the stirring significantly affects the surface morphology and flow properties of the microspheres. The study demonstrated that the rpm also has significant effects on the release pattern of the drug from the microsphere dosage form, and its optimization is important. With an increase of stirring rate from 600 rpm to 1000 rpm, the morphology of the microspheres became unsatisfactory as the surface became rougher and flow properties declined. Using 600 rpm, we found the microspheres with the most acceptable flow properties, satisfactory surface morphology, and highest sustaining release effects. So, DF1 is the best formulation among all the batches of microspheres prepared. But lower rpm was found to be inconvenient for microsphere preparation. So, we can conclude that stirring speed is a significant formulation parameter to be optimized for obtaining microsphere formulations with desired properties.
Acknowledgement
We are grateful to the Bangladesh Council of Scientific and Industrial Research (BCSIR), Centre for Advanced Research (CARS), University of Dhaka and University of Asia Pacific, Bangladesh for the research facilities. We would like to thank Dr. Md. Aftab Ali Shaikh, Professor, Department of Chemistry, University of Dhaka and Chairman, Bangladesh Council of Scientific and Industrial Research for his immense support and contribution in providing the research facilities for the work.
Conflict of Interest
This research has no conflict of interest.
Funding Source
The research has been funded by Bangladesh Council of Scientific and Industrial Research
References
- Lei L, Zhu Y, Qin X, et al. Magnetic biohybrid microspheres for protein purification and chronic wound healing in diabetic mice. Chem Eng J. 2021;425:130671. doi:10.1016/j.cej.2021.130671
CrossRef - Lengyel M, Kállai-Szabó N, Antal V, Laki AJ, Antal I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci Pharm. 2019;87(3). doi:10.3390/scipharm87030020
CrossRef - Haznedar S, Dortunç B. Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int J Pharm. 2004;269(1):131-140. doi:10.1016/j.ijpharm.2003.09.015
CrossRef - Arefin P, Hasan I, Islam MS, Reza MS. Formulation and In vitro Evaluation of Eudragit RL 100 Loaded Fexofenadine HCL Microspheres. Bangladesh Pharm J. 2016;19(1):58-67. doi:10.3329/bpj.v19i1.29240
CrossRef - Suzuki D, Horigome K, Kureha T, Matsui S, Watanabe T. Polymeric hydrogel microspheres: design, synthesis, characterization, assembly and applications. Polym J. 2017;49(10):695-702. doi:10.1038/pj.2017.39
CrossRef - Arefin P, Habib MS, Ahmed NU, et al. Microspheres and microcapsules: A review of their manufacturing techniques for Pharmaceutical industries. Indian J Nov Drug Deliv. 2020;12(4):177-185.
- Arefin P, Habib MS, Chakraborty D, Bhattacharjee SC, Das S. An overview of microcapsule dosage form. Int J Pharm Chem Anal. 2020;7(4):155-160. doi:10.18231/j.ijpca.2020.025
CrossRef - Xiong B, Chen Y, Liu Y, Hu X, Han H, Li Q. Artesunate-loaded porous PLGA microsphere as a pulmonary delivery system for the treatment of non-small cell lung cancer. Colloids Surfaces B Biointerfaces. 2021;206:111937. doi:10.1016/j.colsurfb.2021.111937
CrossRef - Limpa SI, Islam Z, Reza MS. Comparative Evaluation of Bromhexine HCl Mucoadhesive Microspheres Prepared by Anionic, Cationic and Nonionic Polymers. Bangladesh Pharm J. 2020;23(2):117-124. doi:10.3329/bpj.v23i2.48331
CrossRef - Zhu Y, Chen S, Zhang C, et al. Novel microsphere-packing synthesis, microstructure, formation mechanism and in vitro biocompatibility of porous gelatin/hydroxyapatite microsphere scaffolds. Ceram Int. August 2021. doi:10.1016/j.ceramint.2021.08.111
CrossRef - Wang H, Yan K, Chen J. Preparation of hydroxyapatite microspheres by hydrothermal self-assembly of marine shell for effective adsorption of Congo Red. Mater Lett. 2021;304:130573. doi:10.1016/j.matlet.2021.130573
CrossRef - Wenzhi S, Dezhou W, Min G, Chunyu H, Lanlan Z, Peibiao Z. Assessment of nano-hydroxyapatite and poly (lactide-co-glycolide) nanocomposite microspheres fabricated by novel airflow shearing technique for in vivo bone repair. Mater Sci Eng C. 2021;128:112299. doi:10.1016/j.msec.2021.112299
CrossRef - Arefin P, Hasan I, Reza MS. Design, characterization and in vitro evaluation of HPMC K100 M CR loaded Fexofenadine HCl microspheres. Springerplus. 2016;5(1). doi:10.1186/s40064-016-2322-2
CrossRef - Li G, Yu Y, Han W, et al. Solvent evaporation self-motivated continual synthesis of versatile porous polymer microspheres via foaming-transfer. Colloids Surfaces A Physicochem Eng Asp. 2021;615:126239. doi:10.1016/j.colsurfa.2021.126239
CrossRef - Yu Y, Li G, Han W, et al. An efficient preparation of porous polymeric microspheres by solvent evaporation in foam phase. Chinese J Chem Eng. 2021;29:409-416. doi:10.1016/j.cjche.2020.09.002
CrossRef - Agrawal H, Joshi R, Gupta M. Optimization of pearl millet-derived bioactive peptide microspheres with double emulsion solvent evaporation technique and its release characterization. Food Struct. 2021;29:100200. doi:10.1016/j.foostr.2021.100200
CrossRef - Song X, Xiao W, Wang P, Liao B, Yan K, Zhang J. Hollow glass microspheres-based ultralight non-combustible thermal insulation foam with point-to-point binding structure using solvent evaporation method. Constr Build Mater. 2021;292:123415. doi:10.1016/j.conbuildmat.2021.123415
CrossRef - He X, Ji Y, Xie J, Hu W, Jia K, Liu X. Emulsion solvent evaporation induced self-assembly of polyarylene ether nitrile block copolymers into functional metal coordination polymeric microspheres. Polymer (Guildf). 2020;186:122024. doi:10.1016/j.polymer.2019.122024
CrossRef - Zhu W, Ren J, Wang Z, Han D, Yang H, Guo X. Synthesis of “brain-like” hierarchical porous microspheres by emulsion-solvent evaporation. Mater Lett. 2015;155:130-133. doi:10.1016/j.matlet.2015.04.132
CrossRef - Zafar A, Afzal M, Quazi AM, et al. Chitosan-ethyl cellulose microspheres of domperidone for nasal delivery: Preparation, in-vitro characterization, in-vivo study for pharmacokinetic evaluation and bioavailability enhancement. J Drug Deliv Sci Technol. 2021;63:102471. doi:10.1016/j.jddst.2021.102471
CrossRef - Liu Z, Bu R, Zhao L, et al. Hydrogel-containing PLGA microspheres of palonosetron hydrochloride for achieving dual-depot sustained release. J Drug Deliv Sci Technol. August 2021:102775. doi:10.1016/j.jddst.2021.102775
CrossRef - Pavon C, Aldas M, Rayón E, Arrieta MP, López-Martínez J. Deposition of gum rosin microspheres on polypropylene microfibres used in face masks to enhance their hydrophobic behaviour. Environ Technol Innov. 2021;24:101812. doi:10.1016/j.eti.2021.101812
CrossRef - Cerón AA, Nascife L, Norte S, et al. Synthesis of chitosan-lysozyme microspheres, physicochemical characterization, enzymatic and antimicrobial activity. Int J Biol Macromol. 2021;185:572-581. doi:10.1016/j.ijbiomac.2021.06.178
CrossRef - Shasho H, Sakur AA, Trefi S. SEPARATION AND ASSAY OF FOUR ANTIHISTAMINE DRUGS DIPHENHYDRAMINE, CHLORPHENIRAMINE, CYPROHEPTADINE AND FEXOFENADINE IN PHARMACEUTICAL FORMS BY A SINGLE HPLC METHOD. Int J Pharm Pharm Sci. 2018;10(4):53. doi:10.22159/ijpps.2018v10i4.24819
CrossRef - Bielory L. Fexofenadine hydrochloride in the treatment of allergic disease: a review. J Asthma Allergy. September 2008:19. doi:10.2147/JAA.S3092
CrossRef - Ahn JH, Kim J, Rehman NU, Kim HJ, Ahn MJ, Chung HJ. Effect of rumex acetosa extract, a herbal drug, on the absorption of fexofenadine. Pharmaceutics. 2020;12(6):1-14. doi:10.3390/pharmaceutics12060547
CrossRef - Kaiser HB, Rooklin A, Spangler D, Capano D. Efficacy of Loratadine Compared with Fexofenadine or Placebo for the Treatment of Seasonal Allergic Rhinitis. Clin Drug Investig. 2001;21(8):571-578. doi:10.2165/00044011-200121080-00006
CrossRef - Jalil R, Nixon JR. Microencapsulation using Poly(L-Lactic Acid) I: Microcapsule Properties Affected by the Preparative Technique. J Microencapsul. 1989;6(4):473-484. doi:10.3109/02652048909031167
CrossRef - Gandra S. Enhanced Intestinal Absorption And Bioavailability Via Proniosomes For Bazedoxifene Acetate Drug. Int J Pharm Pharm Sci. November 2020:31-42. doi:10.22159/ijpps.2020v12i12.39576
CrossRef - Dey S, Pramanik S, Malgope A. Formulation and Optimization of Sustained Release Stavudine Microspheres Using Response Surface Methodology. ISRN Pharm. 2011;2011:1-7. doi:10.5402/2011/627623
CrossRef - Sharma M, Kohli S, Dinda A. In-vitro and in-vivo evaluation of repaglinide loaded floating microspheres prepared from different viscosity grades of HPMC polymer. Saudi Pharm J. 2015;23(6):675-682. doi:10.1016/j.jsps.2015.02.013
CrossRef - Swami SB, Sawant AA, Khandetod YP. Microencapsulation techniques used for bioactive food ingredients : An overview. 2020;9(7):40-49.
- Kemala T, Budianto E, Soegiyono B. Preparation and characterization of microspheres based on blend of poly(lactic acid) and poly(ɛ-caprolactone) with poly(vinyl alcohol) as emulsifier. Arab J Chem. 2012;5(1):103-108. doi:10.1016/j.arabjc.2010.08.003
CrossRef - Huh Y, Cho H-J, Yoon I-S, et al. Preparation and evaluation of spray-dried hyaluronic acid microspheres for intranasal delivery of fexofenadine hydrochloride. Eur J Pharm Sci. 2010;40(1):9-15. doi:10.1016/j.ejps.2010.02.002
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.