How to Cite | Publication History | PlumX Article Matrix
Polymeric Micelles: A Novel Approach towards Nano-Drug Delivery System
Rutuja Hemant Vinchurkar and Ashwin Bhanudas Kuchekar*
and Ashwin Bhanudas Kuchekar*
School of Pharmacy, Dr. Vishwanath Karad MIT World Peace University, Pune- 411 038, India
Corresponding Author E-mail: ashwin.kuchekar@mitwpu.edu.in
DOI : http://dx.doi.org/10.13005/bbra/2947
ABSTRACT:
Nano delivery systems, polymeric micelles represent one of the most promising delivery platforms for therapeutic compounds. It has shown that a poorly soluble molecule which has high potency and remarkable toxicity can be encapsulated with the polymeric micelle. There are various poorly soluble drugs used in micellar preparations, mostly for their anti-cancer activity. Drugs in the inner core protect the drug from degradation and allow drug accumulation in the tumour site in the case of cancer treatment. Block copolymers are chosen based on the physicochemical characteristics of medicinal drugs. The amphiphilic block copolymer structure has both lipophilic and hydrophilic blocks, which enclose tiny hydrophobic molecules. It is a targeted drug delivery method because of its high effectiveness for drug retention in tissue, prevention of enzymes from degradation, and improvement of the cellular absorption mechanism. In an experimental environment, variations in temperature and solvent polarity stimulate copolymer micelle self-assembly. This is a thermodynamically guided procedure in which self-assembly happens by converting polymeric micelles. These aggregates go from a non-equilibrium to a thermodynamically equilibrium state, and they stay stable for a long time. The balance of thermodynamic and kinetic forces is critical in micelles self-assembly because the kinetic process predicts assembly behaviour and hierarchical structure. The purpose of this special issue is to provide an updated overview of micelles, a number of polymers and drugs commonly used in micellar preparation and their application.
KEYWORDS: Anti-Cancer Drugs; Block Polymers; Nano Delivery; Polymeric Micelles
Download this article as:| Copy the following to cite this article: Vinchurkar R. H, Kuchekar A. B. Polymeric Micelles: A Novel Approach towards Nano-Drug Delivery System. Biosci Biotech Res Asia 2021;18(4). |
| Copy the following to cite this URL: Vinchurkar R. H, Kuchekar A. B. Polymeric Micelles: A Novel Approach towards Nano-Drug Delivery System. Biosci Biotech Res Asia 2021;18(4). Available from: https://bit.ly/3Gggc8W |
Introduction
The assembly of polymeric micelles in an aqueous medium is caused by an amphiphilic block copolymer with a core-shell structure with a size range of 10 to 100nm. It has gained much attention for research and is one of the most studied systems1, 2. A micelle’s core can be used to encapsulated drugs that have a low water solubility, thus improving drug solubility, stability, and pharmacokinetic profile as well as stabilizing compounds that are degradable. Targeted drug delivery systems have great efficiency in retaining drugs in the tissues, conserving enzymes, and enhancing uptake mechanisms at the cellular level3,4. Additionally, the EPR effect is used to deliver targeted and localized treatments, which are also advantageous in terms of drug safety profiles 5. A variety of morphologies of subunits like rods, vesicles, and hierarchical morphology result in variations in assembly of polymeric micelles at higher levels as well as kinetic trapping of copolymer micelles in thermodynamic transitions. The purpose of this article is to provide a brief overview of preparation and introduction to micelle for self-assembly drug delivery systems.
Strategies of Drug Targeting 76
Passive Targeting
Drug delivery approaches focused at systemic circulation are referred to as passive delivery systems. Because of the ability of certain colloid to be taken up by Reticulo Endothelial Systems (RES), notably in the liver and spleen, they can be used as a passive hepatic drug target (Fig. 1).
Inverse Targeting
The approach is known as inverse targeting because it seeks to circumvent passive uptake of colloidal carrier by RES. The normal function of the RES is suppressed by pre-injecting massive amounts of blank colloidal carriers or macromolecules like dextran sulphate to accomplish inverse targeting. This strategy results in RES saturation and the inhibition of defence mechanisms. This method of targeting drug(s) to non-RES organs is quite effective.
Active targeting
Instead of spontaneous RES absorption, a drug-carrying carrier system is supplied to a particular location by surface modification in this manner (Fig. 1). Surface modification techniques include coating the surface with a bioadhesive, non-ionic surfactant, specific cell or tissue antibodies (monoclonal antibodies), or albumin protein. Active targeting can be modified on multiple levels, as follows:
First order targeting (organ compartmentalization) – The drug carrier system is only distributed to the capillary bed of a pre-determined target site, organ, or tissue.
Second order targeting (cellular targeting) – The delivery of a medicine to a specific cell type, such as tumour cells, with pinpoint accuracy (& not to the normal cells).
Third order targeting (intercellular organelles targeting) – Drug delivery to the intracellular organelles of the target cells.
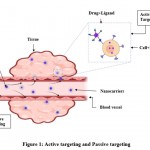 |
Figure 1: Active targeting and Passive targeting |
Block copolymers
Block copolymers are used to encapsulate therapeutic substances into micelles through several interactions which help to shield the medication from the outside surroundings while also assisting in the improvement of the pharmacokinetic profile 6. For smaller molecules such as nucleic acids, proteins and molecular substances, block copolymers are mainly used 7. Depending upon the physicochemical properties of therapeutic compounds, the desired block polymers are used. The lipophilic blocks and hydrophilic blocks are present in the amphiphilic block copolymer structure which encapsulates small hydrophobic molecules 8-10. The core and corona of the polymeric micelles form in such a way that the therapeutic medication is lacking during spontaneous self-assembly of micelles during self-assembly of amphiphilic block copolymers. The small drugs are water-insoluble conjugates with core-forming blocks by the chemical interaction of block copolymers, which then self-assembles to form a polymeric micelle having the drug in the core part. Charged blocks are required for electrostatic interactions in biopolymers for encapsulation in polymeric micelles 11, 12. Amphiphilic block copolymers have hydrophilic shells and a hydrophobic core that, when coming into contact with aqueous media, form micelles by self-assembly. This shell protects the drug in the core and avoids aggregation and precipitation of micelles. In the preparation of polymeric micelles, (P)-hydrophilic and (Q)-hydrophobic blocks are necessary, which are most commonly used to make (P-Q) diblock and (P-Q-P) triblock copolymers. The Q-block, having a hydrophobic character, has been used recently for encapsulation of drugs which have less water solubility as it shows great interaction with poorly soluble drugs 13. Kozlov et al. demonstrated that block copolymers show complex interactions as they have both hydrophilic and hydrophobic blocks present in them, and they are interdependent as they contribute to the microenvironment in the formation of micelles containing less soluble drugs 14. The Luxenhofer group showed that in the case of polymeric micelles with high drug loading capacity, hydrophilic blocks play a key role in polymer and therapeutic drug interactions 15. Each copolymer block serves a critical function in micelle production. The total quantity of loaded drug into the polymeric micelles can influence the external appearance of the micelle, the size of the micelle, and stability in aqueous media. This complex interdependency and length of block structure give a unique capacity for solubilization of therapeutic drugs. However, it is more challenging to understand the interactions and the design of block copolymers 13.
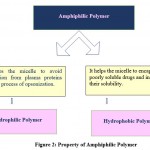 |
Figure 2: Property of Amphiphilic Polymer |
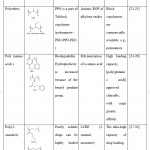 |
Table 1: Hydrophobic polymers used for creating amphiphilic block copolymer |
PDLLA- poly(D,L-lactide), PLGA- poly(D,L-lactide-co-glycolide), PCL- poly(Ɛ-caprolactone), PPO- poly(propylene oxide), PBLA- poly(β-benzyl-l-aspartate), PBLG- poly(γ-benzyl-α l-glutamate), PBuOzi- poly(2-n-butyl-2-oxazine), PPrOzi- poly(2-n-propyl-oxazine), PBuOx- poly(2-n-butyl-2-oxazoline), PiPrOx- poly(2-isopropyl-2-oxazoline), PPrO- poly(2-n-propyl-2-oxazoline)
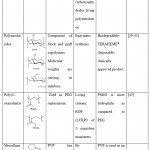 |
Table 2: Hydrophilic polymers used for creating amphiphilic copolymers |
PEG- polyethyleneglycol, mPEG- methoxy-PEG, OH-PEG- hydroxyl-PEG, PMeOx- poly(2-methyl-2-oxazoline), PEtOx- poly(2-ethyl-2-oxazoline), PVP- poly(vinylpyrrolidone), PDMA- poly(N,N-dimethylacrylamide), HPMA- poly[N-(2-hydroxypropyl)methacrylamide], PMMA- poly(methyl methacrylate)
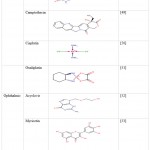 |
Table 3: Poorly soluble drugs used in preparation of micelle formulation |
Methods of preparation
Micelles are prepared with different methods, which are mainly dependent upon the physicochemical properties of the block copolymer [57]. An appropriate method is selected as per the solubility of a block copolymer of the micelle in the aqueous solution. The following are the methods of preparation of polymeric micelles71
Direct Dissolution
Precipitation/Evaporation
Oil in Water Emulsion
Thin Film Hydration
Ultrasonication
Dialysis
Freeze Drying
Direct Dissolution
It is the most commonly used method for micelle formation. Block copolymers with high aqueous solubility are used. In an aqueous medium, the drug is dissolved along with the polymer. It requires stirring and heating for drug loading in the micelle. Dehydration of core-forming blocks initiates the micelle formation. This technique involves the preparation of micelles using a drug and a polymer, mostly poloxamer. Separately, they are dissolved in an aqueous solution. Micelles are formed when both solutions are combined together with an acceptable drug-polymer ratio. Water-soluble copolymers have the advantage of easy drug and copolymer dispersion in water or a buffer. Low encapsulation efficiency is a disadvantage.
Precipitation/evaporation
In this method, a suitable volatile solvent is selected to dissolve both the therapeutic substance and polymer. After dissolution, a slow aqueous phase is incorporated into the mixture. It is then stirred continuously in order to remove the organic media. Withdrawal of the organic phase initiates the formation of the micelles. Precipitation creates pure and homogeneous material, which is its main advantage. It has several disadvantages, including the need for separation of the product after precipitating, and producing a large volume of salt-containing solutions. In addition, if the precipitation is done discontinuously, it is difficult to maintain a consistent product quality.
Oil in water emulsion
Lipophilic drug is solubilized in chloroform, dichloromethane or ethyl acetate solvents which are water-immiscible. Distilled water is added to form an emulsion which is oil in water type. The organic solvent is slowly evaporated to obtained drug-loaded micelles (Fig. 3). An advantage of polymeric micelles is their ease of preparation and uniform size. The difficulty in removing free drugs and organic solvents is a significant drawback.
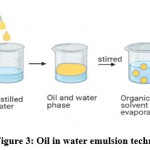 |
Figure 3: Oil in water emulsion technique. |
Thin Film Hydration
Lipophilic drug substances in an organic phase, the copolymer is dissolved. The solvent is evaporated using a rotary evaporator which results in the formation of dry film. Aqueous media is added to the film and it is stirred and sonicated as a result of which drug-loaded micelles are formed (Fig 4).The easiest technique for making liposomes is lipid film hydration, which does not need the use of any expensive or difficult equipment and does not need the use of high pressure or temperature. Drawback is the efficiency of encapsulation is poor for micellar preparation.
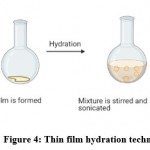 |
Figure 4: Thin film hydration technique |
Ultrasonication
Hydrophilic drugs with amphiphilic polymers are solubilized in aqueous media whereas the organic phase in the hydrophobic rotating evaporator is used to extract the medication. The finished product solution is sonicated to get drug-loaded micelles (Fig 5).Studies such as those conducted by Nakabayashi et al. suggest that nanoemulsions formed by ultrasonication are stable and transparent without surfactant. However, disadvantage of this method is that it can only prepare small batches of nanoemulsions70.
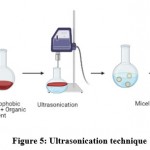 |
Figure 5: Ultrasonication technique |
Dialysis
This method is used when an amphiphilic copolymer has low water solubility. Lipophilic drugs with copolymers are added and mixed in the same organic solvent media as dimethyl sulfoxide (DMSO), N, N-Dimethylformamide (DMF), acetonitrile (ACN), tetrahydrofuran (THF), acetone or dimethylacetamide are used for solubilization purposes. Aqueous media is added to the mixture which stimulates the micelle formation, then it is dialyzed in opposition to water for some period to remove organic solvents (Fig 6).The dialysis method is suitable for hydrophobic drugs and copolymers soluble in organic solvents. Major disadvantage is that it is difficult to remove organic solvents and free drugs from the block copolymer in organic solvents.
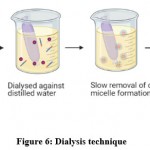 |
Figure 6: Dialysis technique |
Freeze drying
Copolymer, along with therapeutic drugs, is dissolved in a mixture of aqueous solvents with organic solvents like water and Ter-butanol and subjected to lyophilization. The obtained freeze-dried mixture is reconstituted by adding an injectable vehicle that spontaneously forms drug-loaded micelles. Drugs and copolymers are dissolved in a mixture of water and organic solvent, which is advantageous. Risk of residual organic solvents is a disadvantage.
Self-assembly of copolymers
Self-assembly of copolymer micelles is activated by changes in temperature and polarity of solvent in an experimental condition. It is a thermodynamically steered process in which self-assembly occurs by converting from a condition of non-equilibrium polymeric micelles to a state of thermodynamic equilibrium, and these aggregates are steady for a prolonged period (Fig 7). The force that propels the copolymer micelles assembly may be divided into three categories. oleophobic interaction, electrostatic interaction and molecular forces.
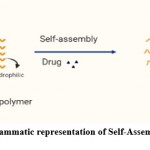 |
Figure 7: Diagrammatic representation of Self-Assembly of Copolymer. |
Solvophobic Interactions
Most commonly, solvophobic interaction is used when non-ionic copolymers are used in micelle preparation. This interaction is increased by a change in solvent, adding a solvophobic agent or by changing the temperature [58]. This type of self-assembly can be categorised into two manners, i.e., theoretical simulation-driven assembly, core-core coupling assembly, corona-corona coupling assembly, and electrostatic interactions.
Core-core coupling assembly
It is most appropriate for polymeric micelles with rod coils as the core and corona forming chain are anisotropically distributed and also parallelly stacked and this influences both rod and coil chain distribution and these loosely located corona forming chains at binding sites can be specified regions.
Corona-corona coupling assembly
Micelles are linked together via corona binding. This attraction reduces the solubility of chains of corona forming units. Compartmentalized corona micelles as well as Janus micelles are the perfect candidates for this.
Theoretical simulation-driven assembly
It provides information about the micelle and its every effect, like attractive and repulsive interactions between the core and the corona. Simulation helps us to understand the polymer chain arrangement. Theoretical simulation gives an approximation of energy variation before and after the assembly of aggregates on a microscopic scale 59.
Electrostatic interactions
Electrostatic forces are one of the primary forces during the formation of polyelectrolyte-based micelles and are one of the most widely researched micelle formation techniques. Various mechanisms that have been observed are as follows
pH-induced copolymeric assembly
It is the most simplistic, yet efficient method for the fabrication of desired nanomaterials. Zhou and colleagues described a pH-induced self-assembling co-polymer vesicle with a hydrophobic core of hyperbranched copolymer poly (3-ethyl-3-oxetanemethanol)-star-PEO (HBPO-star-PEO), an electroneutral layer of PEO, and a positively charged HBPO-star-poly (2-(dimethylamino) ethyl methacrylate (HBPO with an increasing pH from 1M NaOH to 3M NaOH, the copolymer changed its conformation from isotropic to anisotropic due to the formation of PDMAEMA patches that served as binding sites for vesicles to aggregate. The anisotropic micelles formed by pH-induced self-assembly can be further researched and can serve as a direction for future progress 60.
Counter ions triggered assembly
The addition of counter ions in polyelectrolyte-based copolymeric vesicles triggers self-assembly effectively. They can be used for preparing micelles with biological properties, e.g., Chen and his group prepared micelles using a negatively charged plasmid as a base. The negative charge on the plasmid was used as a core in which a saturated deionized CO2 water to PEG-block-poly (4-vinylpyridine) (PEG-b-P4VP) methanol solution was added. The self-assembly progressed through two stages, the first being the one in which DNA wrapped itself around the polymer, leading to the formation of a monodispersed form of ‘beads on a string’. In the second stage, multiple monomers led to the formation of complex polymeric nano-rings. They added spheres in later research containing carboxylate groups on their surface. In this solution, the rings could interact with spheres that resemble ‘host-guest interactions on a nano-scale 61.
Co-assembly by opposite charges
Liu and colleagues reported the co-assembly of copolymer nanofibers bearing carboxyl groups (CNFs) from poly (2-cinnamoyloxyethylmethacrylate)-block-poly (tert-butyl acrylate) (PCEMA-b-PtBA) and nano-cylinders bearing amino groups (ANCs) from poly (tert-butyl acrylate)-block-poly (2- A solution of ANC was added to the CNF, and when the mass ratio of ANC: CNF reached 5:1, wrapping of ANC took place around the CNF. This process is induced by opposite charges of carboxyl and amino groups. It results in the formation of a multi-layered composite cylinder with a PCEMA core, inner-shell PDMAEMA, intermediate-shell PCEMA, and outer-shell PtBA. These cylinders can be utilised as medication delivery devices or even as a contrast agent for imaging systems 62.
Molecular force-directed interactions
Molecular forces are called short-range forces, which include crystallinity, covalent bonds, and host-guest interactions. When the copolymer has a reactive functional group, the molecular forces between these groups and the crystallisation of the chain can lead to the aggregation of copolymer micelles. Crystallization driven self-assembly (CDSA) is the most broadly studied self-assembly in which the core termini possess crystalline faces which stand active for further growth and is composed of crystallised PFS chains. The initial fibre-like micelles are used to prepare micelle seeds, which the termini of get epitaxially crystallised by the unimers, which leads to the formation of uniformly structured fibre-like micelles 63.
Gene therapy
Purpose of Gene therapy has demonstrated to have a lot of promise in treating human diseases caused by gene mutations, such as cancer. When polymer microparticles are compared to polyplexes (complexes of polymer and DNA), polyplexes, in most cases, are more stable. The tiny size of these particles makes them ideal for delivering nucleic acids. Encapsulation, therefore, protects genes much better against serum breakdown than chemical modification. The absence of tumor-selective, safe, efficacious, and non-toxic gene transfer vectors is the fundamental impediment to the actual implementation of cancer gene therapy74. An ideal gene therapy vector should be inert and able to be used as a payload in the cell at the target spot, resulting in effective transfection. The vector should have a buffering capacity for improved transfection, a small enough size and stability to avoid aggregation in the blood, the ability to efficiently target cells and release the DNA intracellularly, and the ability to import the DNA into the nucleus.As non-viral gene carriers, cationic polymers provide the possibility to create carrier systems with unique properties that can be fitted to any system. Micelles can provide the capacity to load genes/gene-drugs into distinct micelle compartments based on the coreshell structure of polymeric micelle systems75.
Classification of polymeric micelles
Different kinds of polymeric micelles exist depending upon their amphiphilic character and the solvent parameters such as type of solvent, concentration of polymer, ionic strength, pH etc. Different micelles can be obtained (Fig 8.) When in the amphiphilic block copolymer, the length is longer for the hydrophilic block and the length is shorter for the hydrophobic block, which gives spherical micelles. In normal micelles, the hydrophobic tail is at the core part and the outer head part has a lipophobic character. The hydrophobic tail flocks to the inside for minimal interaction with aqueous fluids, while the hydrophilic head stays on the outside for maximum contact. It is the most commonly used polymeric micelles as it is easy to prepare as compared to other types of micelles. When the hydrophobic block of micelles is longer, it will try to form rods and lamellae morphologies. Typically, because of the hydrophobic interactions, the blocks form the inner core. When charged block copolymers are used, the electrostatic interaction takes place. Polyion complex micelles are formed as a result of this process. In reverse micelles, the core has a hydrophilic head and the outer part has a hydrophobic tail. These types of micelle formation occur mostly in oil-water mixtures where the amount of water is very small, so the hydrophilic head flocks to the interior, thus water resides inside the micelle. In the case of metal-ligand, hydrogen bonding is used to coordinate and create the complex. It can contribute to the process of micellization. Multi-compartment micelles can be formed by using triblock copolymers which give several well-differentiated compartments, and both the core and the corona can be compartmentalized, enabling concomitant drug loading and release. In triblock copolymer, if the hydrophobic end is small and it has a long hydrophilic chain, then it forms flower-like polymeric micelles 64.
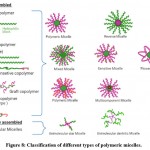 |
Figure 8: Classification of different types of polymeric micelles. |
Challenges and future development
The micelle subunits improve the flexibility of the self-assembly system despite the fact that the mechanism for it is still limited. This is because in self-assembly, many obstacles need to be notified and you still need to get deep inside knowledge. In micelles self-assembly, the balance of thermodynamic and kinetic is very important because the process of kinetics predicts the assembly behaviour and hierarchical structure. The ongoing studies have mostly concentrated on the variation in structures that are seen in aggregates, so the main hurdle is to have an effective path to study the morphological development through the self-assembly process. The goal of future research is to determine the most effective kinetic technique for micelle self-assembly.
Supramolecular polymerization has received significant heed, involving a one-dimensional theory of microscale self-assembly of copolymer micelles. A few research groups have studied the polymerization in supramolecular micelles with various micelles, and Winnik and Manner found that living polymerization reaction characteristics are similar to those of one-dimensional CDSA 65. Lin’s team obtained polypeptide-based polymerization of copolymeric micelles that follows the fundamental rule of the mechanism of step polymerization with characteristics of second-order reaction in polymeric micelles 66. In supramolecular chemistry, the formation of advanced theory on supramolecular polymerization can become an advancing lead.
Theoretical simulations have come up as an effective method to look into micelle assembly as they give information about the microscopic and mesoscopic copolymer assembly of micelles. Zhou et al. found that via condensation–coalescence mechanisms, micelles can be arranged into high-ordered superstructures, but they are still limited and the only study has withdrawn help from the simulation. As a result, simulations are being considered more in the future to solve vexing problems 67.A.B Kuchekar et al utilized statistical PB design for identify influencing variables such as HP β-CD and Eudragit S100 that can be used for investigation.68A.B Kuchekar et al found most significant factors influencing mean particle size and zeta potential of the polymeric micelle formulation.A.B Kuchekar et al conducted preliminary batches employed to screen the significant formulation and process variables of CTB loaded polymeric micelles by Quality-by-Design (QbD) approach69. To treat Influenza virus infection, a multifunctional PLA-b-PEG copolymer modified methyl-b-neuraminic acid (mNA) has been developed by Amisha et. as drug delivery micelles[72].The backbone resonance assignment for NS4A was discovered in micelles. Full-length Dengue NS4A was created for structural investigation, according to Yan Li et al73.
Conclusion
The techniques of micelle formation, polymers utilised, alternative ways of self-assembly, and current advancements in copolymer micelles are included in this study. In general, a micelle should possess an anisotropically distributed composition of polymer subunits. Electrostatic, solvophobic, and molecular force interactions can all be used to control micelle self-assembly. All of these rules can be used to provide instructions in this area and also provide stimulation for future studies. Due to a lack of in-depth understanding of assembly kinetics, there are many challenges ahead and limited information is available on theoretical simulation assembly of micelles.
In order to develop and promote the advances in supramolecular chemistry, we believe this strategy of hierarchical assembly should continue.
Acknowledgement
Each author expresses thanks to School of Pharmacy, Dr. Vishwanath Karad, MIT, WPU, Kothrud for allowing them to work on this review topic.
Conflict of interest
The authors declare no conflicts of interest.
Funding Sources
No specific grant was received from any funding agency.
References
- M. A. Moses, H. Brem, and R. Langer.Advancing the field of drug delivery: taking aim at cancer. Cancer Cell 2003;4(5):337–41.
CrossRef - J. Patel, J. Amrutiya, P. Bhatt, A. Javia, M. Jain, and A. Misra.Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J Microencapsul 2018;35(2):217–204.
CrossRef - C. Caddeo et al.Nanocarriers for antioxidant resveratrol: Formulation approach, vesicle self-assembly and stability evaluation. Colloids Surf. B: Biointerfaces 2013;111:332–327.
CrossRef - A. Kumar, A. Ahuja, J. Ali, and S. Baboota.Curcumin-loaded lipid nanocarrier for improving bioavailability, stability and cytotoxicity against malignant glioma cells. Drug Delivery 2016;23: 229–214.
CrossRef - Y. Shi, R. van der Meel, X. Chen, and T. Lammers.The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020;10:7924–7921.
CrossRef - K. Kataoka, A. Harada, and Y.Nagasaki. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 2001;47:131-113.
CrossRef - H. Cabral, K. Miyata, K. Osada, and K. Kataoka.Block copolymer micelles in nanomedicine applications. Chem Rev 2018;118:6892-6844.
CrossRef - A. V. Kabanov et al.The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles: micelles as microcontainers for drug targeting. FEBS Lett 1989;258:345-343.
CrossRef - R. Savić, A. Eisenberg, and D. Maysinger.Block copolymer micelles as delivery vehicles of hydrophobic drugs: micelle–cell interactions. J Drug Target. 2006;14:355-343.
CrossRef - H. Lee, F. Zeng, M. Dunne, and C. Allen.Methoxy poly(ethylene glycol)-block-poly(deltavalerolactone) copolymer micelles for formulation of hydrophobic drugs. Biomacromolecules. 2005;6:3128-3119.
CrossRef - Y. Kakizawa and K. Kataoka.Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev. 2002;54:222-203.
CrossRef - A. V. Kabanov and V. A. Kabanov.Interpolyelectrolyte and block ionomer complexes for gene delivery: physico-chemical aspects. Advanced Drug Delivery Reviews. 1998;30(1-3):60-49.
CrossRef - D. Hwang et al.Novel poly (2-oxazoline) block copolymer with aromatic heterocyclic side chains as a drug delivery platform. J Control Release. 2019;307:271-261.
CrossRef - M. Y. Kozlov, N. S. Melik-Nubarov, E. V. Batrakova, and A. V. Kabanov. Relationship between pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules. 2000;33:3313-3305.
CrossRef - M. M. Lübtow, H. Marciniak, A. Schmiedel, M. Roos, C. Lambert, and R. Luxenhofer. Ultrahigh to ultra-low drug-loaded micelles: Probing host-guest interactions by fluorescence spectroscopy. Chemistry a European Journal. 2019;25:12610-12601.
CrossRef - K. Shiraishi et al.Determination of polymeric micelles’ structural characteristics, and effect of the characteristics on pharmacokinetic behaviors. Journal of Controlled Release. 2015;203:84-77.
CrossRef - M. Qiao, D. Chen, X. Ma, and Y. Liu. Injectable biodegradable temperature-responsive PLGA–PEG–PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Internatinal Journal of Pharmaceutics. 2005;294(1-2):112-103.
CrossRef - R. Ghasemi et al.MPEG-PLA and PLA-PEG-PLA nanoparticles as new carriers for delivery of recombinant human Growth Hormone (rhGH). Scientific Reports. 2018;8:13-1.
CrossRef - E. A. Rainbolt, K. E. Washington, M. C. Biewer, and M. C. Stefan.Recent developments in micellar drug carriers featuring substituted poly (ε-caprolactone) s. Polymer Chemistry. 2015;6:2381-2369.
CrossRef - X. L. Zheng, B. Kan, Z. Y. QuinPreparation of MPEG–PLA nanoparticle for honokiol delivery in vitro. International Journal of Pharmaceutics. 2010;386(1-2):267-262.
CrossRef - J. Herzberger et al.HPolymerization of ethylene oxide, propylene oxide, and other alkylene oxides: synthesis, novel polymer architectures, and bioconjugation. Chem Rev 2016; 116:2243-2170.
CrossRef - W. Daniel, S. E. Stiriba, and F. Holger.Hyper branched polyglycerols: from the controlled synthesis of biocompatible polyether polyols to multipurpose applications. Acc Chem Res2010;43:141-129.
CrossRef - Y. K. Choi, Y. H. Bae, and S. W. Kim.Star-shaped poly (ether-ester) block copolymers: Synthesis, characterization, and their physical properties. Macromolecules1998; 31:8774-8766.
CrossRef - T. Thambi, H. Y. Yoon, K. Kim, I. C. Kwon, C. K. Yoo, and J. H. ParkBioreducible block copolymers based on poly (ethylene glycol) and poly (γ-benzyl L-glutamate) for intracellular delivery of camptothecin. Bioconjug Chem. 2011;22:1931-1924.
CrossRef - N. Kamaly, B. Yameen, J. Wu, and O. C. FarokhzadDegradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem Rev. 2016;116:2663-2602.
CrossRef - C. M. González-Henríquez, M. A. Sarabia-Vallejos, and J. Rodríguez-HernándezStrategies to fabricate polypeptide-based structures via ring-opening polymerization of N-carboxyanhydrides. Polymers. 2017;9:551.
CrossRef - M. S. Haider, M. M. Lübtow, S. Endres, V. Aseyev, A. C. Pöppler, and R. Luxenhofer.Think Beyond the Core: The Impact of the Hydrophilic Corona on the Drug Solubilization Using Polymer Micelles, 2019.
CrossRef - M. M. Lübtow, L. Hahn, M. S. Haider, and R. Luxenhofer. Drug specificity, synergy and antagonism in ultrahigh capacity poly (2-oxazoline)/poly (2-oxazine) based formulations. J Am Chem Soc. 2017;139:10983-10980.
CrossRef - M. M. Lübtow, M. S. Haider, M. Kirsch, S. Klisch, and R. Luxenhofer. Like Dissolves Like? A comprehensive evaluation of partial solubility parameters to predict polymer-drug compatibility in ultrahigh drug-loaded polymer micelles. Biomacromolecules. 2019;20:3056-3041.
CrossRef - A. C. Pöppler, M. M. Lübtow, J. Schlauersbach, J. Wiest, L. Meinel, and R. Luxenhofer.Loading-dependent structural model of polymeric micelles encapsulating curcumin by solidstate NMR spectroscopy. Angew Chem Int Ed Engl. 2019;58:18546-18540.
CrossRef - Z. He et al. A high capacity polymeric micelle of paclitaxel: Implication of high dose drug therapy to safety and in vivo anti-cancer activity. Biomaterials. 2016;101:309-296.
CrossRef - T. Lorson et al.Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials. 2018;178:280-204.
CrossRef - R. Luxenhofer et al.Doubly amphiphilic poly(2-oxazoline)s as high-capacity delivery systems for hydrophobic drugs. Biomaterials. 2010;31:4979-4972.
CrossRef - F. Li, S. Li, A. El Ghzaoui, H. Nouailhas, and R. Zhuo. Synthesis and gelation properties of PEG− PLA− PEG triblock copolymers obtained by coupling monohydroxylated PEG− PLA with adipoyl chloride. Langmuir. 2007;23:2783-2778.
CrossRef - B. Bogdanov, A. Vidts, A. Van Den Bulcke, R. Verbeeck, and E. Schacht.Synthesis and thermal properties of poly (ethylene glycol)-poly (ϵ-caprolactone) copolymers. Polymer. 1998;39:1636-1631.
CrossRef - S. Cui et al.Amphiphilic star-shaped poly (sarcosine)-block-poly (ε-caprolactone) diblock copolymers: one-pot synthesis, characterization, and solution properties. J Mater Chem B. 2017;690-679.
CrossRef - Y. Deng et al.Poly (εcaprolactone)-block-polysarcosine by ring-opening polymerization of sarcosine Nthiocarboxyanhydride: synthesis and thermoresponsive self-assembly. Biomacromolecules. 2015;16:3274-3265.
CrossRef - A. Birke, J. Ling, and M. Barz.Polysarcosine-containing copolymers: Synthesis, characterization, self-assembly, and applications. Prog Polym Sci. 2018;81:208-163.
CrossRef - K. O. Doh and Y. Yeo.Application of polysaccharides for surface modification of nanomedicines. Ther Deliv. 2012;3:1456-1447.
CrossRef - Y. Il Jeong et al.Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly (DL-lactide-coglycolide) copolymer. Int J Nanomedicine. 2011;6:15-14.
CrossRef - D. H. Kim et al.Antitumor activity of sorafenib-incorporated nanoparticles of dextran/poly (dl-lactide-co-glycolide) block copolymer. Nanoscale Res Lett. 2012;7:15-6.
CrossRef - M. S. Verma, S. Liu, Y. Y. Chen, A. Meerasa, and F. X. Gu. Size-tunable nanoparticles composed of dextran-b-poly (D, L-lactide) for drug delivery applications. Nano Res. 2012;5:61-49.
CrossRef - R. Luxenhofer et al.Poly (2‐ oxazoline) s as Polymer Therapeutics. Macromol Rapid Commun. 2012;33:1631-1613
CrossRef - T. Chen et al. Multi-functional chitosan polymeric micelles as oral paclitaxel delivery systems for enhanced bioavailability and anti-tumor efficacy. International Journal of Pharmaceutics. 2020;30(578):119-105.
CrossRef - C. Liang et al. Π electron stabilized polymeric micelles potentiate docetaxel therapy in advanced-stage gastrointestinal cancer. 2021;266:432-120.
CrossRef - H. Cabral and K. KataokaProgress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:476–465.
CrossRef - N. A. N. Hanafy, M. El-Kemary, and S. Leporatti. Micelles structure development as a strategy to improve smart cancer therapy. Cancers 2018;10:238.
CrossRef - M. Dasgupta, E. Judy, and N. Kishore.Partitioning of anticancer drug 5-fluorouracil in micellar media explored by physicochemical properties and energetics of interactions: Quantitative insights for implications in drug delivery, Colloids and Surfaces B: Biointerfaces 2018; 187:730-110.
CrossRef - W. He, Y. Du, W. Zhou, T. Wang, M. Li, and X. Li. Core-crosslinked nanomicelles based on crosslinkable prodrug and surfactants for reduction responsive delivery of camptothecin and improved anticancer efficacy. European Journal of Pharmaceutical Science. 2020;150:340-105.
CrossRef - W. Zhuang, B. Ma, G. Liu, X. Chen, and Y. Wang. A fully absorbable biomimetic polymeric micelle loaded with cisplatin as drug carrier for cancer therapy, Regenerative Biomaterials. 2018;5(1):8–1.
CrossRef - K. Wang et al.Oxaliplatin-incorporated micelles eliminate both cancer stem-like and bulk cell populations in colorectal cancer. International Journal of Nanomedicine. 2011;6:3218-3207.
CrossRef - A. Varela-Garcia, A. Concheiro, and C. Alvarez-Lorenzo. Soluplus micelles for acyclovir ocular delivery: formulation and cornea and sclera permeability. 2018;1:552(1-2):47-39.
CrossRef - F. Sun et al. New micelle myricetin formulation for ocular delivery: improved stability, solubility and ocular anti-inflammatory treatment. Drug Delivery. 2019;26(1): 585–575.
CrossRef - H. Dong, Y. Li, S. Cai, R. Zhuo, X. Zhang, and L. Liu. A facile one-pot construction of supramolecular polymer micelles from a-cyclodextrin and poly(e-caprolactone). Angew Chem Int Ed. 2008;47:5576–5573.
CrossRef - H. L. Lin, W. T. Cheng, L. C. Chen, H. O. Ho, S. Y. Lin, and C. M. Hsieh.Honokiol/Magnolol-Loaded Self-Assembling Lecithin-Based Mixed Polymeric Micelles (lbMPMs) for Improving Solubility to Enhance Oral Bioavailability, International Journal of Nanomedicine. 2021;26(16):665-651.
CrossRef - H. K. Manjili, H. Malvandi, M. S. Mousavi, E. Attari, and H. Danafar.In vitro and in vivo delivery of artemisinin loaded PCL–PEG–PCL micelles and its pharmacokinetic study, Artificial Cells, Nanomedicine and Biotechnology an International Journal. 2018; 46(5):936-926.
CrossRef - A. V. Samrot et al.Production and Characterization and application of nanocarriers made of polysaccharides, proteins, bio-polyesters and other biopolymers: A review. Int J Biol Macromol. 2020;165:3105–3088.
CrossRef - Y. Lu, J. Lin, L. Wang, L. Zhang, and C. Cai.Self-assembly of copolymer micelles: higher-level assembly for constructing hierarchical structure. Chemical Reviews 2020;120(9):4140–4111.
CrossRef - B. Fang, A. Walther, A. Wolf, Y. Xu, J. Yuan, and A. H. E. Müller.Undulated multicompartment cylinders by the controlled and directed stacking of polymer micelles with a compartmentalized corona. Angew Chem Int Ed. 2009;48:2880−2877.
CrossRef - H. Jin, Y. Zhou, W. Huang, and D. Yan.Polymerization-like multilevel hierarchical self-assembly of polymer vesicles into macroscopic superstructures with controlled complexity. Langmuir. 2010;26:14519−14512.
CrossRef - A. Hanisch, A. H. Gröschel, M. Förtsch, T. I. Löbling, F. H. Schacher, and A. H. E. Müller.Hierarchical self-assembly of miktoarm star polymers containing a polycationic segment: A general concept. Polymer. 2013;54:4537−4528.
CrossRef - X. Li, G. Liu, and D. Han.Wrapping amino-bearing block copolymer cylinders around carboxyl-bearing nanofibers: A case of hierarchical assembly. Soft Matter. 2011;7:8223−8216.
CrossRef - K. J. M. Bishop, C. E. Wilmer, S. Soh, and B. A. Grzybowski.Nanoscale forces and their uses in self-assembly. Small. 2009;5:1630-1600.
CrossRef - S. M. N. Simões, A. R. Figueiras, F. Veiga, A. Concheiro, and C. Alvarez-Lorenzo Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert Opin Drug Delivery. 2015;12(2):318-297.
CrossRef - J. B. Gilroy et al. Monodisperse cylindrical micelles by crystallization-driven living self-assembly. Nat Chem. 2010;2:570−566.
CrossRef - Z. Zhuang et al. Hierarchical nanowires synthesized by supramolecular stepwise polymerization. Angew Chem Int Ed. 2016;55:12527−12522.
CrossRef - Y. Zhou, X. Ma, L. Zhang, and J. Lin. Directed assembly of functionalized nanoparticles with amphiphilic diblock copolymers. Phys Chem Phys. 2017;19:18766−18757.
CrossRef - A.Kuchekar, A.Pawar. Screening of factors using plackettburman design in the preparation of capecitabine-loaded nano polymeric micelles.Int J Pharm Pharm Sci.2014;6(4):489-496.
- Kuchekar, A. Pawar. Capecitabine loaded polymeric micelles: Formulation, characterization and cytotoxicity study.International Conference on Advanced Nanomaterials & Emerging Engineering Technologies:4:412-415.
- K. Nakabayashi, T. Fuchigami, and M. Atobe, “Tandem acoustic emulsion, an effective tool for the electrosynthesis of highly transparent and conductive polymer films,” Electrochim. Acta. 2013;110: 593–598.
CrossRef - S. M. N. Simões, A. R. Figueiras, F. Veiga, A. Concheiro, and C. Alvarez-Lorenzo, “Polymeric micelles for oral drug administration enabling locoregional and systemic treatments,” Expert Opinion on Drug Delivery. 2015;12(2): 297-318.
CrossRef - M. Chakravarty and A. Vora, “Nanotechnology-based antiviral therapeutics,” Drug Delivery and Translational Research. 2021
CrossRef - Y. Li, M. Y. Lee, Y. R. Loh, and C. B. Kang, “Secondary structure and membrane topology of dengue virus NS4A protein in micelles,” Biochim. Biophys. Acta – Biomembr., 2018;1860(2):442-450.
CrossRef - S. Tiwari, M. Gupta, and S. P. Vyas, “Nanocarrier Mediated Cytosolic Delivery of Drug, DNA and Proteins,” Proc. Natl. Acad. Sci. India Sect. B Biol. Sci., 2012;82(S1):127–150
CrossRef - D. K. Chatterjee et al., “Convergence of nanotechnology with radiation therapy-insights and implications for clinical translation,” Transl. Cancer Res., 2013; 2(4):256–268.
- Bhargav E, Madhuri N, Ramesh K, Anand manne, Ravi V. Targeted Drug DeliveryA Review, World J. Pharm. Pharm. Sci. 2013;3(1):150 -169

This work is licensed under a Creative Commons Attribution 4.0 International License.





