How to Cite | Publication History | PlumX Article Matrix
Cocrystal Formulation: A Novel Approach to Enhance Solubility and Dissolution of Etodolac
Sapana P. Ahirrao* , Mayur H. Sonawane, Deepak S. Bhambere
, Mayur H. Sonawane, Deepak S. Bhambere , Pavan B. Udavant
, Pavan B. Udavant , Eknath D. Ahire
, Eknath D. Ahire , Rupali Kanade
, Rupali Kanade and Dinesh kuber
and Dinesh kuber
MET’s Institute of Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, India-422 003
DOI : http://dx.doi.org/10.13005/bbra/2971
ABSTRACT:
Etodolac (ETD) is a non-steroidal anti-inflammatory drug (NSAID) given in rheumatoid arthritis treatment. As it comes under BCS class II drug hence it exhibits low water solubility. Also, its dissolution rate-limited oral absorption results in delayed onset of action. The Novel approach in the solubility enhancement field; crystal engineering was preferred to prepare pharmaceutical cocrystals of etodolac with GRAS (generally recognized as safe) molecules. Pharmaceutical cocrystals of etodolac were prepared with p-hydroxybenzoic acid and glutaric acid with the drug: coformer ratio 1:1 and 1:2. Cooling cocrystallization was used to prepare etodolac cocrystals. Cocrystal formulations were characterized by saturation solubility study, in-vitro dissolution studies, and stability study. Cocrystal was also characterized by analytical parameters like Fourier transform infrared spectroscopy (FTIR), powder X-ray diffraction (PXRD), and differential scanning calorimetry (DSC). Optimized Cocrystal formulation dissolved more rapidly and their equilibrium solubility is greater than the plain drug.
KEYWORDS: Cocrystals; Crystallization; Etodolac; Dissolution; X-ray diffraction
Download this article as:| Copy the following to cite this article: Ahirrao S. P, Sonawane M. H, Bhambere D. S, Udavant P. B, Ahire K. D, Kanade R, Kuber D. Cocrystal Formulation: A Novel Approach to Enhance Solubility and Dissolution of Etodolac. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Ahirrao S. P, Sonawane M. H, Bhambere D. S, Udavant P. B, Ahire K. D, Kanade R, Kuber D. Cocrystal Formulation: A Novel Approach to Enhance Solubility and Dissolution of Etodolac. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/382RoFr |
Introduction
The study of pharmaceutical co-crystals in the context of crystal engineering continues to be an exciting and rewarding endeavor which allows modifications to be introduced to the crystalline form of an API, which in turn can alter its physicochemical properties, without compromising its intended biological activity. Zaworotko and co-employees described those as ‘co-crystals which might be fashioned among a molecular or ionic energetic pharmaceutical ingredient (API) and a co-crystal former that could be a strong below ambient conditions.’ Majority of co-crystals are built with robust hydrogen bonds and there is possibility that the proton involved in the hydrogen bonding interaction is transferred from the donor (acid) to the acceptor (base) to form a salt. Pharmaceutical solid forms exist in different forms like dissimilar polymorphs of an API, hydrates or solvates, in addition to salts, co-crystals, and amorphous materials, inclusive of amorphous dispersions. Co-crystals are investigated to increase the solubility, bioavailability, and/or other physical or chemical deficits of a taken API1. Cocrystals is homogenous crystalline phase with well-defined stoichiometry found to be novel technique for solubility enhancement due to their ability to modify the solubility properties of nonionizable drugs that cannot otherwise form pharmaceutical salts. Compared to polymorphs, cocrystals have the ability to increase solubility by orders of magnitude above the drug solubility and also in contrast to amorphous pharmaceutical forms; cocrystals can achieve thermodynamic stability in the solid state while providing large solubility advantage over a plain drug2.
The discovery of new drugs carried out in pharmaceutical companies is a time-consuming and costly process 3. Among the newly discovered drugs, more than 60% of new drug molecules exhibit poor aqueous solubility 4. Various researchers have developed different approaches for solubility enhancement of poorly soluble/water-insoluble drugs like salt formation, solid dispersion, size reduction, and complexation 5-12.
Salt formation is one of the basic methods used to transform the physical characteristics of APIs and almost half of the BCS Class II drugs are formulated as salts to improve solubility. However, a requirement for the Salt formation method is that the API ought to own a suitable (simple or acidic) ionizable site. But, co-crystals suggest a dissimilar ways, where any API, in spite of of acidic, basic, or ionizable groups, might hypothetically be co-crystallized. Hence cocrystal formation is considered to be a convenient and novel crystal engineering technique in the arena of solubility enhancement. Co-crystals are formulated by various techniques such as slowly evaporation at room temperature 13, 14, reaction co-crystallization 15, 16, cooling co-crystallization 17, 18, grinding method 19, 20, and supercritical fluid technique 21, 22. By considering the pharmaceutical application of crystal engineering, it can be concluded that co-crystal may be a useful and successful strategy in improving the apparent solubility (combination of dissolution and solubility) of BCS Class II and class IV drugs which is the major problem in the expansion of optimized formulations of new chemical entities (NCEs) discovered in pharmaceutical industries 23, 24.
The current study deals with cocrystals formation of BCS class II drug; etodolac. The prescribed dose of etodolac is 300 mg orally twice to thrice a day and multiple administrations, making it difficult for patient compliance and convenience. Limited dissolution and poor absorption of etodolac associated with pharmacokinetic variation in rheumatoid arthritis patients. In the present work, etodolac cocrystals were prepared using suitable coformers; p-hydroxybenzoic acid and glutaric acid by varying drug: coformer ratio of 1:1 and 1:2 by cooling crystallization method. The physical nature of nebivolol hydrochloride and prepares cocrystals have been characterized via way of differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and IR spectroscopy.
Material and Methods
Materials
Etodolac has been received as a gift sample from IPCA Lab, Mumbai. All other chemical compounds and solvents used in this research had been analytical grade and procured from Loba Chemie Pvt. Ltd., Mumbai.
Etodolac Solubility Analysis
Excess quantities of etodolac, etodolac glutaric acid cocrystals were added in 10 ml of distilled water in a volumetric flask sealed with aluminum foil. The volumetric flasks have been kept in a shaker at 37±0.5°C for 24 hours. The solutions have been filtered via a 0. 45 μm Millipore filter and the filtrate became analyzed spectrophotometrically (Shimadzu Co, Japan) at 248 nm.
Synthesis of Etodolac Cocrystals
Trial batches of etodolac cocrystal were prepared using coformers; p-hydroxybenzoic acid and glutaric acid which have the ability for the formation of hydrogen bonding (good proton donor and acceptor). But cocrystal formulation batches of glutaric acid results in increased aqueous solubility and dissolution rate of etodolac22. Hence glutaric acid is considered to be a more appropriate coformer for further etodolac cocrystal formulation. The cooling crystallization method was preferred to prepare etodolac cocrystal where etodolac and glutaric acid were taken in ratios of 1:1 and 1:2. Etodolac was dissolved in methanol and glutaric acid was dissolved in distilled water. The drug solution was added to the coformer solution. The resulting mixture was kept in the refrigerator overnight and filtered to obtain the cocrystal. The solution was then filtered to remove insoluble.
Drug-Excipients Compatibility Study
Thermal evaluation of the etodolac was carried out using differential scanning calorimeter ( Mettler Lab Star Switzerland).Samples of pure drug, glutaric acid and cocrystal formulation kept in glass vials which were hermetically sealed aluminum and heated from 20° to 300°C and the heating rate was 10°C/min and using consistent purging dry nitrogen flow (100 ml/min).25. Further drug excipient compatability was performed by Infrared spectroscopy using Shimadzu FTIR spectrometer by diffused reflectance method. The scanning range was 400 to 4000 cm–1.
Particle Morphology and Surface Roughness Analysis
The external morphology of co-crystals of etodolac turned into decided with the aid of using SEM (Joel® JSM-6390LV) method. The product for evaluation turned into sprinkled over the double adhesive tape connected with an aluminium stub and at last, the stub containing the product turned into located with inside the chamber. The product turned into scanned arbitrarily at 10 kV acceleration voltage and the photomicrographs have been taken 25,26.
Characterization of Physical/Chemical State of Drug Present
Powder X-ray diffraction data recording was done by means of a powder X-ray diffractometer (Bruker AXS D8 Advance, Germany). Conditions used for measurement: Si(Li) PSD detector, Cu X-ray source (λ = 1.5406 Å), 3° to 135° angular range. Powder X-ray diffraction data obtained compared with bulk drug and co-formers 25,27.
Intrinsic Dissolution Rate Measurement
Etodolac and selected co-crystal equivalent to drug dose etodolac glutaric acid cocrystal were subjected to dissolution study in phosphate buffer pH 6.8 at 37±0.5 °C and 50 RPM using Type II (paddle type) dissolution apparatus. The samples had been withdrawn at 10 mins time c programming language for 60 mins and changed with a clean dissolution medium. Immediately, samples had been filtered through Whatman filter paper, diluted appropriately, and analyzed with the aid of using UV spectrophotometer. (Shimadzu Co, Japan).
Stability Study of Prepared Formulation
In the stability analysis, samples of cocrystals had been placed in USP type I glass vials and hermetically sealed with rubber plugs and aluminum caps. Vials had been stored in a stability chamber and maintained storage condition according to ICH guidelines (40 ± 5 °C and 75% RH). The samples of cocrystals (n = 3) had been taken out on the interval of 0, 1, 2, and three months and the physical properties, drug content, and solubility had been determined.
Results and Discussion
Solubility Analysis
Solubility of etodolac and etodolac glutaric acid cocrystals in distilled water are mentioned in the following table.
Table 1: Obtained Solubility of Etodolac and Cocrystals
| Sample title | Solubility* (mg/ml) |
| Etodolac | 0.3178 ±0.025 |
| Etodolac glutaric acid cocrystals with drug:coformer ratio 1:1 | 1.150±0.030 |
| Etodolac glutaric acid cocrystals with drug:coformer ratio 1:2 | 1.220 ±0.028 |
* Mean ± SD, n = 3
Solubility of pure etodolac showed 0.3178 mg/ml concentration at 24 h with continuous stirring. Co-crystals of etodolac glutaric acid with the drug: coformer ratios 1:1 and 1:2 showed an increase in aqueous solubility of 2.150mg/ml and 2.220 mg/ml respectively (Table 1). Etodolac glutaric acid cocrystals with a 1:1 ratio show a 3.61-fold increase and Etodolac glutaric acid cocrystals with a 1:2 ratio show a 3.83-fold increase in solubility which indicates that cocrystal formulation would have significant potential in the field of solubility enhancement of poorly soluble drugs 27,28.
Drug-Excipients Compatibility Study
DSC data recorded for plain etodolac, glutaric acid, and its cocrystals are shown in Fig. 5.
DSC thermogram for plain etodolac showed a sharp endothermic peak at 149.07 °C (consequent to its melting point) and glutaric acid showed only one sharp endothermic peak at 96.21°C corresponding’s to its melting point. Etodolac glutaric acid cocrystals showed a sharp endothermic peak at 149.45 °C which indicates that there is a minor shift in peak when compared with the endotherm of the pure drug sample. The vanishing of the endothermic peak leads to the melting of glutaric acid is due to its solubility at a lower concentration in the cocrystal formulation.
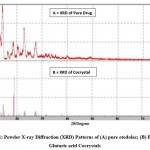 |
Figure 1: Powder X-ray Diffraction (XRD) Patterns of (A) pure etodolac; (B) Etodolac Glutaric acid Cocrystals |
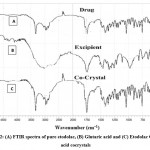 |
Figure 2: (A) FTIR spectra of pure etodolac, (B) Glutaric acid and (C) Etodolac Glutaric acid cocrystals |
Interaction among drug and cocrystal was studied by FTIR Spectroscopy. FTIR spectra of a drug, glutaric acid, and cocrystal are shown in figure 4. Characteristic peaks of etodolac infrared spectra due to various functional groups like C=C, C-H, C=O, N-H, O-H are shown in table 2.
In addition to the characteristic peaks of etodolac, few additional peaks were observed in cocrystals of etodolac due to the construction of hydrogen bonding between drug and co-former in cocrystals. In etodolac glutaric acid cocrystals, a peak was found in the range of 1475-1600 cm-1due to a carboxylic acid moiety which designate the presence of C=O stretching. Another peak was observed in the range of 1330-1450 cm-1 which point out the presence of C-O stretching. A peak in the range of 2950-3050 cm-1 stretch shows hydrogen bonding.
Table 2: FTIR Data of etodolac
| Range | Absorption | Type of bond |
| 3000–3400 | 3057cm-1 | C=C stretching |
| 1400–1500 | 1458.18 cm-1 | C-H stretching |
| 1600-1800 | 1710 cm-1 | C=O stretching |
| 2800-3000 | 2980 cm-1 | O-H stretching |
| 1600-1800 | 1745 cm-1 | COOH stretching |
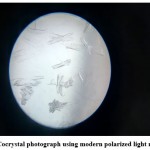 |
Figure 3: Cocrystal photograph using modern polarized light microscope |
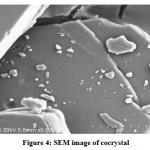 |
Figure 4: SEM image of cocrystal |
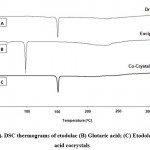 |
Figure 5: (A). DSC thermograms of etodolac (B) Glutaric acid; (C) Etodolac glutaric acid cocrystals |
Table 3: Stability data of optimized cocrystal formulation
| Time Interval | Solubility* (mg/ml) of cocrystals formulation with a drug:coformer ratio 1:1 | Solubility* (mg/ml) of cocrystals formulation with a drug:coformer ratio 1:2 | Drug content* (%) of cocrystals formulation with a drug: coformer ratio 1:1 | Drug content* (%) of cocrystals formulation with a drug: coformer ratio 1:2 |
| Initial | 1.150±0.020 | 1.220 ±0.015 | 98.87±0.15 | 99.10±0.1 |
| One month | 1.152±0.029 | 1.228±0.022 | 99.07±0.80 | 99.02±0.1 |
| Two month | 1.148±0.035 | 1.30±0.018 | 99.30±0.60 | 98.80±0.1 |
| Three Month | 1.149±0.015 | 1.301±0.023 | 98.90±0.35 | 99.15±0.1 |
Table 4: In-vitro dissolution study data of cocrystals throughout stability study
| Time Interval | In-vitro dissolution rate (%) of co-crystals formulation with a drug:coformer ratio of 1:1 | In-vitro dissolution rate (%) of co-crystals formulation with a drug:coformer ratio of 1:2 |
| Initial | 80.99±0.650 | 91.10±0.501 |
| One month | 79.97±0.915 | 90.80±0.810 |
| Two month | 81.09±1.080 | 91.05±0.110 |
| Three Month | 80.25±0.885 | 91.50±0.850 |
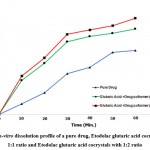 |
Figure 6: In-vitro dissolution profile of a pure drug, Etodolac glutaric acid cocrystals with 1:1 ratio and Etodolac glutaric acid cocrystals with 1:2 ratio |
Particle Morphology and Surface Roughness Analysis
Cocrystal photograph obtained using Modern polarized light microscope shown in Figure 3 and SEM image shown in Figure 4. Surface properties and cocrystal formation are indicated from the SEM image. A microscopic photograph indicates the formation of needle-like crystals as shown in Figure 3.
Characterization of Physical/Chemical State of Drug Present
Powder X-ray diffraction data recorded for plain etodolac, glutaric acid, and its cocrystals is shown in Fig.2t The XRD pattern of cocrystals with etodolac: glutaric acid ratio 1:1 and cocrystals with etodolac: glutaric acid ratio 1:2 shows the generation of new additional peaks with increased intensities. X-ray diffraction pattern of pure drug shows 3 strongest peaks at 13.580°, 14.452°and 22.948° at 2θ; series of sharp and intense diffraction peaks were emphasized the crystalline state of pure Etodolac. X-ray diffraction pattern of cocrystal shows sharp peaks at 14.440°, 18.540°, 18.760° and 18.998° at 2θ. When the X-ray diffraction pattern of pure drug and cocrystal was compared, additional peaks were observed at 18.540°, 18.760°, and 18.998° which designated the development of cocrystals.
Intrinsic Dissolution Study
In-vitro dissolution data of plain drug and etodolac cocrystal system given in Figure no.1. Dissolution of cocrystal and the plain drug became conducted in pH 6.8 phosphate buffer and cocrystal exhibited a quicker dissolution rate than plain drug. The dissolution of etodolac became 50.24% in 60 mins while cocrystal with a drug: coformer ratio of 1:1 confirmed 79.99 and cocrystal with a drug: coformer ratio of 1:2 confirmed 90.10% drug release in 60 mins. At each time point quantity of drug released from cocrystal became constantly greater than that of plain drug. As per the results obtained, a good enhancement in the dissolution rate in co-crystals was observed in comparison with pure drugs.
Stability Study
Stability study of cocrystals was conducted at 40 ± 5 °C/75 ± 5% RH for the period time of three months. During this period, cocrystal remained stable without any significant changes which were confirmed after physicochemical characterization. The results found for drug content and solubility throughout the stability experimentation is revealed in Table 2 and Table 3.
Conclusion
Cocrystals formulation of Etodolac was formulated with glutaric acid by cooling cocrystallization showed a significant increase in solubility and dissolution rate as compared to the parent compound. Optimized cocrystal formulations were characterized by numerous analytical methods FTIR, DSC, and XRD which confirmed the formation of etodolac glutaric acid cocrystal. From results obtained from solubility, dissolution, and stability studies (refer to Table 1, figure1, Table 3, and Table 4) it can be concluded that the cocrystal formulation is effective in increasing the solubility and dissolution rate of etodolac. Etodolac and glutaric acid in equimolar ratio forms a co-crystal by cooling cocrystallization method. An Etodolac Glutaric acid cocrystal significantly increases in solubility with a dissolution rate 2 – 3 times faster than that of pure etodolac. The enhancement of aqueous solubility and dissolution rate of etodolac with co-crystallization may be a potential way to solving the bioavailability problem of etodolac. Therefore, It is the novel approach for solubility enhancement of poorly water soluble drug candidate with minimum excipients, easy method development and minimum requirement of solvent in case of organic based solvent approach.
Acknowledgement
Authors are thankful to IPCA laboratories, Mumbai, Maharashtra, India, for providing sample of etodolac and R. C. Patel college of Pharmacy, Shirpur for their help in DSC, and Diya Lab; Mumbai for performing PXRD analysis. The authors are also thankful to Dr. S. J. Kshirsagar, Principal, and management of METs Institute of Pharmacy, Nashik, Maharashtra, India, for providing laboratory facilities.
Conflict of Interest
The authors declared that there is no conflict of interest.
Funding Source
No specific grant was received from any funding agency.
References
- Wouters, J., Et Al., Alternative Solid Forms: Co-Crystals. Polymorphism In The Pharmaceutical Industry: Solid Form And Drug Development, 2018: P. 61-90.
CrossRef - Kuminek, Gislaine, Et Al. Advanced Drug Delivery Reviews, “Cocrystals To Facilitate Delivery Of Poorly Soluble Compounds Beyond-Rule-Of-5.” 101 (2016): 143-166.
CrossRef - Taylor, D., The Pharmaceutical Industry And The Future Of Drug Development. 2015.
CrossRef - Almeida E Sousa, L., Et Al., Supersaturation Potential Of Salt, Co-Crystal, And Amorphous Forms Of A Model Weak Base. Crystal Growth & Design, 2016. 16(2): P. 737-748.
CrossRef - Fu, Q., Et Al., Salt Formation Of Two BCS II Drugs (Indomethacin And Naproxen) With (1R, 2R)-1, 2-Diphenylethylenediamine: Crystal Structures, Solubility And Thermodynamics Analysis. Journal Of Molecular Structure, 2019. 1185: P. 281-289.
CrossRef - Kalepu, S. And V. Nekkanti, Insoluble Drug Delivery Strategies: Review Of Recent Advances And Business Prospects. Acta Pharmaceutica Sinica B, 2015. 5(5): P. 442-453.
CrossRef - Fong, S.Y.K., A. Ibisogly, And A. Bauer-Brandl, Solubility Enhancement Of BCS Class II Drug By Solid Phospholipid Dispersions: Spray Drying Versus Freeze-Drying. International Journal Of Pharmaceutics, 2015. 496(2): P. 382-391.
CrossRef - Van Den Mooter, G., The Use Of Amorphous Solid Dispersions: A Formulation Strategy To Overcome Poor Solubility And Dissolution Rate. Drug Discovery Today: Technologies, 2012. 9(2): P. E79-E85.
CrossRef - Dizaj, S.M., Et Al., Nanosizing Of Drugs: Effect On Dissolution Rate. Research In Pharmaceutical Sciences, 2015. 10(2): P. 95.
- Khadka, P., Et Al., Pharmaceutical Particle Technologies: An Approach To Improve Drug Solubility, Dissolution And Bioavailability. Asian Journal Of Pharmaceutical Sciences, 2014. 9(6): P. 304-316.
CrossRef - Maragos, S., Et Al., Effect Of Cyclodextrin Complexation On The Aqueous Solubility And Solubility/Dose Ratio Of Praziquantel. AAPS Pharmscitech, 2009. 10(4): P. 1444-1451.
CrossRef - Manne, A.S.N., Et Al., Hot Liquid Extrusion Assisted Drug-Cyclodextrin Complexation: A Novel Continuous Manufacturing Method For Solubility And Bioavailability Enhancement Of Drugs. Drug Delivery And Translational Research, 2021. 11(3): P. 1273-1287.
CrossRef - Sládková, V., Et Al., Application And Comparison Of Cocrystallization Techniques On Trospium Chloride Cocrystals. Crystal Growth & Design, 2014. 14(6): P. 2931-2936.
CrossRef - Guo, M., Et Al., Pharmaceutical Cocrystals: A Review Of Preparations, Physicochemical Properties And Applications. Acta Pharmaceutica Sinica B, 2021.
CrossRef - Biscaia, I.F.B., Et Al., Obtaining Cocrystals By Reaction Crystallization Method: Pharmaceutical Applications. Pharmaceutics, 2021. 13(6): P. 898.
CrossRef - Malamatari, M., Et Al., Experimental Cocrystal Screening And Solution Based Scale-Up Cocrystallization Methods. Advanced Drug Delivery Reviews, 2017. 117: P. 162-177.
CrossRef - Yu, Z.Q., P.S. Chow, And R.B. Tan, Operating Regions In Cooling Cocrystallization Of Caffeine And Glutaric Acid In Acetonitrile. Crystal Growth & Design, 2010. 10(5): P. 2382-2387.
CrossRef - Maryam, K., Creating Cocrystals: A Review Of Pharmaceutical Cocrystal Preparation Routes And Applications Cryst. Growth Des, 2018. 18: P. 6370-6387.
CrossRef - Wang, X., Et Al., Drug-Drug Cocrystals: Opportunities And Challenges. Asian Journal Of Pharmaceutical Sciences, 2021. 16(3): P. 307-317.
CrossRef - Yamamoto, K., S. Tsutsumi, And Y. Ikeda, Establishment Of Cocrystal Cocktail Grinding Method For Rational Screening Of Pharmaceutical Cocrystals. International Journal Of Pharmaceutics, 2012. 437(1-2): P. 162-171.
CrossRef - Pando, C., A. Cabanas, And I.A. Cuadra, Preparation Of Pharmaceutical Co-Crystals Through Sustainable Processes Using Supercritical Carbon Dioxide: A Review. RSC Advances, 2016. 6(75): P. 71134-71150.
CrossRef - Ribas, M.M., Et Al., Curcumin Cocrystals Using Supercritical Fluid Technology. The Journal Of Supercritical Fluids, 2019. 152: P. 104564.
CrossRef - Mcnamara, D.P., Et Al., Use Of A Glutaric Acid Cocrystal To Improve Oral Bioavailability Of A Low Solubility API. Pharmaceutical Research, 2006. 23(8): P. 1888-1897.
CrossRef - Ahire, E., Et Al., Parenteral Nanosuspensions: A Brief Review From Solubility Enhancement To More Novel And Specific Applications. Acta Pharmaceutica Sinica B, 2018. 8(5): P. 733-755.
CrossRef - Ahire, E., Et Al., Nanocrystal Based Orally Disintegrating Tablets As A Tool To Improve Dissolution Rate Of Vortioxetine. Bulletin Of Faculty Of Pharmacy, Cairo University, 2020. 58(1&2): P. 11-20.
- Elder DP, Holm R, De Diego HL. Use Of Pharmaceutical Salts And Cocrystals To Address The Issue Of Poor Solubility. International Journal Of Pharmaceutics. 2013 Aug 30;453(1):88-100.
CrossRef - Rai SK, Allu S, Nangia AK. Salts And Cocrystal Of Etodolac: Advantage Of Solubility, Dissolution, And Permeability. Crystal Growth & Design. 2020 May 15;20(7):4512-22.
CrossRef - Parashar P, Gautam S, Singh N, Kanoujia J, Yadav A, Singh I, Dwivedi M, Saraf SA. Evaluating Anti-Inflammatory Potential Of Etodolac Co-Crystals: Nitric Oxide, IL-6 And TNF-A As Potential Markers.

This work is licensed under a Creative Commons Attribution 4.0 International License.





