How to Cite | Publication History | PlumX Article Matrix
Formulation Development and Evaluation of Microemulsion Based Lornoxicam Gel
Nilima A. Thombre* , Pooja S. Niphade
, Pooja S. Niphade , Eknath D. Ahire
, Eknath D. Ahire  and Sanjay J. Kshirsagar
and Sanjay J. Kshirsagar
Department of Pharmaceutics, MET’s Institute of Pharmacy, Bhujbal Knowledge City, Adgaon, Nasik-3, Affiliated to Savitribai Phule Pune University, Maharashtra, India- 422003
Corresponding Author E-mail: nilimat_iop@bkc.met.edu
DOI : http://dx.doi.org/10.13005/bbra/2968
ABSTRACT:
The objective of the existing research was formulation development and preparation of microemulsion loaded emulgel in augmenting the topical delivery of Lornoxicam. Emulsion and gel combined are recognized as Emulgels. Gelling agents Carbopol 940, 974, 980 were used to formulating Lornoxicam emulgel. The drug release from emulgel was determined depending on the different gelling agents. Clove oil for internal phase and Polyethylene glycol 200 was applied as co-surfactant and Tween 20 as permeation enhancer. Prepared emulgels were evaluated for in-vitro and in- vivo anti-inflammatory activity using Albino mice and stability studies. The optimized batch of emulgel gave better results when compared with the marketed Diclofenac sodium gel. 90.42% drug release was detected in 8 hr. Prepared formulation was free from skin irritation and detected with 62.02% inhibition of edema. Correspondingly, prepared formulations were found to be stable. Developed microemulsion increased solubility of drugs, so that less soluble drugs can be applied in the formulation. Carbopol 980 (1.5 %) gave better results for emulgel. Therefore, microemulsion-loaded Lornoxicam Emulgel detected with better analgesic effect.
KEYWORDS: Carbopol 980; Clove oil, Emulgels; Lornoxicam; Microemulsion; PEG 200
Download this article as:| Copy the following to cite this article: Thombre N. A, Niphade P. S, Ahire E. D, Kshirsagar S. J. Formulation Development and Evaluation of Microemulsion Based Lornoxicam Gel. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Thombre N. A, Niphade P. S, Ahire E. D, Kshirsagar S. J. Formulation Development and Evaluation of Microemulsion Based Lornoxicam Gel. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3IlXWvD |
Introduction
For the localized effect of drugs, topical drug delivery systems are preferred. Topical drug delivery systems used for dermal and transdermal diseases. The advantage of the topical route is formulation is directly applied to the skin and increases the permeability of drugs 1. By using topical drug delivery systems disadvantages of an oral route can be reduced. A targeted drug delivery system increases patient compliance. Inflammation is a local protective response of the body tissue injury 2,3.
An emulsion is a heterogeneous system that contains one liquid in another which is not miscible, prepared a coarse dispersion, and stabilize by adding emulsifying agent Hoar and Schulman introduced the microemulsion. Isotropic mixtures of oil, water, and surfactant and sometimes co-surfactant known as Microemulsion. Microemulsions are thermodynamically stable. Droplet size is >0.15 micron and solution is transparent. Microemulsions require a higher amount of surfactant, in the range of 6-8% by total weight contrasting with a value of 2-3% for emulsions. Phase diagram used for obtaining the microemulsion region. J. Willard Gibbs proposed the phase diagram rules. Titration method used for phase diagram and External phase titrated with internal phase. Cloudiness is obtained at the end of the titration. Lastly, the coarse emulsion will be prepared and titrated by a co-surfactant till a transparent solution is obtained. The co-surfactant shows the microemulsion region in a different ratio which is stable, called as “pseudo-component” 4-7.
A liquid or semi-liquid thick, clear substance known as Gel. Gel and emulsion combined are called as Emulgels. A gelling agent is an important agent to prepare emulgel. Lornoxicam is a BCS class- 2 drug, contains low solubility and high permeability. And in the oral route, it shows the disadvantages like gastric irritation, first-pass metabolism. So that, here is used topical drug delivery system for Lornoxicam by using microemulsion system. Microemulsion increases the solubility and also improves the permeability of the drug 8-11.
Materials and Methods
Lornoxicam was supplied by Glenmark Pharma. Ltd. Sinner (MH, India), Clove oil, Polyethylene glycol 200 supplied by Thomas Baker, Tween 20 supplied by Thomas Baker, Mumbai (MH, India), Carbopol 940 supplied by Lobie Chemical Mumbai (MH, India), Carbopol 974 and Carbopol 980 was supplied by Lubrizol Pvt. Limited Mumbai (MH, India). Methyl paraben, propyl paraben, Triethanolamine was supplied by Thomas Baker, Mumbai.
Drug and Excipients compatibility studies
Drug and excipients characterization was started with the determination of melting point by using Thiele’s tube method. The drug was filled in capillary at the closed side and capillary kept in liquid paraffin Thiele’s tube. And temperature recorded at which drug melts. Further drug spectra were determined by using UV-visible spectrophotometer 12. The maximum wavelength of Lornoxicam was analyzed between 400-200nm. The standard calibration curve for the Lornoxicam stock solution was prepared by 10 mg of Lornoxicam dissolved in 100ml methanol. The 2, 4, 6, 8, 10 ppm concentrations were prepared and diluted by stock solution. 379 nm used as maximum wavelength to obtain absorbance using methanol as blank. The FT-IR spectra of Lornoxicam, clove oil, Tween 20 were recorded using FTIR (SHIMADZU). 4000-400 cm-1 range of frequency was scanned 12.
Solubility determination of Lornoxicam
Solubility determination was performed by using the shake flask method. Solubility was obtained for oils, surfactants and co-surfactant. 3 ml solvent was taken in the test tube with saturated drug and the mixture was kept in a cyclometer for 10 min. for mixing purposes. Kept for 72 hrs. In an isothermal shaker at 37+1 c. At 5000 rpm samples were centrifuged 15 min and filter through membrane filtration (0.45 micrometer). The supernatant of samples were examined by using UV 13,14.
Preparation of Microemulsion
Tween 20, clove oil, PEG 200 were utilized for formulation depending on the maximum solubility of the drug. The titration method was used for the pseudo-ternary phase diagram. 1:1, 1:2, 1:3, 2:1, and 3:1 different ratio taken for surfactant and co-surfactant. 90:10, 80:20, 70:30, and 60:40 ratio taken for oil and sample mixture and Water added slowly and mixed by vortex mixture. Cloudiness was observed at the end point. Surfactant and co-surfactant ratio plotted as a triangle which shows in the dotted region. The pseudo ternary phase diagram gives different region for the preparation of microemulsion. Lornoxicam added oil, surfactant and co-surfactant mixture in different percentages given in table 2 and slowly add water with continuous stirring. At constant stirring and temperature, microemulsion was prepared 2,15.
Characterization of prepared microemulsion
Dilution Test and Dye Test
On addition of water in microemulsion, O/W found to be stable whereas W/O detect with breaking of microemulsion. In the dye test, suddan red added in microemulsion and observed on a microscope. The globule observed red and ground was detected as colorless which confirmed developed microemulsion as O/W 2.
Measurement of Globule size, PDI and Zeta Potential
Zetasizer Nano-ZS (Malvern Instruments, UK) was used for the determination of globule size. 1 ml of microemulsion was diluted with doubled distilled water and by zeta cell globule size was obtained. For stability zeta potential was determined. The measurement was performed at 25°C 2,10.
Measurement Viscosity, Phase Separation and pH determination
Brookfield Viscometer (DV-E viscometer LV) was applied for measurement of the viscosity by using spindle S-18. Sample were taken in the beaker and the spindle were placed in the beaker containing sample. For the phase separation study, microemulsion centrifuge in centrifugation (Remi Motor, Mumbai) at 5000 rpm for 30 min. By using digital pH meter the pH of the prepared microemulsion was determined and calibrated by using phosphate buffer. All readings was determined in triplicate and average of triplicate was taken for more accuracy 16,17.
Stability Studies
Accelerated stability study was done for prepared batches at temperature 40 ±2°C and 75±5% RH for 3 months. 0, 30, 60, and 90days at this time points sample taken and examined for any physical changes. 16.
Method of preparation of Emulgel
Four microemulsions formulations were prepared and composition having S/Cos ratio 2:1 was taken for the preparation of gels, Carbopol 940, Carbopol 974 and Carbopol 980 three galling agent was used in different concentrations (0.5-2 %). Microemulsion loaded gel was prepared by using microemulsion as base. In one beaker PEG 200 was taken and preservatives added in that to make oily phase and drug added in this phase. The water was added in Tween 20 to prepare aqueous phase. The oil phase added in aqueous phase with continuous stirring to prepare clear microemulsion. Carbopol 980 was dispersed in distilled water for 24 hr. In 6-6.5 pH was adjusted by using triethanolamine. Gelling phase was added in microemulsion in 1:1 ratio with continuous stirring to obtain transparent microemulsion based gel 18-20.
Characterization of microemulsion based Gel
Physical Appearance and determination of pH
The color, homogeneity, consistency was determined. By Digital pH meter pH of gel was obtained. 1ml of gel diluted with 9ml of distilled water and readings were taken.21.
Rheological study
Rheological study was performed for the determinations of formulations rheological characteristics. Viscosity is the important evaluation parameter for the topical dosage form. Therefore, viscosities of formulations were determined by using Brookfield viscometer at 370c by using Spindle number S-64 22-23.
Spreadability
The spreadability apparatus was used for the test. The two glass slides (10 × 10 cm) were placed in this apparatus. 0.5gm sample was kept in glass slides. 100 gm weight kept on upper slide and measure the diameter or length which was of pre-marked circle. The time required to spread the sample was recorded 24. Formula-
S=MxL/T
Where,
M= weight
L=length or diameter
T=time
Extrudability determination
The extrudability test was determined to study how much pressure or force is required to expel material from tubes. The gel was extruded from aluminum collapsible tube by applying weights and measured the area for calculation 21. The formula:
Extrudability=Weight (gm)/Area (cm2)
Globule size and its distribution in emulgel
Malvern Zetasizer instrument was used to determine the globule size. For obtained clear solution sample was mixed in water. The homogeneous dispersion of gel sample was then placed for the analysis and readings were recorded 25,26.
Stability Studies
At 40°C and 75% RH samples were kept for 3 months. Any physical/chemical changes in the formulation was studied at the end of study27.
In-vitro diffusion study
Modified Franz diffusion cell was used. A glass cylinder used with 10 cm height for diffusion cell. In receptor chamber the phosphate buffer pH 6.8 was kept and in donar receptor gel was applied. The cell was kept in contact with receptor chamber at 32±1°C. The samples were examined at different time points and analyzed at 276 nm on UV. 28,29.
Skin irritation test
0.5 gm of gel sample applied on skin 1” x 1” (2, 54 x 2, 54 cm) square. The mice were used for skin irritation test. After a 24 hour, the animals were examined for presence of any erythema or swelling 26.
Anti-inflammatory study
For anti-inflammatory study albino mice were used. 15 mice were weighed and maintained them on fasting overnight. Control, standard and test 3 group were prepared and in each group 5 animals were taken. 1% (w/v) carrageenan was injected on left hind paw to induce edema. 0.5 gm of gel applied on the skin. At 0, 30, 60, 120 and 180 minute thickness of paw was measured by using vernier caliper. Microemulsion loaded gel and marketed Diclofenac gel were compared with carrageenan. By using ANOVA test results were calculated 29.
Result and Discussion
Drug and Excipients compatibility studies
The drug maximum wavelength was checked with UV-Spectrophotometer and characteristic sharp peak were obtained at the 376 nm in phosphate buffer 6.8 solution and 379 nm in methanol solution. 400-200 nm maximum wavelength was analyzed for the samples. 2, 4, 6, 8, 10 ppm concentrations were prepared and diluted with methanol. In the both solution, linearity was detected as well as obtained spectra was matched with the reported spectra of Lornoxicam 30. The FT-IR characterization of Lornoxicam was also recorded and C-O, C-C, N-H, C-H, C=O functional groups were identified stretching was recorded and its matches with the previously reported data. FT-IR spectra also determined for the clove oil and tween 20 excipients and both are found same as reported one 31,32.
 |
Figure 1: A) Calibration curve of Lornoxicam B) FT-IR spectra of Lornoxicam C) FTIR spectra of clove oil D) FTIR spectra of tween 20 |
Solubility study of Lornoxicam
The oil applied as most important excipients in microemulsion. Solubility of all different oils shown in below table. The clove oil showed higher solubility as compared to other oils. Solubility study of different surfactant was mentioned in Table 1. Tween 20 showed higher solubility. Highest solubility of drug was found in PEG 200 and hence it was selected as co-surfactant for further study. Table 1 summarized the solubility study of the Lornoxicam drug 33.
Table 1: Lornoxicam drug solubility (mg/ml) in oil, co-surfactant, surfactant
|
Sr. No |
Oils |
Solubility |
Surfactant |
Solubility |
| 1 | Peppermint Oil | 0.88 | Tween20 | 3.39 |
| 2 | Olive Oil | 1.02 | Tween80 | 1.71 |
| 3 | Cinnamon Oil | 1.83 | Span20 | 1.12 |
| 4 | Clove Oil | 6.39 | Span80 | 1.43 |
| 5 | Eucalyptus oil | 0.41 | Transcutol | 1.33 |
| 6 | Labrafil M 2125 | 1.60 | PEG400 | 3.36 |
| 7 | Labrafil M 2125 | 1.08 | PEG200 | 4.811 |
| 8 | Soybean Oil | 1.94 | Ethanol | 0.56 |
| 9 | – | – | Propylene Glycol | 1.02 |
Preparation of Microemulsion
From pseudo-ternary phase diagram, Tween 20 as surfactant, Polyethylene glycol taken as co-surfactant and clove oil was taken as oily phase. Microemulsion formation area was found to be greater with and decreased in surfactant & Co-surfactant ratio at 2:1. The ratio 2:1 of surfactant : co-surfactant containing tween 20 and PEG 200 showed more microemulsion region with stability. Table 2 showed the composition of microemulsion formulation 34.
Table 2: Composition of microemulsion formulations
| Sr. No. | Ingredients (%w/w) | ME1 | ME2 | ME3 | ME4 |
| 1. | Lornoxicam | 0.5 | 0.5 | 0.5 | 0.5 |
| 2. | Clove oil | 20 | 20 | 20 | 15 |
| 3. | Tween 20 | 35 | 33 | 30 | 35 |
| 4. | PEG 200 | 15 | 17 | 15 | 15 |
| 5. | Water | 30 | 30 | 35 | 35 |
| 6. | Triethanolamine | Q.S. | Q.S. | Q.S. | Q.S. |
Characterization of Microemulsion
Dilution Test and Dye Test
The dilution test is based on the solubility of an external phase of microemulsion. O/W microemulsion can be diluted with water. After dilution of microemulsion with water there is no phase separation indicates that prepared microemulsions were o/w type. Dye test was performed as mentioned in the methods section. The scattered globules appear red with colorless continuous phase and O/W type of emulsion was detected. Figure 2 showed the scattered globules with red color.
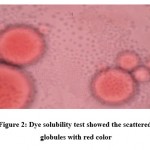 |
Figure 2: Dye solubility test showed the scattered globules with red color |
Globule size, Zeta Potential and polydispersibility index
As per Figure 3 A, a globule size was obtained at 217 nm. Zeta potential is important for stability of formulations and the standard value is up to -30mv. In current formulation, -22mv zeta potential (Figure 3 B) was obtained which indicated the formulation with stability. Polydispersibility index measure the heterogeneity or homogeneity of samples. Standard value of PDI is 0.0 to 1.0. The obtained PDI value for the formulation was 0.300 showed that formulation is stable and particles are uniformly dispersed in system 35,36.
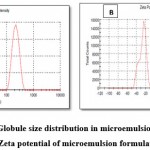 |
Figure 3: A) Globule size distribution in microemulsion formulation B) Zeta potential of microemulsion formulation |
Measurement of Viscosity, Phase separation and pH determination
Viscosity is resistance to flow, which is an important physicochemical property for topical preparations because it influences spreadability and drug release as well as jellification. The viscosities of all developed microemulsions were measured at 25°C at 12 rpm using spindle s- 18. M1 formulation showed higher viscosity of 126.2 cps which could be due to presence of larger amount of oil phase. M4 formulation showed least viscosity of 104 cps. The viscosity had an inverse relationship between concentration of oil and S/Cos. There is no phase separation of microemulsion after centrifugation at 3000 rpm for 1 hr. as shown in Figure 4 and was confirmed stability of microemulsion. pH determination was done by using Digital pH meter and all formulations were detected in range of 6.6 to 6.8. Thus obtained pH of developed formulations are well suited for pH of skin.
 |
Figure 4: Phase separation of microemulsion |
Stability studies microemulsion
Accelerated stability study was performed for 3 months of formulations at temperature 40±2°C & 75±5% RH. Samples were maintained for 0, 30, 60, and 90days. After storage of the specified months, the formulations were analyzed & all formulations were found stable at accelerated stability study.
Formulation Batches of Emulgels
Table 3 mentioned the summarized data of different composition of excipients for formulation development and preparation of Emulgels.
Table 3: Formulation batches of Emulgels (% Wight by weight)
| Sr. No. | Component | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
| 1 | Lornoxicam | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 2 | Carbopol 940 | 1.5 | 2.0 | – | – | – | – | – | – |
| 3 | Carbopol 974 | – | – | 1.5 | 1.75 | 2.0 | – | – | – |
| 4 | Carbopol 980 | – | – | – | – | – | 1.5 | 1.75 | 2.0 |
| 5 | Clove oil | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| 6 | Tween 20 | 33.3 | 35 | 33.3 | 35 | 33.3 | 33.3 | 35 | 33.3 |
| 7 | PEG 200 | 16.6 | 15 | 16.6 | 15 | 16.6 | 16.6 | 15 | 16.6 |
| 8 | Propyl Paraben | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 9 | Methyl Paraben | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| 10 | Triethanolamine | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. |
| 11 | Water | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. |
Evaluations for Microemulsion based Emulgels
Physical Appearance and determination of pH
Upon the physical observation, it was observed that all formulations detected with milky white appearance, homogeneous with no grittiness, no creaming and semi-solid in consistency. The pH values were all found in range of 6-7. All pH values are mentioned in Table 4.
Table 4: Microemulsion based emulgels pH
| Sr. No. | Formulations | pH value |
| 1 | F1 | 6.51±0.03 |
| 2 | F2 | 6.44±0.03 |
| 3 | F3 | 6.35±0.05 |
| 4 | F4 | 6.54±0.03 |
| 5 | F5 | 6.36±0.01 |
| 6 | F6 | 6.50±0.01 |
| 7 | F7 | 6.22±0.01 |
| 8 | F8 | 6.44±0.01 |
± SD (n=3)
Rheological behavior of gel
The rheological behavioral study was performed as per prescribed procedure in the methods section. Formulation developed with Carbopol 980 (1.5-2%) was detected with increased viscosity in prepared formulations. Figure 5 indicated the viscosity for different formulations. All formulations detected with shear thinning property.
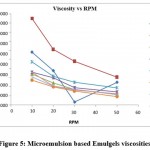 |
Figure 5: Microemulsion based Emulgels viscosities |
Spreadability study
20.76 gm.cm/sec spreadability coefficient was obtained for F4 formulation. So, data is obtained because of less viscous preparation.
Extrudability test
As per the outcome of Extrudability test for emulgel, formulation F2, F5, And F8 were contained highest amount of Carbopol 940, Carbopol 974 and Carbopol 980 i.e. 2.0 %. From this, increase in viscosity reduces the extrudability. Prepared formulations detected with better extrudability due to low viscosity in nature.
Globule size and Globule size distribution
At 244 nm wavelength globule size and globule size distribution was observed. When percentage of surfactant less then less globule size will also observed. Figure 6 showed the globule size distribution of prepared emulgel formulation.
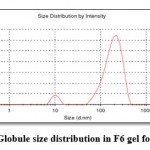 |
Figure 6: Globule size distribution in F6 gel formulation |
Stability study of Emulgels
F6 formulation was examined for accelerated stability and there is no any agglomeration or chemical instability was observed. Finally after 3 months of accelerated stability study, developed Emulgel (F6) formulation detected with good stability and did not showed any major physical/chemical changes in the emulgel.
In-vitro permeability or diffusion test
Figure 7 mentioned drug release pattern of drug from emulgels. Formulations F3 and F6 showed drug release in 8 hrs as 88.42 % and 90.47% respectively. Thus, formulation EG6 was detected with good in-vitro diffusion as compared to others.
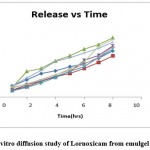 |
Figure 7: In vitro diffusion study of Lornoxicam from emulgel formulations |
Diffusion models for Emulgel formulations
The release kinetics of the different formulations were shown in Figure 8 by Korsmeyer- Peppas model. The release of drug followed erosion type of diffusion. As per the outcome of the study, it can be considered that the drug release was increased with increase in time exponentially. Figure number 8 indicates diffusion kinetic models for all formulation and Korsmeyer-Peppas kinetic treatment for Emulgel containing Lornoxicam.
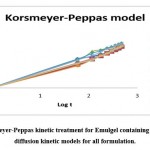 |
Figure 8: Korsmeyer-Peppas kinetic treatment for Emulgel containing Lornoxicam and diffusion kinetic models for all formulation. |
Skin irritation test
Figure 9 showed the outcome of skin irritation test. Mice was used for the skin irritation test. After 24 hrs, it was observed that, there is no skin irritation such as redness or rashes on the skin. Its means prepared final formulation Emulgel can be considered as safe for the application on the skin.
 |
Figure 9: Skin irritation test conducted on albino mice for microemulsion based emulgel. |
Anti-inflammatory study
The standard group was treated with Diclofenac sodium gel, emulgel F6 formulation as test sample and carrageenan as control. 69.92 % & 62.08 % was observed percent inhibition for paw volume of emulgel for anti-inflammatory activity. < 0.0001 P value was observed by ANOVA test which showed formulation is significant. Table No 5 mentioned the outcome of anti-inflammatory activity.
Table 5: Microemulsion based emulgels Anti-inflammatory activity
| Sr. No |
Group Taken |
Paw Volume (mm) |
Percent Inhibition | ||||
| 0 (min) | 30 (min) | 60 (min) | 120 (min) | 180 (min) | |||
| 1 | Control | 2.23±0.
1317 |
4.26±0.
2648 |
4.47±0.
2973 |
4.6±0.29
71 |
4.92±0.
3192 |
0 |
| 2 | Standard | 2.68±0.
1186 |
4.34±0.
2447 |
3.8±0.2
007 |
3.33±0.1
549 |
3.12±0.
2776 |
69.92 |
| 3 | Test | 2.25±0.
0648 |
4.242±0
.1814 |
3.82±0.
2366 |
3.52±0.2
033 |
3.38±0.
2290 |
62.08 |
*(n=3)
Conclusion
Microemulsion system provides solubilization of hydrophobic drugs, thus imparts in enhancing the availability of the drug in the formulation. Firstly stable microemulsion was prepared and it was developed into Emulgel as a final formulation. Carbopol 980(1.5 %) gave better results as compared to other batches of emulgels. Prepared Emulgel was evaluated for their different characterization properties including, physical appearance, and pH of the formulation, rheological behaviour of the formulation, spreadability and extrudability study as well as globule size of emulgel and its distribution in the formulation. In the regards of all characterization aspects, the prepared Emulgel was shown the positive results. 90.42% drug release was detected by formulation batch F6 in 8 hr. The prepared Emulgel formulation was free from any skin irritation and detected with 62.02% inhibition of edema. Therefore, microemulsion loaded Lornoxicam emulgel gave better analgesic effect and utilized as anti-inflammatory activity.
Acknowledgment
Author would like to acknowledge to Trustees, Bhujbal Knowledge City, MET’s institute of pharmacy, Nashik, Maharashtra, India for providing the necessary facilities to carry out current research work.
Conflict of Interest
The authors declare the no conflicts of interest
Funding Sources
The authors received no external funding for this research
References
- Biswal B, Karna N, Nayak J, Joshi V. Formulation and evaluation of microemulsion based topical hydrogel containing lornoxicam. Journal of Applied Pharmaceutical Science. 2014 Dec;4(12):077-84.
CrossRef - Biswal B, Karna N, Nayak J, Joshi V. Formulation and evaluation of microemulsion based topical hydrogel containing lornoxicam. Journal of Applied Pharmaceutical Science. 2014;4(12):077-084.
- Verma S, Vaishnav Y, K Verma S, K Jha A. Anhydrous Nanoemulsion: An Advanced Drug Delivery System for Poorly Aqueous Soluble Drugs. Current Nanomedicine (Formerly: Recent Patents on Nanomedicine). 2017;7(1):36-46.
- Singh D, Sachan AK, Kumar S. Formulation and evaluation of topical gel delivery of lornoxicam. World J Pharm Pharm Sci. 2018;7(6):884-896.
- Shah V, Sharma M, Gandhi K, Suthar V, Parikh RK. Quality by Design (QbD) approach for optimization of microemulsion based topical gel. Marmara Pharmaceutical Journal. 2016;20(3):415-424.
CrossRef - Yuan Y, Li S-m, Mo F-k, Zhong D-f. Investigation of microemulsion system for transdermal delivery of meloxicam. International journal of Pharmaceutics. 2006;321(1-2):117-123.
CrossRef - Urmaliya H, Gupta M, Agrawal A, Jain NK, Dubey A. Formulation development and evaluation of microemulsion gel of Ketoconazole as an antifungal agent. Pharmacia: An Int J of Pharm Sci. 2016;2:120-130
- Alexander A, Khichariya A, Gupta S, Patel RJ, Giri TK, Tripathi DK. Recent expansions in an emergent novel drug delivery technology: Emulgel. Journal of Controlled Release. 2013;171(2):122-132.
CrossRef - Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Critical Reviews™ in Therapeutic Drug Carrier Systems. 1999;16(5).
CrossRef - Ahire E, Thakkar S, Borade Y, Misra M. Nanocrystal based orally disintegrating tablets as a tool to improve dissolution rate of Vortioxetine. Bulletin of Faculty of Pharmacy, Cairo University. 2020;58(1&2):11-20.
- Sowmya C, Reddy S, Sivaprasad N, Kumar B. Preparation and Evaluation of ofloxacin microemulsion gel. International Journal of. 2012.
- Yener G, Üner M, Gönüllü Ü, et al. Design of meloxicam and lornoxicam transdermal patches: Preparation, physical characterization, ex vivo and in vivo studies. Chemical and Pharmaceutical Bulletin. 2010;58(11):1466-1473.
CrossRef - Kharwade M, Achyuta G, Subrahmanyam C, Babu PS. Solubility behavior of lornoxicam in binary solvents of pharmaceutical interest. Journal of solution chemistry. 2012;41(8):1364-1374.
CrossRef - Li F, Song S, Guo Y, et al. Preparation and pharmacokinetics evaluation of oral self-emulsifying system for poorly water-soluble drug Lornoxicam. Drug delivery. 2015;22(4):487-498.
CrossRef - Nancya S. Development and Evaluation of Microemulsion for Transdermal Delivery of Lornoxicam, Periyar College of Pharmaceutical Sciences for Girls, Tiruchirappalli, Tamil …; 2012.
- Naeem M, Rahman NU, Khan JA, Sehti A, Nawaz Z. Development and optimization of microemulsion formulation using Box-Behnken design for enhanced transdermal delivery of Lornoxicam. Latin American Journal of Pharmacy. 2013;32(8):1196-1204.
- Ahire E, Thakkar S, Darshanwad M, Misra M. Parenteral nanosuspensions: a brief review from solubility enhancement to more novel and specific applications. Acta Pharmaceutica Sinica B. 2018;8(5):733-755.
CrossRef - Mahaparale SP, Gaware V. Formulation and evaluation of lornoxicam emulgel.
- Ashara K, Soniwala M, Shah K. Emulgel: A novel drug delivery system. Journal of Pakistan Association of Dermatology. 2017;26(3):244-249.
- Yadav SK, Mishra MK, Tiwari A, Shukla A. Emulgel: a new approach for enhanced topical drug delivery. Int J Curr Pharm Res. 2016;9(1):15-19.
CrossRef - Baibhav J, Gurpreet S, Rana A, Seema S. Development and characterization of clarithromycin emulgel for topical delivery. Int J Drug Dev Res. 2012;4(3):310-323.
- Mohamed MI. Optimization of chlorphenesin emulgel formulation. The AAPS journal. 2004;6(3):81-87.
CrossRef - Thombre NA and Gide PS. Rheological characterization of galactomannans extracted from seeds of Caesalpinia pulcherrima. Carbohydrate Polymers. 2013; 94: 547– 554
CrossRef - Sultana SS, Parveen P, Rekha MS, Deepthi K, Sowjanya C, Devi AS. Emulgel-a novel surrogate approach for transdermal drug delivery system. Ind Am J Pharm Res. 2014;4:5250-5265.
- Panwar A, Upadhyay N, Bairagi M, Gujar S, Darwhekar G, Jain D. Emulgel: a review. Asian J Pharm Life Sci. 2011;2231:4423.
- Varma VNSK, Maheshwari P, Navya M, Reddy SC, Shivakumar H, Gowda D. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharmaceutical Journal. 2014;22(6):591-599.
CrossRef - Singla V, Saini S, Joshi B, Rana A. Emulgel: A new platform for topical drug delivery. International Journal of Pharma and Bio Sciences. 2012;3(1):485-498.
- Hamed R, Basil M, AlBaraghthi T, Sunoqrot S, Tarawneh O. Nanoemulsion-based gel formulation of diclofenac diethylamine: design, optimization, rheological behavior and in vitro diffusion studies. Pharmaceutical development and technology. 2016;21(8):980-989.
CrossRef - Pandey SS, Maulvi FA, Patel PS, et al. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: in vitro and in vivo studies. Colloids and Surfaces B: Biointerfaces. 2020;186:110681.
CrossRef - Xiao B, Zhao F, Gao Y. Characterization and application of lornoxicam, europium (III), and lysozyme fluorescence. Instrumentation Science & Technology. 2016;44(3):282-293.
CrossRef - Suhasini M, Sailatha E, Gunasekaran S, Ramkumaar G. Vibrational and electronic investigations, thermodynamic parameters, HOMO and LUMO analysis on Lornoxicam by density functional theory. Journal of Molecular Structure. 2015;1100:116-128.
CrossRef - Patel P, Ahir K, Patel V, Manani L, Patel C. Drug-Excipient compatibility studies: First step for dosage form development. The Pharma Innovation. 2015;4(5, Part A):14.
- Gadade D, Kulkarni D, Rathi P, Pekamwar S, Joshi S. Solubility enhancement of lornoxicam by crystal engineering. Indian Journal of Pharmaceutical Sciences. 2017;79(2):277-286.
CrossRef - Badawi AA, Nour SA, Sakran WS, El-Mancy SMS. Preparation and evaluation of microemulsion systems containing salicylic acid. Aaps Pharmscitech. 2009;10(4):1081-1084.
CrossRef - Suryawanshi SR, Thakare NP, More DP, Thombre NA. Bioavailability enhancement of ondansetron after nasal administration of Caesalpinia pulcherrima-based microspheres. Drug Delivery.2015; 22(7): 894-902.
CrossRef - Thombre NA, Gide PS. Floating-bioadhesive gastroretentive Caesalpinia pulcherrima-based beads of amoxicillin trihydrate for Helicobacter pylori eradication. Drug Delivery. 2016; 23(2): 405–419
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





