Manuscript accepted on : 08-03-2022
Published online on: 15-03-2022
Plagiarism Check: Yes
Reviewed by: Dr. Rajamanikkam K 
Second Review by: Dr. Shivangi Mathur 
Final Approval by: Dr. rer. Nat. Hesham Ali El- Enshasy
Insight into the Potential Cyanobacterial Metabolites and Their Screening Strategies
Department of Botany, St. Joseph’s College (Autonomous), Bengaluru-27
Corresponding Author E-mail: niveshika87@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2983
ABSTRACT:
Cyanobacteria are the first oxygenic photosynthesis performing prokaryotes. They are considered to have fast growth, amenability to genetic modifications towards photo-autotrophy. Have the ability to grow under heterotrophic conditions with minimum available sunlight to obtain energy by utilizing organic carbon as its substrate. Cyanobacteria are diversely spread in marine, freshwater, terrestrial habitats which differ from each other concerning their structural and functional metabolism. It produces bioactive compounds which are toxic to animals as well as humans which are produced in freshwater habitats whereas marine species of cyanobacteria produce secondary metabolites which are involved in the production of new drugs and also show potential in various fields such as Biotechnological applications, pharmacology, agriculture sustainability, and environmental remediation. Cyanobacteria also produce non-toxic compounds which help in protecting plants by producing phytohormones, siderophores, and UV protective or absorbing compounds. Marine cyanobacterial bioactive compounds are involved in several bioactivities such as antiviral, antialgal, antiprotozoal, antifungal activities, etc. Freshwater species are involved in forming harmful cyanobacterial blooms which are highly toxic to animals as well as humans (Ex: cyanotoxins, hepatotoxins, etc). Different strategies are used to detect the cyanobacterial compounds under in-vivo and in-vitro cultures. To analyze the quality and safety of water, screening methods are necessary to detect possible toxic compounds present in the environmental habitats. Screening methods include microscopy assay, physiological methods, chemical methods, biochemical-based methods, and molecular-based methods. All these methods of screening help in characterizing, identifying the cyanobacterial toxins and also have a few limitations in their reliability, sensitivity, and limit in the detection of the compounds.
KEYWORDS: Biotechnological application; Cyanobacteria; Secondary metabolites; Screening strategies
Download this article as:| Copy the following to cite this article: Pooja S, Niveshika N. Insight into the Potential Cyanobacterial Metabolites and Their Screening Strategies. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Pooja S, Niveshika N. Insight into the Potential Cyanobacterial Metabolites and Their Screening Strategies. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3MRkwj2 |
Introduction
Cyanobacteria is a gram-negative photoautotrophic bacterium that is said to be the first prokaryote to get involved in oxygenic photosynthesis1,2. They are considered an ancient group of photosynthetic prokaryotes3. Also, their ability towards photo-autotrophy, fast growth, and amenability to genetic modifications are quite immense4–8. Cyanobacteria are the primitive prokaryotes to perform the oxygenic photosynthesis using both the photosystems P1 and P2 (P1= P700 nm and P2= P680 nm) to produce energy, water, carbon dioxide, inorganic compounds for their growth. They also have the capability of growing under heterotrophic conditions with minimum sunlight to obtain energy by utilizing organic carbon as a substrate9–12. The cyanobacterial classification was proposed in the year 1985 and divided into 4 orders namely: Chroococcales, Nostocales, Oscillatoriales, Stigonematales, and their phyla also identified such as Chroococcales, Gloeobacterales, Pleurocapsales7. Later it was classified into 5 orders. They are Chroococcales, Pleurocapsales, Nostocales, Oscillatoriales, Stigonematales13 as shown in (Table 1). The relative quantity of biomolecules was separated from the filamentous form of cyanobacteria: Nostocales order (Genera includes Lyngbya, Oscillatoria, and Symphloca5.
Table 1: Cyanobacteria are classified in 5 orders.
| Sl. No | Orders | Species | Habitat | Form |
| 1 | Chroococcales | Microcystis sp | Freshwater | Unicellular |
| Synechococcus sp | Marine water | |||
| Synechocystis sp | Freshwater | |||
| 2 | Pleurocapsales | Hyella caespitosa | Marine water | Unicellular |
| 3 | Nostocales | Anabaena sp | Freshwater | Filamentous |
| Nostoc sp | Terrestrial | |||
| 4 | Oscillatoriales | Oscillatoria sp | Freshwater | Filamentous |
| Lyngbya majuscula | Tropical marine water | |||
| 5 | Stigonematales | Fischerella muscicola | Freshwater | Filamentous |
Cyanobacteria are populated diversely in marine, freshwater habitats, or terrestrial environments in terms of morphology (morphologically, cyanobacteria can be unicellular or filamentous with having spherical, rod, spiral shapes and they often grow in large colonies Ex: Oscillatoria, Nostoc, Spirulina7), physiology and metabolism and it can also survive in extreme conditions such as hot spring water, geotherms as seen in Synechococcus species, high temperature and so on13,14.
Cyanobacteria produce harmful blooms under eutrophic conditions due to the presence of high levels of phosphorus and nitrogen13,15. Cyanotoxins are harmful to animals as well as humans and are produced in freshwater habitats whereas the marine habitat species of cyanobacteria produce bioactive compounds which are involved actively in the production of new drugs such as antiviral, antibiotic, anticancer, etc.3,16. Screening of un-purified extracts has been proven to be an active method in identifying organisms that produce potentially useful compounds17.
Secondary Metabolites from Cyanobacteria
Over some time, the cyanobacteria have evolved to produce various secondary metabolites to get through the changes in the surrounding environment such as high temperature, high pH, high dissolved phosphorus, and nitrogen18. Secondary metabolites are often called Natural products with low molecular weight organic molecules that have multiple biological activities19. Cyanobacteria are known to produce around 1100 unique secondary metabolites20. Among these, almost 300 nitrogen-containing secondary metabolites have been reported from the marine prokaryotic cyanobacteria5. Exceptionally, marine cyanobacteria have a very rich origin of bioactive compounds compared to freshwater and terrestrial cyanobacteria due to the varied bioproducts obtained from these marine species. These secondary metabolites show potential in various fields such as biotechnological applications, pharmacology, agriculture sustainability, and environmental remediation. The cyanobacterial secondary metabolites play an important function in the improvement of drugs mainly in the field of cancer and infection21. Improved knowledge and understanding of cyanobacterial metabolites and their utilization in drug development has raised a new perspective of utilizing cyanobacteria in the field of medicine9,22. Cyanobacteria are found to be involved in activities like an antibiotic, antiviral, anticancer, cytotoxicity, anti-inflammatory, enzyme inhibitor, free radical scavenger2,23. Figure 1 shows different cyanobacterial metabolites.
Secondary metabolites are the essential origin of new composition which leads to medicating in many crucial sickness areas22. When cyanobacterial species grow rapidly to form harmful blooms, they can produce large amounts of unique and bioactive secondary metabolites24. Secondary metabolites from cyanobacteria perform numerous roles such as defensive mechanisms against other organisms including antibiotics, fungicides, etc., as a metal transporting agent, as facilitators of symbiosis, as photo-protectants, as antioxidants, as allelochemicals in signaling14,25.
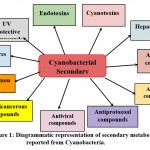 |
Figure 1: Diagrammatic representation of secondary metabolites reported from Cyanobacteria. |
Toxic Secondary Metabolites from Cyanobacteria
Cyanobacterial toxins are well studied and vastly associated with the health effects on the human and healthy environment. The first Scientific literature on the toxicity of secondary metabolites in cyanobacteria was reported by George Francis due to increased deaths of livestock in South Australia after drinking water which contained high consumption of cyanobacteria in lake Alexandra26. In Cyanobacteria, secondary metabolites are divided into toxic metabolites and non-toxic secondary metabolites. Here, non-toxic cyanobacterial compounds usually help in plant growth, cell division, and the release of nutrients. Toxic secondary metabolites are based on which organ they affect (animals and humans). Unicellular freshwater species such as Microcystis aeruginosa have a very high source of peptide metabolites and have adverse effects on human health27. Toxic metabolites show a bad impact on both animals and humans. Some lakes were infected by cyanobacterial blooms causing diseases by releasing their toxic substances that lead to different sorts of problems to the cattle, fishes, humans, etc24. Bloom forming species of cyanobacteria are known to produce the secondary metabolites which belong to different classes, among them are the neurotoxins and hepatotoxins27. The majority of the toxic secondary metabolites (Ex: cyanotoxins) were produced from the freshwater habitats of cyanobacteria such as harmful blooms which lead the humans (to fall sick) and the aquatic organisms (lethal deaths)28. The production of the potent neurotoxins (nervous system) and hepatotoxins (liver cells) show potentially dangerous consequences 29. Benthic forms of cyanobacteria form clusters or mats as a part of the biofilms which helps in photo-autotrophy30. There are a few toxic secondary metabolites from cyanobacteria: Cyanotoxins such as hepatotoxins, neurotoxins, dermatotoxins, etc18.
Cyanotoxins
Cyanotoxins are natural toxins produced from freshwater cyanobacteria due to a decrease in nutrient sources31. Cyanotoxins weigh around 299Da produced by cyanobacterial species viz. Aphanizomenon isatschenkoi, Anabaena circinalis, Cylindrospermopsin raciborskii, Planktothrix species29. This acts as a channel blocker as it blocks the VGSC in neurons32 in the environment, there is a threat to animal and human health33. Cyanotoxins are harmful to higher animals, humans (rarely)34. Cyanotoxins are rarely ingested by humans in high amounts but if a person is exposed for a long period it might lead to chronic effects which promote the development of further carcinogenic problems35. Compared to other cyanotoxins, hepatotoxins are naturally faced by humans and animals in bloom formation of cyanobacteria29. Research on their toxicological and ecotoxicological properties revealed that there are possible ways to employ naturally toxic bioactive metabolites called Allelochemical drugs37. Allelopathy involves using the bioactive metabolites by one species to slow down the growth of sympatric species which shows potential to compete with the resource such as algaecides, herbicides, and insecticides26. The cyanotoxins are natural algaecides that slow down the growth rate in other cyanobacteria18. Cyanotoxins are divided into alkaloid neurotoxins and cyclic-peptide hepatotoxins. Deaths from these toxins are most likely due to intrahepatic hemorrhagic and hypovolemic shock36. Hepatotoxins and neurotoxins are intracellular cyanobacterial metabolites. They are produced by only particular strains of the cyanobacterial species38.
Hepatotoxins
The most common cyanotoxins in freshwater are hepatotoxins produced by a few cyanobacterial species belonging to Microcystis, Anabaena, Nostoc, Planktothrix, Nodularia, etc39. The variation in the structure of hepatotoxin has been found in the filamentous brackish water cyanobacterial species such as Nodularia spumigena36. Sometimes, the temperature influences the growth and production of the hepatotoxin whereas warmer temperature may directly or indirectly promote the growth and levels of the cellular toxicity in hepatotoxins40. Hepatotoxins are taken up by hepatocytes triggering illness. Hepatotoxins are responsible for the poisoning of wildlife, humans worldwide29. Most commonly the cyanobacteria encounter hepato-toxicosis involving the hepatotoxins41. The release of the hepatotoxin from the cyanobacterial species also has an allelopathic effect on other phytoplanktons. Hepatotoxins are highly stable under sunlight and resistant to UV radiations 42. Hepatotoxins affect massive hemorrhages and disruption in the liver as well as kidneys and exposure to this toxin occurs mostly through the diet or by direct contact with the contaminated water. It is known to have high resistance to digestion in the gastrointestinal tracts of eukaryotes because the presence of peptide bonds linked to D-amino acids are not sustainable to normal enzymes. Thereby it shows a high risk to consumers of animal products that have been contaminated 43. Hepatotoxin is comprised of microcystin (cyclic peptide) produced by Microcystis aeruginosa, and also by some other genera such as Oscillatoria sp, Anabaena sp, Nostoc sp, etc. and Nodularin (pentapeptide) by Nodularia spumigena and Cylindrospermopsin produced by Cylindrospermopsis raciborskii 41. Both the microcystin and nodularin have a significant impact on water quality44 also have similar toxicity mechanisms but the morbidity of cylindrospermopsin may rely on metabolic modification in liver29.
Microcystin (MC)
Microcystin is a cyclic heptapeptide and highly hepatotoxic non-ribosomal peptide produced by a few cyanobacterial species37. Their growth in freshwater leads to animal deaths and most of these microcystins are found growing in brackish water or freshwater habitat20. The biosynthesis of MCs is increased under high light and red-light conditions37. Hepatotoxic blooms are seen all over the world and microcystins are found common in these blooms36. Hepatotoxic microcystins are produced by bloom-forming species through a complex of non-ribosomal peptide synthetase (NRPS) and polyketide synthase (PKS)17. These have been studied most commonly and found to slow down the growth of other photoautotrophs40. Microcystin-LR is the virulent member of MC variants and it has the potential to treat cancer due to cytotoxicity45. Microcystin-LR weighs around 994 Da and its structure is Cyclo (-D-Ala-L-Leu-D-MeAsp-L-Arg-Adda-D-Glu-Mdha) here, Adda is 3-amino-9-methoxy-2,6,8, trimethyl-10-phenyl deca-4,6-dienoic acid; D-MeAsp is D-erythro-Beta-iso aspartic acid; Mdha is N-Methyl-dehydroalanine 22,36,46. Uptake of MC-LR into the liver can be suppressed by bile salts competitively such as taurocholate or by a competitive substance named antamanide, sulfo-bromo-phthalein, rifampicin, etc.37 Microcystin inhibits the subclasses of threonine or serine amino acids, phosphatases type PP (1 and 2A) with a broad range of protein substrates (targeted)2. Primary targets for MCs are eukaryotic protein phosphatase47.
Nodularin (NOD)
Nodularia produces the nodularin by cyanobacterial species Nodularia spumigena, this genus is well known due to its toxicity nature which is specific to form the cyanobacterial blooms (highly toxicant)49. Nodularin weighs around 824 Da and is termed as NODLN36. It is a diazotrophic filamentous cyanobacteria50. Nodularin surroundings extend from slight brackish water, saline-alkaline inland lakes, and few are found rarely in the freshwater habitats and soil51. Nodularin is closely related to microcystin19. Nodularin is synthesized by a multi-enzyme compound containing NRPS and PKS as domains50 Nodularin shares a highly similar biosynthetic pathway of Microcystin15. Toxicological characteristics of nodularin are to inhibit the threonine or serine protein phosphatases such as PP1 and PP2A37. Exposure to nodularin causes oxidative stress in wildlife such as mussels, fishes, marine fucus vesiculosus52.
Cylindrospermopsin (CYN)
Cylindrospermopsin is an alkaloid hepatotoxin isolated from freshwater cyanobacterial species named Cylindrospermopsis raciborskii, Aphanizomenon ovalisporum and its natural analogues are cytotoxins which provokes hepatotoxicity in humans53. Cylindrospermopsin weighs around 415.43Da54. The precursor of CYN is formed by the unique activity of L-arginine-glycine amidinotransferase Cry A15. CYN is classified as hepatotoxin because of its nature to damage the hepatocyte and liver such as Lipidosis, hemorrhage, necrosis. CYN is also produced by benthic forms of cyanobacterial species (Lyngbya wollei)30. CYN is the most frequently studied hepatotoxin after Microcystin so far and their distribution is increasing day by day, they are known to be an effective cytotoxin that affects different organs and their functions in animals, plants causing fatal deaths in cattle and outbreaks in human health situations by causing the illness49. In vitro, their decomposed products are toxic to humans and their presence of harmful characters in CYN is huge and primarily it includes the cytotoxicity55. It inhibits the glutathione, protein synthesis, and cytochrome P450, also characterized by a tricyclic carbon skeleton attached to the hydroxymethyl uracil, a sulfate group, Guanidine15,54. There are 3 different variants of CYN that have been identified such as 7-deoxy cylindrospermopsin, Cylindrospermopsin, 7-cylindrospermopsin based on the presence of substituents on 7th carbon53. Mode of action and uptake mechanism is known in Cylindrospermopsin15. In rodents, it has affected liver, kidney, heart damage including their hepatocellular vacuolation, cytotoxicity, etc.49. The main harmful effect after exposure to CYN is the inhibition of protein synthesis due to reaction with eukaryotic translation protein synthesis55. Their biological activity involves the interruption of various metabolic pathways and also causes oxidative stress by increasing the concentration of free oxygen radicals54.
Neurotoxins
Neurotoxins are alkaloids43. Cyanotoxins affect the nervous system either in the central or peripheral region due to chemical and biological agents known as neurotoxins. The chemical structure of neurotoxins is closely related to CYN56. Many of the neurotoxic substances from marine cyanobacteria are known to target the voltage-gated Sodium channels (VGSC)15. Neurotoxins of the cyanobacteria are composed of 2 groups with two low molecular weight alkaloids such as anatoxins (anatoxin-a, homoanatoxin-a, anatoxin-a(s)) and saxitoxins. Neurotoxins destroy the natural growth of the neural impulses to muscles causing paralysis and respiratory failure leading to animal death29. The mode of action is well defined based on their targeting organs56. Marine cyanobacteria are considered as a communicator of potent neurotoxins which acts as either activator or blocker34. The new classes of cyanobacterial metabolites are defined by their exceptional integration of NRPS & PKS-subunits with long unusual 15-carbon linear polyketide chain, tri-heterocyclic ring consisting of 2 α-methyl thiazolines and a thiazole33. The PKS extends the residue of isoleucine with an uncertain number of extra amino acids to form the cyclic structure. Ingestion of neurotoxins causes death in animals due to respiratory fatigue and failure53. Symptoms of this toxin cause staggering, muscle fasciculation, gasping, convulsions, and opisthotonos seen in birds. Anatoxin-a and homoanatoxin-a are closely connected in terms of their structure with cholinergic nicotinic agonists which adhere to the neuronal nicotinic acetylcholine receptors38. Anatoxin-a (commonly called as very fast death factor-VFDF)57 is isolated from the freshwater cyanobacteria and it is secondary Bi-cyclic amine with 2-acetyl-9-azabicylo (4,2,1) non-2-ene with a molecular weight of about 165 Da. The production of neurotoxic compound anatoxin-a has been determined from Oscillatoria or Planktothrix occasionally and homoanatoxin-a is produced from stratifying and structurally similar cyanobacteria species called Tychonema bourelly34. These two toxins are potent neurotoxins produced by freshwater cyanobacterial species of different genera such as Anabaena, Aphanizomenon, Oscillatoria, Phormidium, Cylindrospermum36,58.
Neurotoxins such as anatoxin-a alter the neuromuscular transmission18,58. Anatoxin-a is isolated from cyanobacterial species such as Anabaena flos-aquae, Aphanizomenon flos-aquae, Planktothrix which acts as receptor binder binding irreversibly to acetylcholine 28. Anatoxin-a is considered to be the potent drug of postsynaptic cholinergic nicotinic leading to depolarizing neuromuscular blockade. And it provokes the activity of acetylcholine which causes paralysis and muscle cramping29.
Anatoxin-a(s) is a phosphate ester with cyclic N-hydroxy-guanine and their mechanism is to synthesize the organophosphate insecticides such as parathion, malathion, etc. Anatoxin-a (s) is isolated from single cyanobacterial species Anabaena such as A. flos aquae, A. lemmermanni. Anatoxin-a(s) weighs about 252Da56. It inhibits the acetylcholinesterase and interferes in muscle contraction38. This toxin causes muscle fatigue and failure29. This is a neurotoxic alkaloid that is capable of inhibiting the acetylcholine ester 58.
Saxitoxin is also known as paralytic shellfish toxins (PST) which causes the poisoning in shellfish by paralyzing it; these are closely related to alkaloids in terms of morphology 53. PST is fast-acting neurotoxins that slow down the rate of nerve conduction by blocking the sodium (Na) channels without affecting the potassium (K) concentration. It is a neurotoxic guanidinium derivative with two functional groups of amines 28.
Dermatoxins
Cyanotoxins causing skin irritation are known as dermatoxins 18. There are two types of toxins: lyngbyatoxin and aplysiatoxin 46. These two toxins are produced from the Lyngbya majuscula 26. Lyngbyatoxins and aplysiatoxin possess a polyketide backbone containing amino acid constituents16. Lyngbyatoxin and debromoaplysiatoxin are extremely inflammatory and they have structurally different secondary metabolites 37. There is a special activator called protein kinase C (PKC)15. Lyngbyatoxin is known to cause swimmer’s itch. When a person is exposed to this toxin, Lyngbya majuscula produces a few toxins which result in a dermatitis-like condition in swimmers causing inflammation, blisters, and tumors 17. These compounds also act as potent tumor promoters15. Lyngbya majuscula results in contact dermatitis and is found toxic in fishes and grazer species 26.
Endotoxins
Endotoxins are also known as Irritant toxins or Lipopolysaccharides (LPS). LPS is well known for its effect on the alimentary tract of bacteria such as E. coli, Salmonella species, Vibrio cholera, Pseudomonas aeruginosa. Lipopolysaccharides are isolated from the genera such as Microcystis, Anabaena, Spirulina, Oscillatoria. Liposaccharides contain lipid-A, polysaccharide (core region), and outer polysaccharide chain37. Lipopolysaccharide is an obligate region of the outer layer of cells in gram-negative bacteria. It differs from enteric bacteria by lacking phosphates and having a high mixture of unsaturated long fatty acid chains and hydroxy fatty acids. In mammals it is best-known to cause fever, it is involved in septic shock syndrome (SSS) where this is aggravated, toxicant, and induced in the liver and kidney 17. Table 2 showed the toxic secondary metabolites and their causes.
Table 2: Toxic secondary metabolites and their causes.
|
S.no |
Toxic Metabolite | Weight | Causes |
References |
|
| 1 | Cyanotoxins | – | Harmful to higher animals including humans (rarely). There are possible ways to develop bioactive metabolites called allelochemical drugs. Also involved in the formation of toxic cyanobacterial blooms. | 18,29,34,37 | |
| A | Hepatotoxins | Microcystin (MC-LR) | 994Da | Humans may get exposed to MC-LR through their diet which results in liver damage. | 45 |
| Nodularin (NOD) | 824Da | It causes oxidative stress in mussels, fishes, marine fucus vesiculosus. | 52 | ||
| Cylindrospermopsin (CYN) | 415.43Da | Causes fatal deaths in cattle and outbreaks in human health by causing illness. | 49 | ||
| B | Neurotoxins | Anatoxin-a | 165Da | Potent agonist of postsynaptic cholinergic nicotinic leading to depolarizing neuromuscular blockade. | 29 |
| Homoanatoxin-a | – | Cholinergic nicotinic agonist binds to neuronal nicotinic acetylcholine receptors and functions same as Anatoxin-a. | 57 | ||
| Anatoxin-a(s) | 252Da | Synthesizes organophosphate insecticides such as parathion and malathion. | 38 | ||
| Saxitoxin | 299Da | It causes poisoning in shellfish by paralyzing them. | 53 | ||
| C | Dermatotoxins | – | It causes skin irritation. commonly known as Swimmer’s itch. | 17 | |
| D | Endotoxins | – | It causes fever in humans, animals and is concerned with septic shock syndrome (SSS). | 18,26 | |
Non-Toxic Secondary Metabolites From Cyanobacteria
Few cyanobacterial species also produce non-toxic metabolites which help in different ways in protecting them such as phytohormones, siderophores, and UV protective/absorbing compounds18.
Phytohormones
Phytohormones help the plant in terms of their growth, cell division, cell differentiation, and nutrient release. They are produced by Acutodesmus dimorphus, Synechococcus sp, Phormidium corium etc18. Phytohormones such as auxins, indole acetic acid, cytokinin, gibberellins, play significant roles in the germination of the shoot and root lengths 59. Phytohormones regulate different developmental stages and responses of plants under certain environmental conditions60. The release and production of phytohormones by cyanobacterial species have very important ecological consequences 57. The advantage of this is, it has a broad spectrum of activity which is needed for the development of normal plants in both in-vivo and in-vitro cultures13. The production of phytohormones such as auxins, cytokinin, improves the condition of a plant and is active in physiological responses such as wounding, herbivores’ pathogen attacks in salinity, and drought stress. Nostocales of cyanobacteria play a vital role in the paddy fields because of their ability to fix the atmospheric nitrogen and phytohormones supplied for crop growth61.
Siderophores
Siderophores are known as sideramines and are formed by the synthesis of iron chelators62. Siderophores are nothing but to obtain iron in limited conditions. Many bacteria secrete soluble amounts of iron chelators which compete with the environmental iron molecules 40. Siderophores help in the uptake, soluble, transportation of iron and these are complex molecules that solubilize the ferric ions at the range of pH 7.4 25. They have an extremely high affinity towards iron molecules. Iron transport factors are on the boundary line of primary, secondary metabolite compounds because it is required for growth and stimulating the growth under Fe-deficient conditions. These are found in soil that is produced microbially and enterobacterial antibiotics are isolated from human fecal extracts. Micro-organisms show both reduced and advanced affinity towards ferric ions in solubilizing and transporting. Fluorescent siderophores can induce disease suppression because Pseudomonas produces63. Negative mutants of siderophores either promote plant growth under certain conditions or fail to protect against the disease25. It is also involved in antibiotic activity due to the ability to take up Fe-siderophores complexes such as nocardamine and deferri-triacetyl fusigen63
UV-Protective Compounds
For energy production, the cyanobacteria depend on the light, and at a comparable instance, they are exposed to harmful UV radiation15. Micro-organisms show different ways in overcoming this damage with UV light, UV avoidance, UV absorption/sunscreen compounds, synthesis of radiation-absorbing pigments, DNA repairing process15,64. Cyanobacteria produce two sunscreen compounds when they are exposed to harmful UV radiations, they are; scytonemin, mycosporine-like Amino acids (MAAs) 15. With the help of these two compounds, the cyanobacteria can overcome the harmful UV radiations toxicity and are exploited in cosmetic industries18, which was found to defend the skin from UV damage and helped to balance the antioxidant defense system. Sun-screening compounds such as scytonemin and MAAs facilitated cyanobacteria to grow in extreme conditions on bare rocks and in the open ocean 13.
Mycosporine like Amino Acids-(MAAs)
MAAs are water-soluble, colorless compounds with cyclohexane chromophores connected with nitrogen substituents of the amino acids 3. MAAs are isolated from the cyanobacterial genera such as Anabaena, Nostoc, Lyngbya, Synechococcus, Synechocystis18. The basic function of this compound is to assist the cells from alteration by absorbing the UV radiations, it consumes the energy without reactive oxygen species (ROS) scavenging, also protecting the skin from UV damage, defense against oxidative thermal stress 3. Mycosporine-like amino acids are synthesized purely when they are exposed to UV-B19. Cyanobacteria can match the UV protection by exploding the manufacture of MAAs when they are unprotected from harmful UV radiations64. MAAs are involved in antioxidant activity and also absorb light in UV-A and UV-B ranges with a maximum absorbance of 310-360nm13,64. They are present primarily in the cytosol of cells and found on outer cell membranes such as in the Nostoc community and facilitate the photoprotective nature with the ability to spread energy without producing ROS. Four enzymes actively participate in the synthesis of MAAs-shinorine in Anabaena variabilis ATCC29413, dehydroquinase synthase homologue DHQS, O-methyl-transferase O-MT, ATP grasp ligase, an NRPS-like enzyme whereas ATP grasp ligase and NRPS- like enzymes are closely related to amino acid attachment to cyclohexanone region helps in the formation of imine19.
Scytonemin
Scytonemin shows similar features as MAAs and they can block the UV radiations up to 90% 18. Scytonemin is isolated from Lyngbya sp, Anabaena sp,18. It is present in the sheaths of a few cyanobacteria which live in extreme conditions such as on bare rocks and in open oceans 64. Scytonemin (544Da) is a dimeric indole phenolic alkaloid, lipid-soluble, the yellow-brown pigment that acts as a passive sunscreen compound in the protection of cyanobacteria against UV light in marine and freshwater environmental conditions 19,64. The maximum absorbance is in the 380 nm range. By random genetic activations, the gene cluster is liable for producing the scytonemin from Nostoc punctiforme 65. It is restricted only to cyanobacteria and biosynthesized in response to UV-A radiations 19. The biosynthesis pathway starts with tryptophan and tyrosine15. After scytonemin is synthesized, it is eliminated from the outer protective covering of cyanobacteria. Factors to regulate these compounds include UV radiations, Salinity, oxidative stress, nutrient deficiency64. It is chiefly engaged in photoprotection by UV radiations and also has anti-inflammatory activity with no cytotoxicity against non-dividing cells. Scytonemin inhibits polo-like kinase 1 (PLK1) which is present in phosphorylation of activation of proteins such as cdc25C and it allows to repress cell division. So, it is considered a promising compound used in pharmaceutical fields such as Anti-cancer drugs, Sunscreen agents, anti-inflammatory drugs etc3.
Table 3: Non-Toxic Metabolites and their causes.
| S.no | Non-Toxic Metabolite | Causes | References | |
| 1 | Phytohormones | It helps in terms of growth, cell division, cell differentiation, nutrient release. | 60,61 | |
| 2 | Siderophores | It induces the disease suppression and negative mutants of siderophores and is involved either in promoting growth factors or failing to protect against disease. | 25 | |
| 3 | UV protective Compounds | Mycosporine like Amino acids (MAA) | Protects the cells from alteration by riveting UV radiation to consume the energy without reactive oxygen species (ROS) scavenging, protects skin from UV damage, defense against oxidative thermal stress. | 3,64 |
| Scytonemin | It blocks UV radiation up to 90% and biosynthesis in response to UV-A radiation. | 18,19 | ||
Bioactivities of Cyanobacteria
The secondary metabolites of marine cyanobacteria show a wide range of bioactivities which is related to natural environments such as anticancer, anti-infective, anti-inflammatory, antifungal, antialgal, antibacterial, antimicrobial, etc where their functions are not well known but show different roles in pharmaceutical/medical areas such as treating the diseases and biological disorders. Bioactive compounds of marine cyanobacteria have high potentiality in pharma industries.
Anticancer Activity
There is a crucial need to develop brand-new anticancer drugs because tumour cells are processing resistivity against accessible drugs such as Taxanes which is leading to failure in the aiding of cancer treatment (chemotherapeutic). Niveshika et al. (2017) reported a novel cyancompound 9-Ethyliminomethyl-12-(morpholin-4-ylmethoxy)-5,8,13,16-tetraaza -hexacene-2,3-dicarboxylic acid (EMTAHDCA) from freshwater cyanobacteria Nostoc sp. MGL001 has anticancer properties. It was found that cyanocompound EMTAHDCA induced significant cytotoxic response against Dalton’s lymphoma ascites (DLA) cells with an inhibitory concentration (IC50) value of 372.4 ng/mL in a dose and time dependent manner after 24 h of incubation66. Several marine cyanobacterial compounds interact with certain significant molecular targets which are involved in anticancer activity resulting in controlling the death of tumor cells, this targets to start the compounds to slow down the proliferation of cancer cells by inducing apoptotic cell death67. Marine cyanobacteria from the unexplored environments, there is the source of compounds that leads to the discovery of new drugs 67. There are about 62 different isolates from different biomes that tested for anticancer activity and all these isolates belong to 23 genera amongst five orders of cyanobacteria which was identified in Brazil2. More than half of marine cyanobacterial species are potentially utilized for the natural process of secondary metabolite compounds which are impressive in damaging cancer cells by causing apoptosis 5. Several compounds have developed as templates for development of new Anticancer drugs67. These drugs play an important role in treating many deadly and biological disorders19. such as Curacin A and Dolastatin 10 have gone through all pre-clinical and objective trials as potency anticancer drugs.
Curacin A is produced by Lyngbya majuscula and shows the high potentiality of anticancer activity through the suppression of tubulin polymerization57. It is a unique thiazole-containing compound and found to have high potentiality against breast cancer22.
Dolastatin 10 is a bioactive compound that is isolated from Symploca species. It is a pentapeptide compound with 4 (unique) amino acids; dolavaline, dola isoleucine, dolaphenine. This bioactive compound of anticancer activity acts as a potent antiproliferative agent22. An analog of dolastatin 10 is soblidotin TZT-1027, it is different from dolastatin in absence of thiazoline ring from the dolaphenine residue5. It is biosynthesized by NRPS-PKS enzymes to disrupt the microtubule formation57. Cryptophycins are one of the most potent anticancer agents which were produced by marine cyanobacterial species named Nostoc sp GSV2248. Cryptophycins were first identified for their antifungal activity against cryptococci. Later, Moore and his co-workers isolated the same compound from the Nostoc sp and explained their toxicity against tumor cell lines68. This compound found activities against both drug-sensitive and drug-resistant tumor cell lines5,58. Cryptophycins show 1000 times more effective anticancer activity other than any bioactive compounds18,23
Apratoxin A is an effective cytotoxic compound isolated from Lyngbya majuscula species in the marine habitat of cyanobacteria. It is considered a cytotoxic compound due to its action mechanism in weakening the fibroblast growth factor signaling pathway5. Apratoxin A is best-known for causing G1 phase cell cycle arrest resulting in apoptosis22. Many anticancer drugs act as apoptotic modulators to destroy the outcast cells. These apoptotic cells develop complex structural alterations which allow the identification of tumor cells. During the early stages, cells become smaller in size, having dense cytoplasm with thin organelles in it66. Cell extracts from Cyanobium sp; CENA154, Nostoc spp; CENA64 & CENA 69, Oxynema sp; CENA135 have anticancer properties against murine colon cancer line CT-26 and lung cancer 3LL found in Brazilian cyanobacterial species2.
Antibacterial Activity
The secondary metabolites from marine cyanobacteria represent a large source of Bioactive compounds and these are known for their therapeutic effects. Certain marine cyanobacterial compounds are recognized as major producers possessing antibacterial activity. An increasing rise in bacterial strains against antibiotics is encountered in past years. So, to solve this issue there is a huge demand for antibacterial compounds in pharmaceutical fields68. Specifically, the secondary metabolites from marine cyanobacteria have developed a rich source of new therapeutics3. Antibacterial compounds are most effective towards gram-positive and gram-negative bacteria and the discovery of new drugs has become very essential to control bacterial infection during antibacterial resistance. Major antibacterial compounds of cyanobacteria belong to the orders Nostocales and Oscillatoriales 69.
Hapalindole is an indole alkaloid isolated from Fischerella sp. It has shown antibacterial activity against gram-positive, gram-negative bacteria such as E. coli ATCC25992 and Staphylococcus aureus ATCC25923. It is considered as sodium channel modulators1. All the antibacterial alkaloids are indole-containing compounds1. The 12-epi-hapalindole E isonitrile compound’s mode of action is to hinder the RNA polymerase of bacteria70.
Noscomine is a di-terpenoid isolated from the Nostoc commune EAWAG 122b and this compound showed antibacterial activity against Bacillus cereus, Staphylococcus epidermidis and E.coli. Carbamide cyclophanes is a paracyclophane that is detached from the Nostoc sp CAVN 10 shows reasonable antibacterial activity against Staphylococcus resulting in minimum inhibitory concentration (MIC) value of nano molar limit because of its methicillin-resistance 68. Of late, the alkyne containing polyketides-anaephenes were found to be active against Staphylococcus aureus. In peptides, the N-methylation of phenylamine is considered as a crucial factor against antibacterial activity. Niveshika et al (2016) reported novel cyanocompound 9-Ethyliminomethyl-12-(morpholin-4-ylmethoxy)-5,8,13,16-tetraaza -hexacene-2,3-dicarboxylic acid (EMTAHDCA) isolated from Nostoc sp. MGL001 has antibacterial properties71. The cyanocompound exhibited growth inhibiting effects against the gram negative bacterial strains and produced a maximum zone of inhibition at 150 µg/mL concentration71. Even insects have resistance against polluted bacteria by producing antibacterial proteins such as cecropins, attacins, defensins, lysozyme, diptericin, sacrotoxis which causes lysis or bacteriostatic, sometimes by attacking the parasite25.
Antiviral Activity
Increasing viral impedance and their actions on antiviral drugs have transformed into a major issue in the medical area. Several viral diseases are spreading dramatically which are showing impacts on human health and society. Few deadly viruses are namely severe acute respiratory syndrome (SARS), middle east respiratory syndrome (MERS), ebola, swine flu, and now coronavirus disease in 2019 (COVID 19) 72.
Lectins are antiviral proteins that were isolated from cyanobacteria. These are monomer proteins with low molecular weight resulting in the repressive specificity for glycoproteins73. There are three members of lectins that are isolated from cyanobacteria. They are Cyanovirin-N (CV-N), Microvirin (MV-N), Scytovirin (SVN) 74–76. Cyanovirin-N is 101 amino acids long isolated from Nostoc ellipsosporum. CV-N is potent against HIV 1 and 2, in nano-concentrations, Simian immune-deficiency virus (SIV), and other lentiviruses 22. Also involved in inhibition of herpes virus, measles virus in in-vitro culture75. Also recovered to stamp down the division in Hepatitis C virus, Influenza virus77. Microvirin (MV-N) weighs around 14.3 kDa. MV-N is isolated from cyanobacterial Microcystis aeruginosa PCC7806 and shares about 33% of their individuality with effective Anti-Human Immune-Deficiency Virus (HIV)78. Their mode of mechanism helps in the inhibition of virus-cell interaction 76. Scytovirin (SCV) 68 shows antiviral activity by intrusive nature with various tracks in the viral fusion activity. SCV is a 95% amino acid with five disulfide bonds isolated from the binary compound infusion of Scytonema varium22. SCV binds to the covering of glycoprotein HIV (such as gp120, gp160, and gp41) and sets off the low-level nanomolar concentrations68. Calcium spirulan 74kDa is isolated from Arthrospira plantesis which shows the activity against viruses admit HIV-1, Herpes simplex virus type 1 (HSV-1), Human Cytomegalovirus (HCMV), Influenza virus18. The structure of Calcium spirulan consists of sulfated polysaccharides79. Sulfoglycolipids such as sulfolipids have antiviral activity and it is isolated from cyanobacterial genera Lyngbya, Phormidium, Scytonema. This shows the inhibition of HIV 2 DNA polymerization function, reverse transcriptase of HIV1. The presence of fatty acid chains of sulfo glycolipids is mandatory for activity..
Antifungal Activity
Pathogenic fungi are developing resistance to numerous antifungal drugs80. So, there is an urgent need to find out new antifungal compounds which show good resistant 70. Cyano-peptides such as Hassallidin A & B, D, hectochlorin, lyngbyabellin A & B, majusculamide C, laxaphycins, scytophycin, calophycin, etc are identified as antifungal compounds. In cyanobacteria, the majority of antifungal compounds are produced from orders such as Nostocales, Oscillatoriales, Stigonematales81. Hassallidin A & B are cyanobacterial glycosylate lipopeptides are isolated from Hassillia sp. It shows antifungal activity against Candida species with 4.8 μg/mL as MIC value67 whereas Hassallidin D is stranded from Anabaena species shows antifungal activity against Candida albicans, Candida krusei with the MIC value of ≤2.8 μg/mL82. The cyclic tri decapeptides and tolybyssidins A & B are two antifungal compounds that show antifungal activity against the yeast Candida albicans and are isolated from collective refined cyanobacterium Tolypothrix byssoidea EAWAG 19583. The Laxaphycins are well-known antifungal compounds isolated from two cyanobacterial species namely Anabaena laxa 84 and Lyngbya majuscula17. Hectochlorin is isolated from Lyngbya majuscula with unique characters called 2 DHIV units and the gem-di-chloro-group exhibits potent antifungal activity against Candida albicans and anti-proliferative activity for actin stabilization. Hectochlorine structurally resembles Lyngbyabellins A & B57. Hectochlorin A is the acyl CoA synthetase homologue that activates free hexanoic acid and provides hectochlorin synthesis15. Lunatoic acid is an antifungal agent isolated from Cohliobocus lunatus that helps in inducing chlamydospore formation 25. Cryptophycin is first reported as an antifungal compound but later Moore and his co-workers found the same compound from the Nostoc strain exhibiting anticancer activity against tumor cell lines. So, they named this compound anticancer activity58.
Antialgal Activity
Antialgal compounds result in damaging the cellular morphology, SOD (Superoxide dismutase) activity, reduce chlorophyll-content, it also induces ROS etc85. Antialgal activity is highly restricted to certain cyanobacterial genera such as Fischerella, Nostoc, Anabaena, Calothrix, Scytonema containing nitrogen-fixing property with heterocystous filamentous cyanobacteria86. Antialgal compounds possess growth inhibitory activity of algae by targeting their photosynthesis, respiration, enzyme activity, oxidative stress induction87. Fischerella A is an antialgal compound isolated from Fischerella muscicola which has unique enediyne; two heterocyclic moieties show the antialgal activities along with antifungal activity4. Cyanobacterin is isolated from Scytonema hofmannii. It is identified and characterized as chlorinated γ-lactone89. Cyanobacterin is a cyanobacterial toxin that is considered an allelopathic compound that exhibits growth inhibition in cyanobacteria, eukaryotic algae, in various higher plants 26. Cyanobacterin precisely inhibits photosystem II89. Cyanobacterin compounds LU-1 and LU-2 are involved in the inhibition of electron transport in photosystem 2 and they don’t show similarity in terms of morphology. Cyanobacterin LU-1 is isolated from Nostoc linckia and it is found to inhibit cyanobacteria, algae except in non-photosynthetic microbes. Whereas LU-2 inhibits only the cyanobacteria26. The 2’-deoxyadenosine is a bioactive compound isolated from Streptomyces jiujiangensis JXJ 0074T. This bioactive compound damages the vegetative cells by crumpling, collapsing, expanding, perforating, breaking the filamentous cyanobacteria85. Nostocyclamide is a cyclic peptide that appears as an uncoupler of electron transport in photosynthesis and exhibits the inhibition of cyanobacteria. It is considered an allelopathic compound26.
Anti-inflammatory Activity
Secondary metabolites from the marine cyanobacterial species show great participation in producing effective drugs due to their unique structure frameworks and competency with significant biological activity such as anti-inflammatory activity 23. Chemically assorted compounds were found to induce the anti-inflammatory activity66.
The C-phycocyanin is a water-soluble pigment isolated from the marine cyanobacterial species Spirulina platensis and shows hepatoprotective effect due to inhibition of cytochrome P450 by mediated reactions involved in reactive metabolites formation, ability to act as radical scavenging, or sometimes both91. The C-phycocyanin exhibits anti-inflammatory activity due to its noesis to suppress the arachidonic acid metabolism and scavenging free oxygen radicals. This compound shows anti-inflammatory activity along with antioxidant activity91. The spirulina exhibits anti-arthritic effects due to the antioxidant and anti-inflammatory activities of its component phycocyanin92. Different species of marine cyanobacterial genera Lyngbya are known to produce secondary metabolites93. Microcolin A & B is a bioactive compound isolated from the blue-green algae Lyngbya 94. Their mode of mechanism is unidentified but both microcolin A and B are structurally distinguishable in terms of immuno-suppressive drugs. Microcolin A is a lipo-peptide present commonly in the benthic form of cyanobacteria and it can reduce the survival and inhibit the settlement of larvae Porites astreoides by unsettling the natural biomes95. Malyngamide is a lipopeptide isolated from Lyngbya sp93. The majority of Malyngamide S, X are isolated from Bursatella leachii96, Malyngamide O, P is isolated from cyanobacterial species Stylocheilus longicauda97. These compounds show a broad spectrum of bioactivities such as anti-inflammatory, cytotoxicity, antimicrobial etc98. In murine RAW 246.7, the macrophage cell line is treated with LPS and exhibits anti-inflammatory activity by inhibiting induced nitric oxide production100. When the production of nerve growth factor (NGF) and the organic process factors are increased at the central nervous system (CNS), it causes in suppressing the inflammation by shifting immunity response to the anti-inflammation and restrictive mode in the specific brain environment100.
This production in brain cells is induced by pro-inflammatory and anti-inflammatory cytokines like Interleukin-1 (IL-1), (IL-4), (IL-5), tumor necrosis factors (TNF-α), transforming growth factor-beta (TGF-β), interferon beta (IFN-β) by NFkβ signaling 101,102.
Antiprotozoal Activity
Several natural products have been identified in cyanobacteria against the anti-protozoal activity such as malarial parasite plasmodium, protozoan parasites such as Trypanosoma also called sleeping sickness or Chagas disease, and Leishmania (leishmaniasis)103. Many compounds are active in this activity by also showing cytotoxicity by limiting their drug usage. Medicine discovery against anti-protozoal diseases is very dragging because these bioactive compounds from cyanobacterial species are developing resistance towards the specific drugs68. There is imperative demand for more efficient drugs to be discovered because there are no new medicines that are available for tropical disease treatment 104. The antiprotozoal compounds are lipophilic phenolic ambigols which is isolated from Fischerella ambigua, heirridin B is isolated from Phormidium ectocarpi and Cyanobium sp105,106, dolophoenanthridine is an alkaloid calothrixin 107isolated from marine cyanobacteria Calothrix107, the carmabin A, dragomabin, dragonamide A is linear lipopeptides108, the lagunamide A-C is a cyclic depsipeptide and Malyngolide dime is a cyclopeptide is isolated from Lyngbya majuscula 109,110, Venturamide A & B is a cyclic peptide isolated from Oscillatoria sp111, gallinamide A is a linear peptide and this is isolated from Schihzothrix 112,113. All these compounds have antiprotozoal activities which are further used in pharmaceuticals to discover new drugs. Table 4 showed cyanobacterial bioactivities and causes.
Table 4: Cyanobacterial bioactivities and their causes
| S.no | Cyanobacterial Bioactivity | Causes | References |
| 1 | Anticancer activity | Anticancer activity results in controlling the death of tumor cells by inducing apoptotic cell death. Ex: Dolastatin 10, Cryptophycin F, Apratoxin A shows anticancer activity. | 5 |
| 2 | Antibacterial activity | Antibacterial compounds are effective towards Gram-positive and Gram-negative bacteria and the discovery of new drugs has become essential to control the bacterial infection during its resistance. Ex: Hapalindole, Noscomine shows antibacterial activity. | 68,70 |
| 3 | Antiviral activity | Several viral diseases are spreading dramatically which shows impacts on human health and society. Ex: Cyanovirin-N, Microvirin, Scytovirin shows antiviral activity. | 75,76 |
| 4 | Antifungal activity | Pathogenic fungi are developing resistance to numerous antifungal drugs. But few bioactive compounds have shown antifungal activity such as Hassallidin A, B & D, Hectochlorin, Calophycin, etc. | 70,81 |
| 5 | Antialgal activity | Antialgal compounds possess the growth inhibitory activity of algae by targeting their photosynthesis, respiration, enzyme activity, oxidative stress induction.
Ex: Cyanobacterin, Nostocyclamide, etc shows antialgal activity. |
87,88 |
| 6 | Anti-inflammatory activity | Bioactive compounds have great involvement to produce effective drugs due to their competency with significant biological activity. Ex: C-phycocyanin shows anti-inflammatory activity by inhibiting arachidonic acid metabolism. | 91 |
| 7 | Anti-protozoal activity | Drug discovery against anti-protozoal diseases is very slow because bioactive compounds are developing resistance towards specific drugs. But few compounds such as Heirridin B, Carmabin A, venturamide A & B show anti-protozoal activity. | 68,113,114 |
Screening Strategies of Bioactive Compounds from Cyanobacteria
Traditionally, the new drug disclosure is a wet laboratory process that is completely experimental and this process helps to identify the main compound in bioactivity. The new drug discovery and its remedial solutions is an extended process, very costly114. The extracts and refined compounds are time-tested against specific drug targets. Pharmacologists attempt to speed up this screening process by developing new strategies under in-vivo and in-vitro cultures. Viewing of cyanobacteria for pharmacologically bioactive compounds has received considerable attention during past decades115. The screening of isolated compounds from different natural sources is a common way to find out new biologically active compounds. To analyze the quality and safety of water the screening methods are necessary to detect possible toxins present in the environment samples116. Enormous drawing of potency drugs with their targets are being commonly known through a variety of broad speed with the new application including DNA Sequencing, micro-array or 2D gel electrophoresis, mass spectroscopy assays and so on117. Clustscan, NRPS-PKS, NP-searcher are genome screening programs that are used to predict the locations of gene clusters and their constitution of putative products 118. Different screening methods help to identify or detect the presence of toxins in cyanobacteria, namely microscopy assay, physicochemical methods, molecular-based methods, biochemical-based methods, and chemical methods. Every method has particular boundaries in terms of sensibility, reliableness, and limit of sense. All these methods in screening help to characterize, identify cyanobacterial toxins.
Microscopy Assay
This technique is employed for monitoring the cyanobacterial community present in the water bodies; it can be either freshwater or marine water habitat. Microscopy assay is done by cell counting which helps to monitor the cyanobacterial blooms. There are two microscopes used in this analysis as epifluorescence and inverted light microscopy119. In epifluorescence microscopy, the cells are counted in autotrophs by using a 515-560 nm absorption range whereas in heterotrophs the cells are counted by using a blue light source at 420 to 490nm119,120. The inverted light microscopy is used to examine the phytoplanktonic communities in freshwater and marine water environments121. With the help of these two microscopies, the abundance of specific cell colonies in cells/ ML helps to determine the target organisms. Due to dense colonies presence may give false cell estimations and non-homogenous cell distribution. For example, the aggregation of some marine cyanobacterial colonies such as Microcystis doesn’t allow its individuality at a particular level based on its geomorphology122,123. Flow cytometry is used to detect phytoplankton because of its higher sensitivity than any other microscopic approach124. The main disadvantage of this microscopy technique is that there is no possible way to differentiate between toxic and non-toxic cyanobacteria.
Physicochemical Methods
In this method of screening, the cyanobacterial water bodies are used to detect the growth conditions including weather, nutrient availability, presence or absence of photopigments125. Estimation of the presence of nitrogen and phosphorus in water, temperature, and light intensity may favor the cyanobacterial bloom formation in water bodies. Change in weather mainly impacts the bloom growth promotion by modifying seasonal variations in warming of air and water which adds nutrients resulting in increased bloom formation. Cyanobacteria contain the accessory pigments such as Chlorophyll a, phycocyanin, and phycoerythrin126. Chlorophyll-a is present in various organisms, so it is considered a good indicator of total autotrophic phytoplankton127. Phycocyanin and Phycoerythrin are cyanobacterial specific pigments. The phycocyanin is the accessory pigment present in freshwater cyanobacteria128,129 and phycoerythrin is the accessory pigment dominating in marine habitat131. These pigments help to estimate the total biomass of cyanobacteria. The spectrophotometric method is a fast and straightforward pigment quantification based on the relative abundance intensity at a specific wavelength131.
Molecular-based Methods
The specificity, reliability, and speed of this molecular-based method can overcome the disadvantage of Microscopy assay by detecting and quantifying the cyanobacterial toxin encoding genes. Due to its high sensitivity, it enables the early warning of cyanobacterial toxin in water bodies long before the cyanobacterial bloom formation132. So, this method is considered a more efficient monitoring method. It includes polymerase chain reaction (PCR), Real-time quantitative polymerase chain reaction (qPCR), and microarray.
The sequence and diversity of NRPS-PKS cluster genes in-vivo acculturation in cyanobacteria demonstrates the degeneration PCR screening, cloning, sequencing of fragments constitutes as performing to determine potency gene targets in diversified cyanobacterial lineages62. The structure of cyanobacterial metabolites is characterized by using isotopically labeled precursors giving the biogenesis and a specific gene template and it is known by using degenerate PCR amplification. Gerwick and co-workers identified the biosynthetic gene clusters for Apratoxin-a; they used a single cell as PCR to template the whole genome of the producer. Then the genome was sequenced and the cluster was known using various screening47. PCR technique is used to detect the cyanobacterial toxins in marine forms where the host of the primers have developed for aiming the MC gene cluster and also the toxicologic assessment of micro-organisms including the cyanotoxins, nodularin, neurotoxins133. This technique is mainly achieved by amplifying the 16SrRNA gene used for the identification of prokaryotes. This formulation has been utilized to identify different genes associated with CYN manufacture and MC producing from MC strains134,135. The multiple PCR is used to target the 16SrRNA to detect the toxic contamination of MC in regular dietary supplements136.
The qPCR is a quantitative method compared to PCR in terms of sensitivity with the detection limit of Microcystis spp and Cylindrospermopsin raciborskii137,138 and helps in detecting and quantifying the cyanobacterial specific genes 16SrRNA such as Microcystis specific rRNA genes139 There are two different methods used in qPCR technique such as SYBR green and TaqMan63. SYBR green consists of a fluorescent molecule that can intercalate double-stranded DNA. There is a disadvantage of unspecific binding which might occur during the amplification63. The TaqMan method is considered as a short sequence added to the sample. In this method, the amplification is highly specific140.
Microarray is a technique that is utilized to make a screening of gene expression on the genomic scale. Using a microarray chip, it analyses efficiently and quickly by providing quantifiable information on the amount of nucleic acid in the environmental sample141. This technique performs gene expression analysis by targeting the RNA retro transcribed into complementary DNA (cDNA) to identify genetic variation such as Single nucleotides polymorphism (SNP) and its mutation. DNA chip method is formulated to discover the microcystin (MCs) gene (mcy) and nodularin (NOD) synthetases (nda) producing cyanobacterial genera such as Anabaena, Microcystis, Planktothrix, Nostoc, Nodularia142.
Biochemical-based Methods
There are various methods to detect the biochemical properties of cyanobacterial toxins. Among those methods, enzyme-linked immunosorbent assay (ELISA), Ligand binding assay, enzyme inhibition assay. ELISA uses antibodies either monoclonal or polyclonal for quantifying and detecting the cyanobacterial toxins in water samples143,144. ELISA is active along with analytical techniques namely HPLC and LC-MS for more high-fidelity MCs analysis. Because this method is delicate, low-level of expertise needed with limited ridge life may undervalue the denseness of toxins. Antibodies have been generated with different cross quality against MC-LR and this is with success governing the MC content in environmental samples145,146. An indirect competitive ELISA is derived from polyclonal antibodies because there is a good cross-reactivity against a range of purified MC variants146. A direct competitive ELISA is used to detect MCs in water samples also considered as good cross-reactivity with MC variants147.
Measurements are used by two techniques: The radio-isotopic technique based on 32 phosphates radiolabeled substrate148 and the colorimetric assay based on p-nitrophenyl phosphate substrate149. The radio-isotopic technique is dependent on the radiolabeled proteins38. The phosphatase inhibition is also used for speedy observance of the MC producing bloom toxicity41.
Chemical Methods
This method is used to detect the cyanobacterial toxins in water are majorly fluid-based separation such as high-performance liquid chromatography (HPLC), Nuclear magnetic resonance (NMR), and various Mass spectrometric techniques namely triple quadrupole mass spectrometry (LC-MS/MS), Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry, liquid chromatography time of flight (LC-TOF) are available to different cyanobacterial toxins.
Cyanotoxins are chromatographically separated under higher pressure in LC columns by a liquid that was crowded with tiny particles150. Reverse HPLC is used but saxitoxins, β-N-methylamino-L-alanine (BMAA) are polar substances that are separated by hydrophilic interaction LC (HILC)151,152. The need for standardization for many MC variance makes the detection difficult and their results are generally expressed as MC-R equivalents125,153. Liquid chromatography-mass spectrometry (LC-MS) is an analytical technique that helps to detect, identify and confirm the presence of cyanotoxins in environmental samples and it is also considered a highly selective and sensitive technique. This combines the physical detachment capacity of LC mass analysis capabilities of MS and detection of the MC, NOD, CYN is easy. The analysis is performed by ELISA or antithetic LC-MS methods to detect and determine certain isomers154. Detection of BMAA is also difficult due to its smaller molecular structure. There are five different methods or techniques used to detect the BMAA compound in marine cyanobacteria such as HPLC with fluorescence, UV, MS discovery etc155.
LC-MS/MS is an analytical technique that is highly sensitive and selective towards unequivocal detection and quantification of unknown toxins in environmental samples156. CYN has a different molecular structure when compared to others but they are analyzed together by LC-MS/MS by applying particular MS-MS transitions. No other method is found to detect the CYN157. MALDI-TOF is a speedy, exclusive, and delicate analytical method with advanced declarations allowing the exact mass measurement and sensing of compounds founded on their molecular formula. In this ion’s mass to charge ratio is determined in terms of their time measurements158. MCs are peptides, and are readily hypersensitive to sensing with MALDI- TOF MS159. This technique provides support to HPLC by detecting cyanotoxins in minute-quantities such as a cyanobacterial colony not available as purified standards. Unlike LC-MS, LC-TOF-MS has a reward to produce the accurate measurement providing good selectiveness in convoluted samples160. NMR is an analytical technique that studies the magnetic property of certain atomic nuclei and provides detailed information about the structure of the material. This technique is not so useful for quantifiable analysis of cyanotoxins161. Table 5 showed screening methods used to detect the presence of cyanobacterial metabolites.
Table 5: Screening methods used to detect the presence of cyanobacterial metabolites.
| Sl. No | Screening Methods | Uses | References | |
| 1 | Microscopy assay | This method is used to monitor the cyanobacterial community present in water bodies. Two different microscopes are used as epifluorescence and Inverted light microscopy | 120 | |
| 2 | Physicochemical methods | In this method, cyanobacterial water bodies are used to detect the growth conditions including weather, nutrient availability, and presence or absence of photopigments. | 125 | |
| 3 | Molecular based methods | PCR | Used to detect cyanobacterial toxins in marine forms and mainly achieved by amplifying the 16SrRNA gene used for identification of prokaryotes. | 133 |
| qPCR | It is a quantitative method compared to the PCR method and helps in detecting and quantifying cyanobacterial specific genes 16SrRNA. | 138 | ||
| Microarray | It is utilized to form screening of gene reflection on a genomic scale using a microarray chip (provides quantitative information on the amount of nucleic acid present in an environmental sample). | 144 | ||
| 4 | Biochemical based methods | Used to detect biochemical properties of cyanobacterial toxins. ELISA, ligand binding assay, enzyme inhibition assay, and colorimetric assay are used as biochemical methods. | 41,143 | |
| 5 | Chemical methods | Used to detect cyanobacterial toxins in water by liquid separation methods such as HPLC, NMR, Mass spectrometric methods (LC-MS/MS, MALDI-TOF, LC-TOF). Among these NMR is considered to be less efficient for quantitative analysis. | 151,161 | |
Conclusion
Cyanobacteria show the high potentiality to create aggregative populations in the surroundings as they are present as common members of plant communities in marine, freshwater, and brackish water throughout the world. Cyanobacteria are known to produce secondary metabolites which are either useful such as research fields, pharmaceuticals, drug discovery, etc., or harmful by producing toxins that cause cyanobacterial blooms, diseases to animals and wildlife, and even fatal deaths. Numerous secondary metabolites have been identified and sporadic from marine cyanobacterial species which are time-tested for different forms of bioactivities such as anti-cancer, antialgal, antifungal activities, etc. They also offer many advantages as their cultures are readily established and their manufacture can be optimized to give property yields in industrial-level scales. In particular, the application of molecular biology and DNA amplification technology is used to detect the toxins that provide advanced significance in water quality management. Many screening methods are developed to monitor the water assessment of public health risks. The impact of toxins on the water environment is important in the future because of its relation to human health.
Within the scope of this review, several facets of environmental monitoring to detect cyanobacterial toxins by using different screening methods were discussed and analyzed. Different types of complementary approaches were presented such as microscopy assays, physicochemical methods, molecular-based methods, biochemical-based methods, and chemical methods, and their respective advantages and disadvantages were discussed. Among them, the molecular-based method allows first screening of cyanobacterial toxins and gives primary response about the presence of an approached sample in the environment. After concluding the potentially toxic cyanobacteria further methods are carried out as final confirmation of its toxic status.
The review mainly illustrates the cyanobacterial toxins and their causes to the environment, cyanobacterial secondary metabolites which are involved in bioactivities, screening methods to detect the presence of cyanobacterial toxins which prevents future health risk and improves water management.
Acknowledgment
We owe our sincere gratitude to St. Joseph’s College (Autonomous) for providing support for the presentation of the work.
Conflict of Interest
The authors declare no conflict of interest.
Funding Source
There is no funding to report for the present article.
References
- Carpine R, Sieber S. Antibacterial and antiviral metabolites from cyanobacteria: Their application and their impact on human health. Current Research in Biotechnology. 2021;3(February):65-81. doi:10.1016/j.crbiot.2021.03.001
CrossRef - Shishido TK, Popin RV, Jokela J, et al. Dereplication of natural products with antimicrobial and anticancer activity from Brazilian cyanobacteria. Toxins. 2019;12(1):1-17. doi:10.3390/toxins12010012
CrossRef - Demay J, Bernard C, Reinhardt A, Marie B. Natural products from cyanobacteria: Focus on beneficial activities. Marine Drugs. 2019;17(6):1-49. doi:10.3390/md17060320
CrossRef - Niedermeyer TH orst J. Anti-infective Natural Products from Cyanobacteria. Planta medica. 2015;81(15):1309-1325. doi:10.1055/s-0035-1546055
CrossRef - Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007;68(7):954-979. doi:10.1016/j.phytochem.2007.01.012
CrossRef - Jaiswal D, Wangikar PP. Dynamic Inventory of Intermediate Metabolites of Cyanobacteria in a Diurnal Cycle. iScience. 2020;23(11):101704. doi:10.1016/j.isci.2020.101704
CrossRef - Chittora D, Meena M, Barupal T, Swapnil P. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochemistry and Biophysics Reports. 2020;22(January):100737. doi:10.1016/j.bbrep.2020.100737
CrossRef - Santos-Merino M, Singh AK, Ducat DC. New applications of synthetic biology tools for cyanobacterial metabolic engineering. Frontiers in Bioengineering and Biotechnology. 2019;7(FEB). doi:10.3389/fbioe.2019.00033
CrossRef - Baran R, Ivanova NN, Jose N, et al. Functional genomics of novel secondary metabolites from diverse cyanobacteria using untargeted metabolomics. Marine Drugs. 2013;11(10):3617-3631. doi:10.3390/md11103617
CrossRef - dos Santos AM, Vieira KR, Sartori RB, et al. Heterotrophic cultivation of cyanobacteria: Study of effect of exogenous sources of organic carbon, absolute amount of nutrients, and stirring speed on biomass and lipid productivity. Frontiers in Bioengineering and Biotechnology. 2017;5(FEB):1-7. doi:10.3389/fbioe.2017.00012
CrossRef - Singh JS, Kumar A, Rai AN, Singh DP. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Frontiers in Microbiology. 2016;7(APR):1-19. doi:10.3389/fmicb.2016.00529
CrossRef - Pisciotta JM, Zou Y, Baskakov I v. Light-dependent electrogenic activity of cyanobacteria. PLoS ONE. 2010;5(5). doi:10.1371/journal.pone.0010821
CrossRef - Yadav S, Sinha RP, Tyagi MB, Kumar A. Cyanobacterial secondary metabolites. International Journal of Pharma and Bio Sciences. 2011;2(2):144-167. doi:10.1007/978-94-009-0213-8_33
CrossRef - Jones MR, Pinto E, Torres MA, et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Research. 2021;196:117017. doi:10.1016/j.watres.2021.117017
CrossRef - Kehr JC, Picchi DG, Dittmann E. Natural product biosynthesis in cyanobacteria: A treasure trove of unique enzymes. Beilstein Journal of Organic Chemistry. 2011;7:1622-1635. doi:10.3762/bjoc.7.191
CrossRef - Welker M, Dittmann E, von Döhren H. Cyanobacteria as a source of natural products. Methods in Enzymology. 2012;517:23-46. doi:10.1016/B978-0-12-404634-4.00002-4
CrossRef - Burja AM, Banaigs B, Abou-Mansour E, Grant Burgess J, Wright PC. Marine cyanobacteria – A prolific source of natural products. Tetrahedron. 2001;57(46):9347-9377. doi:10.1016/S0040-4020(01)00931-0
CrossRef - Haque F, Banayan S, Yee J, Chiang YW. Extraction and applications of cyanotoxins and other cyanobacterial secondary metabolites. Chemosphere. 2017;183:164-175. doi:10.1016/j.chemosphere.2017.05.106
CrossRef - Mandal S, Rath J. Secondary Metabolites of Cyanobacteria and Drug Development. Published online 2015:23-43. doi:10.1007/978-3-319-12009-6_2
CrossRef - Calcott MJ, Ackerley DF, Knight A, Keyzers RA, Owen JG. Secondary metabolism in the lichen symbiosis. Chemical Society Reviews. 2018;47(5):1730-1760. doi:10.1039/c7cs00431a
CrossRef - Jones AC, Gu L, Sorrels CM, Sherman DH, Gerwick WH. New tricks from ancient algae: natural products biosynthesis in marine cyanobacteria. Current Opinion in Chemical Biology. 2009;13(2):216-223. doi:10.1016/j.cbpa.2009.02.019
CrossRef - Vijayakumar S, Menakha M. Pharmaceutical applications of cyanobacteria-A review. Journal of Acute Medicine. 2015;5(1):15-23. doi:10.1016/j.jacme.2015.02.004
CrossRef - Ali Shah SA, Akhter N, Auckloo BN, et al. Structural diversity, biological properties and applications of natural products from cyanobacteria. A review. Marine Drugs. 2017;15(11). doi:10.3390/md15110354
CrossRef - Gkelis S, Lanaras T, Sivonen K, Taglialatela-Scafati O. Cyanobacterial toxic and bioactive peptides in freshwater bodies of Greece: Concentrations, occurrence patterns, and implications for human health. Marine Drugs. 2015;13(10):6319-6335. doi:10.3390/md13106319
CrossRef - Demain AL, Fang A. The natural functions of secondary metabolites. Advances in biochemical engineering/biotechnology. 2000;69:1-39. doi:10.1007/3-540-44964-7_1
CrossRef - Berry JP. Cyanobacterial Toxins as Allelochemicals with Potential Applications as Algaecides, Herbicides and Insecticides. Marine Drugs. 2008;6(2):117-146. doi:10.3390/md20080007
CrossRef - Dittmann E, Neilan BA, Erhard M, Bo T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. 1997;26:779-787.
CrossRef - Haddad SP, Bobbitt JM, Taylor RB, et al. Determination of microcystins, nodularin, anatoxin-a, cylindrospermopsin, and saxitoxin in water and fish tissue using isotope dilution liquid chromatography tandem mass spectrometry. Journal of Chromatography A. 2019;1599:66-74. doi:10.1016/j.chroma.2019.03.066
CrossRef - Taylor P, Zurawell RW, Chen H, Burke JM, Prepas EE. Journal of Toxicology and Environmental Health , Part B : Critical Reviews Hepatotoxic Cyanobacteria : A Review of the Biological Importance of Microcystins in Freshwater Environments. (July 2013):37-41. doi:10.1080/10937400590889412
CrossRef - Gaget V, Humpage AR, Huang Q, Monis P, Brookes JD. Benthic cyanobacteria: A source of cylindrospermopsin and microcystin in Australian drinking water reservoirs. Water Research. 2017;124:454-464. doi:10.1016/j.watres.2017.07.073
CrossRef - Pereira DA, Giani A. Cell density-dependent oligopeptide production in cyanobacterial strains. FEMS Microbiology Ecology. 2014;88(1):175-183. doi:10.1111/1574-6941.12281
CrossRef - Rodgers KJ, Main BJ, Samardzic K. Cyanobacterial Neurotoxins: Their Occurrence and Mechanisms of Toxicity. Neurotoxicity Research. 2018;33(1):168-177. doi:10.1007/s12640-017-9757-2
CrossRef - Nunnery JK, Mevers E, Gerwick WH. Biologically active secondary metabolites from marine cyanobacteria. Current Opinion in Biotechnology. 2010;21(6):787-793. doi:10.1016/j.copbio.2010.09.019
CrossRef - Kurmayer R, Deng L, Entfellner E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae. 2016;54:69-86. doi:10.1016/j.hal.2016.01.004
CrossRef - Marinho MM, Domingos P, Oliveira AC, et al. Microcystins ( cyanobacteria hepatotoxins ) bioaccumulation in fish and crustaceans from Sepetiba Bay ( Brasil , RJ ). 2003;42:289-295. doi:10.1016/S0041-0101(03)00144-2
CrossRef - Carmichael WW. Cyanobacteria secondary metabolites—the cyanotoxins. Journal of Applied Bacteriology. 1992;72(6):445-459. doi:10.1111/j.1365-2672.1992.tb01858.x
CrossRef - Wiegand C, Pflugmacher S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicology and Applied Pharmacology. 2005;203(3 SPEC. ISS.):201-218. doi:10.1016/j.taap.2004.11.002
CrossRef - McElhiney J, Lawton LA. Detection of the cyanobacterial hepatotoxins microcystins. Toxicology and Applied Pharmacology. 2005;203(3 SPEC. ISS.):219-230. doi:10.1016/j.taap.2004.06.002
CrossRef - Lagoutte B, Tunkelrott M liisa, Dano J. Fast and Direct Extraction of Cell-associated Hepatotoxins from Toxic Cyanobacteria. 2006;86(5). doi:10.2175/106143013X13807328849891
CrossRef - El-Shehawy R, Gorokhova E, Fernández-Piñas F, del Campo FF. Global warming and hepatotoxin production by cyanobacteria: What can we learn from experiments? Water Research. 2012;46(5):1420-1429. doi:10.1016/j.watres.2011.11.021
CrossRef - Heresztyn T, Nicholson BC. Determination of cyanobacterial hepatotoxins directly in water using a protein phosphatase inhibition assay. Water Research. 2001;35(13):3049-3056. doi:10.1016/S0043-1354(01)00018-5
CrossRef - Mankiewicz-Boczek J, Palus J, Gagała I, et al. Effects of microcystins-containing cyanobacteria from a temperate ecosystem on human lymphocytes culture and their potential for adverse human health effects. Harmful Algae. 2011;10(4):356-365. doi:10.1016/j.hal.2011.01.001
CrossRef - Msagati TAM, Siame BA, Shushu DD. Evaluation of methods for the isolation, detection and quantification of cyanobacterial hepatotoxins. Aquatic Toxicology. 2006;78(4):382-397. doi:10.1016/j.aquatox.2006.03.011
CrossRef - Mazur-Marzec H, Meriluoto J, Pliński M. The degradation of the cyanobacterial hepatotoxin nodularin (NOD) by UV radiation. Chemosphere. 2006;65(8):1388-1395. doi:10.1016/j.chemosphere.2006.03.072
CrossRef - Lu N, Ling L, Guan T, et al. Broad-specificity ELISA with a heterogeneous strategy for sensitive detection of microcystins and nodularin. Toxicon. 2020;175:44-48. doi:10.1016/j.toxicon.2019.12.003
CrossRef - Bouaïcha N, Miles CO, Beach DG, et al. Structural diversity, characterization and toxicology of microcystins. Toxins. 2019;11(12):1-40. doi:10.3390/toxins11120714
CrossRef - Grindberg R v., Ishoey T, Brinza D, et al. Single cell genome amplification accelerates identification of the apratoxin biosynthetic pathway from a complex microbial assemblage. PLoS ONE. 2011;6(4). doi:10.1371/journal.pone.0018565
CrossRef - Chen G, Wang L, Li W, Zhang Q, Hu T. Nodularin induced oxidative stress contributes to developmental toxicity in zebrafish embryos. Ecotoxicology and Environmental Safety. 2020;194(174):110444. doi:10.1016/j.ecoenv.2020.110444
CrossRef - Barón-Sola Á, Sanz-Alférez S, del Campo FF. First evidence of accumulation in cyanobacteria of guanidinoacetate, a precursor of the toxin cylindrospermopsin. Chemosphere. 2015;119:1099-1104. doi:10.1016/j.chemosphere.2014.08.046
CrossRef - Lopes VR, Antunes A, Welker M, Martins RF, Vasconcelos VM. Morphological, toxicological and molecular characterization of a benthic Nodularia isolated from Atlantic estuarine environments. Research in Microbiology. 2010;161(1):9-17. doi:10.1016/j.resmic.2009.11.001
CrossRef - Mashile PP, Mashile GP, Dimpe KM, Nomngongo PN. Occurrence, quantification, and adsorptive removal of nodularin in seawater, wastewater and river water. Toxicon. 2020;180(March):18-27. doi:10.1016/j.toxicon.2020.03.009
CrossRef - Lehtimäki N, Shunmugam S, Jokela J, et al. Nodularin uptake and induction of oxidative stress in spinach (Spinachia oleracea). Journal of Plant Physiology. 2011;168(6):594-600. doi:10.1016/j.jplph.2010.09.013
CrossRef - Méjean A, Ploux O. A Genomic View of Secondary Metabolite Production in Cyanobacteria. Vol 65. Elsevier; 2013. doi:10.1016/B978-0-12-394313-2.00006-8
CrossRef - Adamski M, Wołowski K, Kaminski A, Hindáková A. Cyanotoxin cylindrospermopsin producers and the catalytic decomposition process: A review. Harmful Algae. 2020;98(August):101894. doi:10.1016/j.hal.2020.101894
CrossRef - Adamski M, Zimolag E, Kaminski A, Drukała J, Bialczyk J. Effects of cylindrospermopsin, its decomposition products, and anatoxin-a on human keratinocytes. Science of the Total Environment. 2021;765(xxxx). doi:10.1016/j.scitotenv.2020.142670
CrossRef - Hinojosa MG, Gutiérrez-Praena D, Prieto AI, Guzmán-Guillén R, Jos A, Cameán AM. Neurotoxicity induced by microcystins and cylindrospermopsin: A review. Science of the Total Environment. 2019;668:547-565. doi:10.1016/j.scitotenv.2019.02.426
CrossRef - Pearson LA, Dittmann E, Mazmouz R, Ongley SE, D’Agostino PM, Neilan BA. The genetics, biosynthesis and regulation of toxic specialized metabolites of cyanobacteria. Harmful Algae. 2016;54:98-111. doi:10.1016/j.hal.2015.11.002
CrossRef - Gademann K, Portmann C. Secondary Metabolites from Cyanobacteria: Complex Structures and Powerful Bioactivities. Current Organic Chemistry. 2008;12(4):326-341. doi:10.2174/138527208783743750
CrossRef - Gayathri M, Shunmugam S, Thajuddin N, Muralitharan G. Phytohormones and free volatile fatty acids from cyanobacterial biomass wet extract (BWE) elicit plant growth promotion. Algal Research. 2017;26(June):56-64. doi:10.1016/j.algal.2017.06.022
CrossRef - Nowicka B, Ciura J, Szymańska R, Kruk J. Improving photosynthesis, plant productivity and abiotic stress tolerance – current trends and future perspectives. Journal of Plant Physiology. 2018;231:415-433. doi:10.1016/j.jplph.2018.10.022
CrossRef - Pham HTL, Nguyen LTT, Duong TA, et al. Diversity and bioactivities of nostocacean cyanobacteria isolated from paddy soil in Vietnam. Systematic and Applied Microbiology. 2017;40(8):470-481. doi:10.1016/j.syapm.2017.08.001
CrossRef - Ehrenreich IM, Waterbury JB, Webb EA. Distribution and diversity of natural product genes in marine and freshwater cyanobacterial cultures and genomes. Applied and Environmental Microbiology. 2005;71(11):7401-7413. doi:10.1128/AEM.71.11.7401-7413.2005
- Dragan AI, Pavlovic R, McGivney JB, et al. SYBR Green I: Fluorescence properties and interaction with DNA. Journal of Fluorescence. 2012;22(4):1189-1199. doi:10.1007/s10895-012-1059-8
CrossRef - D’Agostino PM, Woodhouse JN, Liew HT, et al. Bioinformatic, phylogenetic and chemical analysis of the UV-absorbing compounds scytonemin and mycosporine-like amino acids from the microbial mat communities of Shark Bay, Australia. Environmental Microbiology. 2019;21(2):702-715. doi:10.1111/1462-2920.14517
CrossRef - Balskus EP, Walsh CT. Investigating the initial steps in the biosynthesis of cyanobacterial sunscreen scytonemin. Journal of the American Chemical Society. 2008;130(46):15260-15261. doi:10.1021/ja807192u
CrossRef - Niveshika, Verma E, Maurya SK, Mishra R, Mishra AK. The Combined Use of in Silico, in Vitro, and in Vivo Analyses to Assess Anti-cancerous Potential of a Bioactive Compound from Cyanobacterium Nostoc sp. MGL001. Front Pharmacol. 2017;8:873. doi:10.3389/fphar.2017.00873
CrossRef - Shishido TK. Cyanobacterial Bioactive Compounds: Biosynthesis, Evolution, Structure and Bioactivity. Vol 2.; 2012. https://helda.helsinki.fi/bitstream/handle/10138/154414/cyanobac.pdf;sequence=1
- Singh RK, Tiwari SP, Rai AK, Mohapatra TM. Cyanobacteria: An emerging source for drug discovery. Journal of Antibiotics. 2011;64(6):401-412. doi:10.1038/ja.2011.21
CrossRef - Sahoo CR, Maharana S, Mandhata CP, Bishoyi AK, Paidesetty SK, Padhy RN. Biogenic silver nanoparticle synthesis with cyanobacterium Chroococcus minutus isolated from Baliharachandi sea-mouth, Odisha, and in vitro antibacterial activity. Saudi Journal of Biological Sciences. 2020;27(6):1580-1586. doi:10.1016/j.sjbs.2020.03.020
CrossRef - Swain SS, Paidesetty SK, Padhy RN. Antibacterial, antifungal and antimycobacterial compounds from cyanobacteria. Biomedicine and Pharmacotherapy. 2017;90:760-776. doi:10.1016/j.biopha.2017.04.030
CrossRef - Niveshika, Verma E, Mishra AK, Singh AK, Singh VK. Structural Elucidation and Molecular Docking of a Novel Antibiotic Compound from Cyanobacterium Nostoc sp. MGL001. Front Microbiol. 2016;7:1899. doi:10.3389/fmicb.2016.01899.
CrossRef - Guan W jie, Ni Z yi, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708-1720. doi:10.1056/nejmoa2002032
CrossRef - Hori K, Miyazawa K, Ito K. Some common properties of lectins from marine algae. Hydrobiologia. 1990;204-205(1):561-566. doi:10.1007/BF00040287
CrossRef - Boyd MR, Gustafson KR, McMahon JB, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus- inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrobial Agents and Chemotherapy. 1997;41(7):1521-1530. doi:10.1128/aac.41.7.1521
CrossRef - Shahzad-ul-Hussan S, Gustchina E, Ghirlando R, Clore GM, Bewley CA. Solution structure of the monovalent lectin microvirin in complex with Manα(1-2) Man provides a basis for anti-HIV activity with low toxicity. Journal of Biological Chemistry. 2011;286(23):20788-20796. doi:10.1074/jbc.M111.232678
CrossRef - Bokesch HR, O’Keefe BR, McKee TC, et al. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42(9):2578-2584. doi:10.1021/bi0205698
CrossRef - O’Keefe BR, Smee DF, Turpin JA, et al. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrobial Agents and Chemotherapy. 2003;47(8):2518-2525. doi:10.1128/AAC.47.8.2518-2525.2003
CrossRef - Huskens D, Fe G, Vermeire K, Kehr J christoph, Balzarini J, Dittmann E. Microvirin , a Novel ␣ ( 1 , 2 ) -Mannose-specific Lectin Isolated from Microcystis aeruginosa , Has Anti-HIV-1 Activity Comparable with That of Cyanovirin-N but a Much Higher Safety Profile *. 2010;285(32):24845-24854. doi:10.1074/jbc.M110.128546
CrossRef - Hayashi T, Hayashi K, Maeda M, Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. Journal of Natural Products. 1996;59(1):83-87. doi:10.1021/np960017o
CrossRef - Mo S, Krunic A, Chlipala G, Orjala J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. Journal of Natural Products. 2009;72(5):894-899. doi:10.1021/np800751j
CrossRef - Jaki B, Orjala J, Sticher O. A novel extracellular diterpenoid with antibacterial activity from the cyanobacterium Nostoc commune. Journal of Natural Products. 1999;62(3):502-503. doi:10.1021/np980444x
CrossRef - Vestola J, Shishido TK, Jokela J, et al. Hassallidins, antifungal glycolipopeptides, are widespread among cyanobacteria and are the end-product of a nonribosomal pathway. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(18):1-9. doi:10.1073/pnas.1320913111
CrossRef - Jaki B, Zerbe O, Heilmann J, Sticher O. Two novel cyclic peptides with antifungal activity from the cyanobacterium Tolypothrix byssoidea (EAWAG 195). Journal of Natural Products. 2001;64(2):154-158. doi:10.1021/np000297e
CrossRef - Frankmölle WP, Larsen LK, Caplan FR, et al. Blue-green alga Anabaena laxa I. Isolation and biological properties. The Journal of Antibiotics. 1992;45(9):1451-1457.
CrossRef - Zhang BH, Chen W, Li HQ, et al. An antialgal compound produced by Streptomyces jiujiangensis JXJ 0074T. Applied Microbiology and Biotechnology. 2015;99(18):7673-7683. doi:10.1007/s00253-015-6584-3
CrossRef - Flores E, Wolk CP. Production, by filamentous, nitrogen-fixing cyanobacteria, of a bacteriocin and of other antibiotics that kill related strains. Archives of Microbiology. 1986;145(3):215-219. doi:10.1007/BF00443648
CrossRef - Dahms HU, Ying X, Pfeiffer C. Antifouling potential of cyanobacteria: A mini-review. Biofouling. 2006;22(5):317-327. doi:10.1080/08927010600967261
CrossRef - Mason CP, Edwards KR, Carlson RE, Pignatello J, Gleason FK, Wood JM. Isolation of chlorine-containing antibiotic from the freshwater cyanobacterium scytonema hofmanni. Science. 1982;215(4531):400-402. doi:10.1126/science.6800032
CrossRef - Gleason FK, Baxa CA. Activity of the natural algicide, cyanobacterin, on eukaryotic microorganisms. FEMS Microbiology Letters. 1986;33(1):85-88. doi:10.1016/0378-1097(86)90191-6
CrossRef - Bhat VB, Madyastha KM. C-phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochemical and Biophysical Research Communications. 2000;275(1):20-25. doi:10.1006/bbrc.2000.3270
CrossRef - Reddy CM, Bhat VB, Kiranmai G, Reddy MN, Reddanna P, Madyastha KM. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochemical and Biophysical Research Communications. 2000;277(3):599-603. doi:10.1006/bbrc.2000.3725
CrossRef - Remirez D, González R, Merino N, Rodriguez S, Ancheta O. Inhibitory effects of Spirulina in zymosan-induced arthritis in mice. Mediators of Inflammation. 2002;11(2):75-79. doi:10.1080/09629350220131917
CrossRef - Malloy KL, Villa FA, Engene N, Matainaho T, Gerwick L, Gerwick WH. Malyngamide 2, an oxidized lipopeptide with nitric oxide inhibiting activity from a Papua New Guinea marine cyanobacterium. Journal of Natural Products. 2011;74(1):95-98. doi:10.1021/np1005407
CrossRef - Mandal AK, Hines J, Kuramochi K, Crews CM. Developing microcolin A analogs as biological probes. Bioorganic and Medicinal Chemistry Letters. 2005;15(18):4043-4047. doi:10.1016/j.bmcl.2005.06.020
CrossRef - Ritson-Williams R, Ross C, Paul VJ. Elevated temperature and allelopathy impact coral recruitment. PLoS ONE. 2016;11(12). doi:10.1371/journal.pone.0166581
CrossRef - Appleton DR, Sewell MA, Berridge M v., Copp BR. A new biologically active malyngamide from a New Zealand collection of the sea hare Bursatella leachii. Journal of Natural Products. 2002;65(4):630-631. doi:10.1021/np010511e
CrossRef - Gallimore WA, Scheuer PJ. Malyngamides O and P from the sea hare stylocheilus longicauda. Journal of Natural Products. 2000;63(10):1422-1424. doi:10.1021/np0000365
CrossRef - Gerwick WH, Tan LT, Sitachitta N. Nitrogen-Containing Metabolites Marine Cyanobacteria. The Alkaloids. 2001;57:75-184.doi: 10.1016/s0099-9598(01)57003-0.
CrossRef - Villa FA, Lieske K, Gerwick L. Selective MyD88-dependent pathway inhibition by the cyanobacterial natural product malyngamide F acetate. European Journal of Pharmacology. 2010;629(1-3):140-146. doi:10.1016/j.ejphar.2009.12.002
CrossRef - Villoslada P, Genain CP. Role of nerve growth factor and other trophic factors in brain inflammation. Progress in Brain Research. 2004;146:403-414. doi:10.1016/S0079-6123(03)46025-1
CrossRef - Awatsuji H, Furukawa Y, Hirota M, Furukawa S, Hayashi K. Interferons Suppress Nerve Growth Factor Synthesis as a Result of Interference with Cell Growth in Astrocytes Cultured from Neonatal Mouse Brain. Journal of Neurochemistry. 1995;64(4):1476-1482. doi:10.1046/j.1471-4159.1995.64041476.x
CrossRef - Awatsuji H, Furukawa Y, Hirota M, et al. Interleukin‐4 and ‐5 as modulators of nerve growth factor synthesis/secretion in astrocytes. Journal of Neuroscience Research. 1993;34(5):539-545. doi:10.1002/jnr.490340506
CrossRef - Gademann K, Kobylinska J. Antimalarial natural products of marine and freshwater origin. Chemical Record. 2009;9(3):187-198. doi:10.1002/tcr.200900001
CrossRef - Simmons TL, Engene N, Ureña LD, et al. Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium Oscillatoria nigro-viridis. Journal of Natural Products. 2008;71(9):1544-1550. doi:10.1021/np800110e
CrossRef - Papendorf O, König GM, Wright AD. Hierridin B and 2,4-dimethoxy-6-heptadecyl-phenol, secondary metabolites from the cyanobacterium Phormidium ectocarpi with antiplasmodial activity. Phytochemistry. 1998;49(8):2383-2386. doi:10.1016/S0031-9422(98)00440-3
CrossRef - Leão PN, Costa M, Ramos V, et al. Antitumor Activity of Hierridin B, a Cyanobacterial Secondary Metabolite Found in both Filamentous and Unicellular Marine Strains. PLoS ONE. 2013;8(7). doi:10.1371/journal.pone.0069562
CrossRef - Crnkovic CM, Krunic A, May DS, et al. Calothrixamides A and B from the Cultured Cyanobacterium Calothrix sp. UIC 10520. Journal of Natural Products. 2018;81(9):2083-2090. doi:10.1021/acs.jnatprod.8b00432
CrossRef - McPhail KL, Correa J, Linington RG, et al. Antimalarial linear lipopeptides from a panamanian strain of the marine cyanobacterium Lyngbya majuscula. Journal of Natural Products. 2007;70(6):984-988. doi:10.1021/np0700772
CrossRef - Tripathi A, Puddick J, Prinsep MR, Rottmann M, Tan LT. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. Journal of Natural Products. 2010;73(11):1810-1814. doi:10.1021/np100442x
CrossRef - Gutiérrez M, Tidgewell K, Capson TL, et al. Malyngolide dimer, a bioactive symmetric cyclodepside from the panamanian marine cyanobacterium lyngbya majuscula. Journal of Natural Products. 2010;73(4):709-711. doi:10.1021/np9005184
CrossRef - Linington RG, González J, Ureña LD, Romero LI, Ortega-Barría E, Gerwick WH. Venturamides A and B: Antimalarial constituents of the Panamanian marine cyanobacterium Oscillatoria sp. Journal of Natural Products. 2007;70(3):397-401. doi:10.1021/np0605790
CrossRef - Linington RG, Clark BR, Trimble EE, et al. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. Journal of Natural Products. 2009;72(1):14-17. doi:10.1021/np8003529
CrossRef - Miller B, Friedman AJ, Choi H, et al. The marine cyanobacterial metabolite gallinamide a is a potent and selective inhibitor of human cathepsin L. Journal of Natural Products. 2014;77(1):92-99. doi:10.1021/np400727r
CrossRef - DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: New estimates of drug development costs. Journal of Health Economics. 2003;22(2):151-185. doi:10.1016/S0167-6296(02)00126-1
CrossRef - Rimsha R, Richa J, Sheela K, Shrivastava PN, Manju J. Bioactive substances of cyanobacteria (Nostoc muscorum ): a review. International Journal of Pharma Sciences and Research. 2014;5(07):320-322. ISSN : 0975-9492
- Akter S, Vehniäinen M, Lamminmäki U. A homogeneous assay for rapid detection of cyanobacterial peptide hepatotoxins: Microcystins and nodularins. Clinica Chimica Acta. 2019;493:S67-S68. doi:10.1016/j.cca.2019.03.150
CrossRef - Kramer R, Cohen D. Functional genomics to new drug targets. Nature Reviews Drug Discovery. 2004;3(11):965-972. doi:10.1038/nrd1552
CrossRef - Challis GL. Mining microbial genomes for new natural products and biosynthetic pathways. Microbiology. 2008;154(6):1555-1569. doi:10.1099/mic.0.2008/018523-0
- Francisco DE, Mah RA, Rabin AC. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Transactions of the American Microscopical Society. 1973;92(3):416-421. doi:10.2307/3225245
CrossRef - Jones JG, Simon BM. An investigation of errors in direct counts of aquatic bacteria by epifluorescence microscopy, with reference to a new method for dyeing membrane filters. Journal of Applied Bacteriology. 1975;39(3):317-329. doi:10.1111/j.1365-2672.1975.tb00578.x
CrossRef - Purwar P, Han S, Lee Y, Saha B, Sandhan T, Lee J. High-resolution cost-effective compact portable inverted light microscope. Journal of Microscopy. 2019;273(3):199-209. doi:10.1111/jmi.12775
CrossRef - Lim CK, Lord G. Current developments in LC-MS for pharmaceutical analysis. Biological and Pharmaceutical Bulletin. 2002;25(5):547-557. doi:10.1248/bpb.25.547
CrossRef - Deng L, Fiskal A, Han X, Dubois N, Bernasconi SM, Lever MA. Improving the accuracy of flow cytometric quantification of microbial populations in sediments: Importance of cell staining procedures. Frontiers in Microbiology. 2019;10(APR):1-13. doi:10.3389/fmicb.2019.00720
CrossRef - Rutten TPA, Sandee B, Hofmann ART. Phytoplankton monitoring by high performance flow cytometry: A successful approach? Cytometry Part A. 2005;64(1):16-26. doi:10.1002/cyto.a.20106
CrossRef - Srivastava A, Singh S, Ahn CY, Oh HM, Asthana RK. Monitoring Approaches for a Toxic Cyanobacterial Bloom BT – Environmental Science & Technology. Environmental Science and Technology. 2013;47(16):8999-9013.
http://pubs.acs.org/doi/abs/10.1021/es401245k%0Ahttp://pubs.acs.org/doi /pdf/10.1021/es401245k%0Ahttp://dx.doi.org/10.1021/es401245k
CrossRef - Bertone E, Chuang A, Burford MA, Hamilton DP. In-situ fluorescence monitoring of cyanobacteria: Laboratory-based quantification of species-specific measurement accuracy. Harmful Algae. 2019;87(January):101625. doi:10.1016/j.hal.2019.101625
CrossRef - Papageorgiou GC, Tsimilli-Michael M, Stamatakis K. The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: A viewpoint. Photosynthesis Research. 2007;94(2-3):275-290. doi:10.1007/s11120-007-9193-x
CrossRef - Eriksen NT. Production of phycocyanin – A pigment with applications in biology, biotechnology, foods and medicine. Applied Microbiology and Biotechnology. 2008;80(1):1-14. doi:10.1007/s00253-008-1542-y
CrossRef - Bastien C, Cardin R, Veilleux É, Deblois C, Warren A, Laurion I. Performance evaluation of phycocyanin probes for the monitoring of cyanobacteria. Journal of Environmental Monitoring. 2011;13(1):110-118. doi:10.1039/c0em00366b
CrossRef - Pagels F, Guedes AC, Amaro HM, Kijjoa A, Vasconcelos V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnology Advances. 2019;37(3):422-443. doi:10.1016/j.biotechadv.2019.02.010
CrossRef - Zavřel T, Chmelík D, Sinetova MA, Červený J. Spectrophotometric determination of phycobiliprotein content in cyanobacterium synechocystis. Journal of Visualized Experiments. 2018;2018(139):1-9. doi:10.3791/58076
CrossRef - Humbert JF, Quiblier C, Gugger M. Molecular approaches for monitoring potentially toxic marine and freshwater phytoplankton species. Analytical and Bioanalytical Chemistry. 2010;397(5):1723-1732. doi:10.1007/s00216-010-3642-7
CrossRef - Moreira C, Ramos V, Azevedo J, Vasconcelos V. Methods to detect cyanobacteria and their toxins in the environment. Applied Microbiology and Biotechnology. 2014;98(19):8073-8082. doi:10.1007/s00253-014-5951-9
CrossRef - Rasmussen JP, Giglio S, Monis PT, Campbell RJ, Saint CP. Development and field testing of a real-time PCR assay for cylindrospermopsin-producing cyanobacteria. Journal of Applied Microbiology. 2008;104(5):1503-1515. doi:10.1111/j.1365-2672.2007.03676.x
CrossRef - Ouahid Y, del Campo FF. Typing of toxinogenic Microcystis from environmental samples by multiplex PCR. Applied Microbiology and Biotechnology. 2009;85(2):405-412. doi:10.1007/s00253-009-2249-4
CrossRef - Te SH, Chen EY, Gin KYH. Comparison of quantitative PCR and droplet digital PCR multiplex assays for two genera of bloom-forming cyanobacteria, Cylindrospermopsis and Microcystis. Applied and Environmental Microbiology. 2015;81(15):5203-5211. doi:10.1128/AEM.00931-15
CrossRef - Furukawa K, Noda N, Tsuneda S, Saito T, Itayama T, Inamori Y. Highly sensitive real-time PCR assay for quantification of toxic cyanobacteria based on microcystin synthetase a gene. Journal of Bioscience and Bioengineering. 2006;102(2):90-96. doi:10.1263/jbb.102.90
CrossRef - Moreira C, Martins A, Azevedo J, et al. Application of real-time PCR in the assessment of the toxic cyanobacterium Cylindrospermopsis raciborskii abundance and toxicological potential. Applied Microbiology and Biotechnology. 2011;92(1):189-197. doi:10.1007/s00253-011-3360-x
CrossRef - Chiu YT, Chen YH, Wang TS, Yen HK, Lin TF. A qPCR-based tool to diagnose the presence of harmful cyanobacteria and cyanotoxins in drinking water sources. International Journal of Environmental Research and Public Health. 2017;14(5). doi:10.3390/ijerph14050547
CrossRef - Soltani Tehrani B, Mirzajani E, Fallahi S, et al. Challenging TaqMan probe-based real-time PCR and loop-mediated isothermal amplification (LAMP): the two sensitive molecular techniques for the detection of toxoplasmosis, a potentially dangerous opportunistic infection in immunocompromised patients. Archives of Microbiology. 2020;202(7):1881-1888. doi:10.1007/s00203-020-01903-1
CrossRef - Pearson LA, Neilan BA. The molecular genetics of cyanobacterial toxicity as a basis for monitoring water quality and public health risk. Current Opinion in Biotechnology. 2008;19(3):281-288. doi:10.1016/j.copbio.2008.03.002
CrossRef - Rantala A, Rizzi E, Castiglioni B, de Bellis G, Sivonen K. Identification of hepatotoxin-producing cyanobacteria by DNA-chip. Environmental Microbiology. 2008;10(3):653-664. doi:10.1111/j.1462-2920.2007.01488.x
CrossRef - Ueno Y, Nagata S, Tsutsumi T, et al. Survey of microcystins in environmental water by a highly sensitive immunoassay based on monoclonal antibodies. Natural Toxins. 1996;4(6):271-276. doi:10.1002/(sici)(1996)4:6<271::aid-nt4>3.0.co;2-a
CrossRef - Yu FY, Liu BH, Chou HN, Chu FS. Development of a sensitive ELISA for the determination of microcystins in algae. Journal of Agricultural and Food Chemistry. 2002;50(15):4176-4182. doi:10.1021/jf0202483
CrossRef - Liu LQ, Xing CR, Yan H, Kuang H, Xu CL. Development of an ELISA and immunochromatographic strip for highly sensitive detection of microcystin-LR. Sensors (Switzerland). 2014;14(8):14672-14685. doi:10.3390/s140814672
CrossRef - Lei X, Song S, Tao H, et al. Development of Indirect Competitive Enzyme-Linked Immunosorbent and Immunochromatographic Strip Assays for Tiamulin Detection in Chicken. ACS Omega. 2018;3(3):3581-3586. doi:10.1021/acsomega.8b00289
CrossRef - Sheng JW, He M, Shi HC, Qian Y. A comprehensive immunoassay for the detection of microcystins in waters based on polyclonal antibodies. Analytica Chimica Acta. 2006;572(2):309-315. doi:10.1016/j.aca.2006.05.040
CrossRef - Lambert TW, Boland MP, Holmes CFB, Hrudey SE. Communications: Quantitation of the Microcystin Hepatotoxins in Water at Environmentally Relevant Concentrations with the Protein Phosphatase Bioassay. Environmental Science and Technology. 1994;28(4):753-755. doi:10.1021/es00053a032
CrossRef - An JS, Carmichael WW. Use of a colorimetric protein phosphatase inhibition assay and enzyme-linked immunosorbent assay for the study of microcystins and nodularins. Toxicon. 1994;32(12):1495-1507. doi:10.1016/0041-0101(94)90308-5
CrossRef - Kohoutek J, Babica P, Bláha L, Maršálek B. A novel approach for monitoring of cyanobacterial toxins: Development and evaluation of the passive sampler for microcystins. Analytical and Bioanalytical Chemistry. 2008;390(4):1167-1172. doi:10.1007/s00216-007-1785-y
CrossRef - Dell’Aversano C, Hess P, Quilliam MA. Hydrophilic interaction liquid chromatography-mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins. Journal of Chromatography A. 2005;1081(2):190-201. doi:10.1016/j.chroma.2005.05.056
CrossRef - Dell’Aversano C, Eaglesham GK, Quilliam MA. Analysis of cyanobacterial toxins by hydrophilic interaction liquid chromatography-mass spectrometry. Journal of Chromatography A. 2004;1028(1):155-164. doi:10.1016/j.chroma.2003.11.083
CrossRef - Ortea PM, Allis O, Healy BM, et al. Determination of toxic cyclic pentapeptides by liquid chromatography with detection using ultra-violet, protein phosphatase assay and tandem mass spectrometry. Chemosphere. 2004;55(10):1395-1402. doi:10.1016/j.chemosphere.2003.11.025
CrossRef - Kleinteich J, Wood SA, Puddick J, Schleheck D, Küpper FC, Dietrich D. Potent toxins in Arctic environments – Presence of saxitoxins and an unusual microcystin variant in Arctic freshwater ecosystems. Chemico-Biological Interactions. 2013;206(2):423-431. doi:10.1016/j.cbi.2013.04.011
CrossRef - Banack SA, Johnson HE, Cheng R, Cox PA. Production of the Neurotoxin BMAA by a Marine. Published online 2007:180-196.
CrossRef - Hedman CJ, Krick WR, Karner Perkins DA, Harrahy EA, Sonzogni WC. New Measurements of Cyanobacterial Toxins in Natural Waters Using High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Journal of Environmental Quality. 2008;37(5):1817-1824. doi:10.2134/jeq2007.0368
CrossRef - Fayad PB, Roy-Lachapelle A, Duy SV, Prévost M, Sauvé S. On-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry for the analysis of cyanotoxins in algal blooms. Toxicon. 2015;108:167-175. doi:10.1016/j.toxicon.2015.10.010
CrossRef - Kostrzewa M, Nagy E, Schröttner P, Pranada AB. How MALDI-TOF mass spectrometry can aid the diagnosis of hard-to-identify pathogenic bacteria–the rare and the unknown. Expert Review of Molecular Diagnostics. 2019;19(8):667-682. doi:10.1080/14737159.2019.1643238
CrossRef - Jang KS, Kim YH. Rapid and robust MALDI-TOF MS techniques for microbial identification: a brief overview of their diverse applications. Journal of Microbiology. 2018;56(4):209-216. doi:10.1007/s12275-018-7457-0
CrossRef - Kang L, Weng N, Jian W. LC-MS bioanalysis of intact proteins and peptides. Biomedical Chromatography. 2019 Jan;34(1):e4633. doi: 10.1002/bmc.4633.
CrossRef - Lin Y, Schiavo S, Orjala J, Vouros P, Kautz R. Microscale LC-MS-NMR platform applied to the identification of active cyanobacterial metabolites. Analytical Chemistry. 2008;80(21):8045-8054. doi:10.1021/ac801049k
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





