How to Cite | Publication History | PlumX Article Matrix
Vishal H Patel1* , Harsha P Soni2
, Harsha P Soni2  and Falguni R Patel3
and Falguni R Patel3
1Department of Microbiology, HVHP Institute of Postgraduate Studies and Research, Kadi. Gujarat India 382715
2Department of Biotechnology, Pramukh Swami Science and H.D Patel Arts College, Kadi. Gujarat India 382715
3Department of Biotechnology and Microbiology, Shree M.M Patel Institute of Science and Research, LDRP campus, Sector-15, Gandhinagar, Gujarat India 382015,
Corresponding Author E-mail: vishalhpatel_89@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2981
ABSTRACT:
The exopolysaccharides (EPSs) are natural polymers of carbohydrates and are excreted by some bacteria outside of their cell walls. The microbial EPS have several biotechnological applications viz. cosmetics, textiles, pharmaceuticals, agriculture, paints and petroleum industries. The wide range of applications and bioactive roles of EPS has triggered increased interest for search unusual and novel EPS.The bacteria from marine ecosystem are also known to secrete novel EPSs. In this context, the main objective of this research is isolation, screening of most potent culturable EPS producing halotolerant bacteria with novel EPS characteristics which can be used in uncommon applications related to the environment. All the bacterial isolates were isolated from coastal regions of Gujarat as it contains 1600 km long costal area, with wide microbial diversity and can serve as a source for promising EPS producers. 9 soil samples were collected from various coastal sites viz. Mundra, Jodiya, Dwarka, Somnath, Diu, Bhavnagar, Khambhat, Dumas and Umargam. Total 59 EPS producing bacterial isolates were obtained in Primary Screening. Based on the results of primary screening, potential morphologically diverse 9 isolates were selected for EPS production in liquid medium. The EPS production ranged from 22.3 to 33.5 mg/ml. The isolate VHP 34 gave best EPS production and was identified as Enterobacter cloacae by 16 s rRNA gene sequencing method. The isolate Enterobacter cloacae VHP-34 was able to grow 0-15% NaCl concentration, hence categorized as Moderately Halotolerant.
KEYWORDS: Exopolysaccharides; Enterobacter cloacae; Gujarat; Marine ecosystem
Download this article as:| Copy the following to cite this article: Patel V. H, Soni H. P, Patel F. R. Isolation, Screening and Molecular characterization of Exopolysaccharide Producing Moderately Halotolerant Bacteria from Various Coastal Sites of Gujarat. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Patel V. H, Soni H. P, Patel F. R. Isolation, Screening and Molecular characterization of Exopolysaccharide Producing Moderately Halotolerant Bacteria from Various Coastal Sites of Gujarat. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3IGj6oY |
Introduction
Exopolysaccharides (EPSs) are high molecular weight carbohydrate polymers that make important element of the extracellular polymers that adhere on microbial cells in the marine terrain. In recent times the demand of natural polymers has increased in medicinal, food and other sectors.1 The marine environment is a rich and largely unexplored source of microbial EPS that can be developed for implicit biotechnological applications.2 Owing to the broad ecological and physiological diversity of marine terrain, an interest on marine microorganisms has surfaced and gained considerable attention.3 Marine organisms have the ability to produce unique bioactive composites which can be used for society and diligence. Furthermore, bioactive compounds, extracellular polysaccharides from marine organisms have found to be fruitful for different industries too.4 Bacteria are of special interest to scientists, as they play an important part in saline as well as hypersaline surroundings and have the eventuality to produce compounds of industrial interest, one of these compounds is the exopolysaccharide (EPS).5
EPS plays a vital role in nutrient uptake, aggregation, adhesion to shells, and biofilms buildup.6 Different Gamma-Proteobacteria were established as EPS producers. Group of some Halophilic EPS producing bacteria are known for protecting membrane integrity. The commonest halophilic EPS producers belong to the genus Halomonas, most diversely H. maura, H. eurihalina, H.ventosae, H. anticariensis, and Halomonas sp.7 The EPSs produced by prokaryotes display a wide structural diversity and thus, have found a wide range of practices in different industrial sectors due to their physical, rheological properties.8
The EPS excreted by marine bacteria have been employed as constituents in food products, drugstore, petroleum industry and emulsification of crude oil, hydrocarbons, vegetable, mineral oils and bioremediation agents in environment management system.9 EPS produced by bacteria exhibiting significant structural diversity with new natural properties are considered as one of the important sources of natural polymers with multiple biotechnological applications.10 The present study is based on the isolation of novel EPS producing marine bacteria from different locations of coastal areas of Gujarat, India and screening the isolates for EPS production using various screening perspectives and identification of most potent culturable EPS producing bacteria.
Materials and Methods
Sample Collection
Soil samples were collected from various coastal sites of Gujarat (Fig. 1) viz. Mundra (22.8298° N, 69.3261° E), Jodiya (22.6942° N, 70.3078° E), Dwarka (22.2506° N, 68.9803° E), Somnath (20.8855° N, 70.4052° E), Diu (20.7144° N, 70.9874° E), Bhavnagar-Gogha beach (21.6767° N, 72.2845° E) Khambhat (22.3181° N, 72.6190° E), Surat Dumas beach (21.1068° N, 72.7115° E) and Umargam (20.1951° N, 72.7461° E) from January to December, 2019. Soil samples, collected in sterile zipper bags, were stored at 4°C.
Soil analysis of various sites
All soil samples were tested for micro as well as macro elements, pH and relative conductivity at Soil testing Laboratory, CORDET, IFFCO- Kalol, Gujarat.
Screening of EPS producing marine bacteria
Primary Screening for Marine EPS producers
Soil samples were serially diluted to 10-3 to 10-4 with sterile distilled water and subsequently, 0.1 ml from all dilutions was spread on two media i.e. modified Glucose yeast extract agar (GYEA) medium. The plates were incubated at 37 °C for 24 h. The modified GYEA medium consists 1 % Peptone, 1 % Yeast extract, 5 % Glucose and 3.5 % NaCl and supported maximum number of isolates hence was used for further analysis.
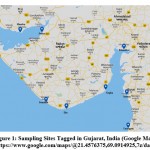 |
Figure 1: Sampling Sites Tagged in Gujarat, India (Google Map). |
Morphological parameters i.e. cell size, shape, arrangement, and pigmentation. EPS production was further checked by using Congo red agar plate assay in which the isolates were cultivated on GYEA medium incorporated with Congo red (0.08% w/v). Dye solution was sterilized individually and added to the medium after autoclaving.
Morphologically distinct colonies were picked and transferred to obtain pure culture of isolates. Each isolates were streaked onto modified GYEA slant and kept at 4ºC until further utilization. The metabolic diversity of selected isolates was studied by routine biochemical test. All the media used for biochemical test were supplemented with 3.5% NaCl.
Secondary Screening for Marine EPS producers
Secondary screening was done on the basis of quantification of EPS production using liquid media. For quantitative estimation of EPS, the overnight grown isolates were inoculated in modified Glucose Yeast extract medium and incubated at 37°C for 5 days on shaker at 150 rpm. At the interval of 24 h, 10 ml broth was withdrawn and centrifuged at 10,000 rpm for 10 min. The supernatant was collected in centrifuge tube and mixed with double volume of chilled Acetone (RANKEM, 99% pure). EPS was separated from the mixture in the form of a slimy sticky precipitate. Precipitates were filtered on a pre-weighted filter paper. The precipitates were allowed to dry overnight at room temperature. The dried filter paper was post-weighed after overnight drying. The increase in the weight of filter paper was measured which indicates the dry mass of EPS produced.11 On the basis of the results of primary and secondary screening, 9 potential EPS producers were selected and their biochemical characterization were performed.12
Salt Tolerance study of Selected Isolate
The isolate VHP-34 was grown on Modified GYEA along with different NaCl concentration from 0% to 30% for studying its salt tolerance. Furthermore VHP-34 was inoculated in modified GYE medium containing salt from 0% to 15% NaCl concentration to check the effect of salt concentration on EPS production and yield.
Molecular Identification of Potent EPS producer
Out of 9 potent EPS producers, highest EPS producing bacterial isolate VHP-34 was selected for further study. Genomic DNA was isolated and quality of DNA was checked on 1.0% Agarose Gel. Fragment of 16S rRNA gene was amplified by PCR and identified by Sanger sequencing method.13
Phylogenetic analysis of Potent EPS producer
The evolutionary history was concluded using the Neighbor-Joining method.14 The optimal tree with the sum of all branches length = 0.04658001 was shown in Figure. 3. The evolutionary distances were estimated with the p-distance method.15 Codon positions included were 1st+2nd+3rd+Noncoding. There were total of 970 positions in the final dataset. Evolutionary analyses were done in MEGA7.16
Results
Soil Analysis of various sites
Results of soil analysis from various sites were presented in Table 1 which reports different parameters like pH, conductivity, organic carbon, phosphate, potassium and different micro and macro elements in ppm. The pH of all soils ranged from 7.8 to 8.8. The conductivity of soil samples from different sites showed high variability with Dumas soil sample having least conductivity and Jodiya soil sample having highest conductivity. The organic carbon was on lower side for all soil samples. The highest P was found for Mundra sample and highest K was found in soils of Mundra as well as Jodiya. The highest Zinc was found in Khambhat sample. The highest Mn and S were found in Umargam sample.
Table 1: Soil sample analysis of various coastal sites of Gujarat.
| Parameters | Mundra | Dwarka | Somnath | Bhavnagar | Khambhat | Diu | Jodiya | Umargam | Dumas |
| pH | 8.4 | 7.9 | 8.0 | 8.3 | 8.3 | 8.8 | 7.8 | 8.3 | 8.8 |
| Conductivity mMos/Cm | 1.85 | 6.9 | 8.4 | 4.9 | 4.4 | 1.27 | 9.9 | 6.9 | 1.77 |
| Organic Carbon (%) | 0.3 | 0.15 | 0.12 | 0.15 | 0.12 | 0.15 | 0.68 | 0.15 | 0.12 |
| P (ppm) | 7.073 | 4.08 | 5.441 | 2.04 | 3.808 | 4.216 | 4.897 | 1.632 | 2.448 |
| K (ppm) | 70.01 | 18 | 68 | 65.99 | 40.029 | 11.011 | 70.014 | 36.011 | 68 |
| Fe (ppm) | 12.6 | 3.74 | 14.22 | 15.36 | 1.14 | 12.62 | 14.9 | 12.6 | 14.33 |
| Zn (ppm) | 0.42 | 0.06 | 0.44 | 0.52 | 2.7 | 0.06 | 1.52 | 0.4 | 0.5 |
| Cu (ppm) | 1.12 | 0.08 | 0.64 | 0.56 | 0.06 | 0.22 | 3.36 | 0.84 | 1.3 |
| Mn (ppm) | 7.14 | 1.72 | 13.32 | 7.82 | 1.76 | 1.1 | 12.4 | 15.03 | 12.88 |
| S (ppm) | 62 | 88 | 93 | 84 | 76 | 24 | 94 | 98 | 74 |
| B (ppm) | 2.26 | 1.27 | 2.5 | 1.7 | 2.55 | 1.83 | 4.24 | 3.33 | 2.32 |
Screening of marine bacteria for EPS production
Primary screening was done on the basis of colony morphology distinct isolates with mucoid, gummy and sticky colonies with slimy capsular material were considered as EPS producers. The EPS producing bacteria were fundamentally selected basis on the development and production of mucoid appearance of the colonies.17, 18 Phenotypic characterization of the isolates was done on the basis of colony morphology and Gram’s staining and KOH method.19 From colony morphology, total 59 different isolates were obtained from various coastal soil samples of Gujarat. The number of isolates selected from individual sites is depicted in Graph 1. The results for Gram’s staining and KOH method of 59 isolates are showed in Table 2; For KOH test isolates were initially cultivated on Nutrient agar. The gram reaction was confirmed by KOH test. The Lipopolysaccharide present in the cell wall of Gram negative bacteria reacts with KOH and make clump, on the basis of clump and string formation isolates can be distinguished as Gram negative. Amongst the 59 isolates, 28 were Gram positive big rods arranged in chain and 31 isolates were Gram negative short rods arranged in cluster.
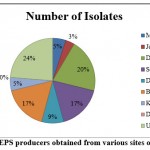 |
Graph 1: EPS producers obtained from various sites of Gujarat |
Further confirmation and selection was carried out using Congo red assay. The EPS production was indicated by black colonies with mucoid and slimy consistency, while non-slime producers usually remained pink.20 The EPS production potential of 59 isolates on modified GYEA and Congo red medium is also shown in Table 2.
Table 2: Morphological characterization of all EPS producing Isolates
| Isolate
No |
Gram’s Reaction | EPS production | ||
| Staining | KOH string formation | GYEA | Congo Red | |
| VHP-1 | -Ve | Present | +++ | +++ |
| VHP-2 | +Ve | Absent | + | + |
| VHP-3 | +Ve | Absent | + | + |
| VHP-4 | +Ve | Absent | + | + |
| VHP-5 | +Ve | Absent | + | + |
| VHP-6 | -Ve | Present | ++ | ++ |
| VHP-7 | -Ve | Present | ++ | ++ |
| VHP-8 | -Ve | Present | ++ | ++ |
| VHP-9 | -Ve | Present | ++ | ++ |
| VHP-10 | -Ve | Present | ++ | ++ |
| VHP-11 | -Ve | Present | ++ | ++ |
| VHP-12 | -Ve | Present | ++ | ++ |
| VHP-13 | +Ve | Absent | + | + |
| VHP-14 | +Ve | Absent | + | + |
| VHP-15 | +Ve | Absent | + | + |
| VHP-16 | +Ve | Absent | + | + |
| VHP-17 | +Ve | Absent | + | + |
| VHP-18 | +Ve | Absent | + | + |
| VHP-19 | +Ve | Absent | + | + |
| VHP-20 | +Ve | Absent | + | + |
| VHP-21 | -Ve | Present | ++ | ++ |
| VHP-22 | -Ve | Present | +++ | +++ |
| VHP-23 | +Ve | Absent | ++ | + |
| VHP-24 | -Ve | Present | ++ | ++ |
| VHP-25 | -Ve | Present | ++ | ++ |
| VHP-26 | -Ve | Present | ++ | ++ |
| VHP-27 | -Ve | Present | ++ | ++ |
| VHP-28 | -Ve | Present | ++ | ++ |
| VHP-29 | +Ve | Absent | +++ | +++ |
| VHP-30 | -Ve | Present | ++ | + |
| VHP-31 | +Ve | Absent | + | ++ |
| VHP-32 | -Ve | Present | +++ | ++ |
| VHP-33 | -Ve | Present | +++ | +++ |
| VHP-34 | -Ve | Present | ++++ | ++++ |
| VHP-35 | +Ve | Absent | + | ++ |
| VHP-36 | -Ve | Present | +++ | ++++ |
| VHP-37 | -Ve | Present | ++ | + |
| VHP-38 | -Ve | Present | +++ | +++ |
| VHP-39 | +Ve | Absent | + | ++ |
| VHP-40 | +Ve | Absent | + | + |
| VHP-41 | +Ve | Absent | + | + |
| VHP-42 | +Ve | Absent | + | + |
| VHP-43 | +Ve | Absent | + | + |
| VHP-44 | +Ve | Absent | + | ++ |
| VHP-45 | +Ve | Absent | + | + |
| VHP-46 | -Ve | Present | ++ | ++ |
| VHP-47 | +Ve | Absent | + | + |
| VHP-48 | -Ve | Present | ++ | ++ |
| VHP-49 | -Ve | Present | ++ | ++ |
| VHP-50 | -Ve | Present | ++ | ++ |
| VHP-51 | +Ve | Absent | +++ | +++ |
| VHP-52 | -Ve | Present | ++ | ++ |
| VHP-53 | +Ve | Absent | +++ | +++ |
| VHP-54 | -Ve | Present | ++ | ++ |
| VHP-55 | -Ve | Present | ++ | ++ |
| VHP-56 | -Ve | Present | ++ | ++ |
| VHP-57 | +Ve | Absent | + | + |
| VHP-58 | +Ve | Absent | + | + |
| VHP-59 | –Ve | Present | ++ | ++ |
Red color on Congo red (GYEA)
++ Pink color on Congo red (GYEA)
+++/++++ Black Congo red (GYEA)
KOH- Potassium Hydroxide
GYEA- Modified Glucose Yeast Extract Agar
Among the 59 isolates obtained from primary screening, 9 morphologically distinct, isolates VHP-1, VHP-22, VHP-29, VHP-33, VHP-34, VHP-36, VHP-38, VHP-51 and VHP-53 were selected on the basis on EPS production on solid media. The growth characteristics of all selected isolates on modified GYEA are depicted in Table 3.
Table 3: Colony Characteristics of Potent EPS producing isolates on modified GYEA
| Isolate→
Colony Morphology↓ |
VHP-1 | VHP-22 | VHP-29 | VHP-33 | VHP-34 | VHP-36 | VHP-38 | VHP-51 | VHP-53 |
| Size | 2 mm | 2 mm | 1 mm | 2 mm | 2 mm | 1 mm | 2 mm | 5 mm | 5 mm |
| Shape | Round | Irregular | Irregular | Round | Round | Round | Round | Irregular | Irregular |
| Edge | Entire | Entire | Wavy | Entire | Wavy | Wavy | Entire | Erose | Wavy |
| Elevation | Raised | Raised | Flat | Pulvinate | Raised | Raised | Pulvinate | Convex | Raised |
| Surface | Smooth | Smooth | Smooth | Smooth | Smooth | Smooth | Smooth | Smooth | Smooth |
| Consistency | Viscous | Viscous | Viscous | Viscous | Viscous | Viscous | Butyrous | Viscous | Viscous |
| Optical | Translucent | Opaque | Opaque | Translucent | Translucent | Translucent | Translucent | Opaque | Opaque |
| Pigment | Red | – | – | – | – | – | – |
Table 4 illustrates Biochemical characterization of 9 isolates. All the isolates were Glucose and Sucrose fermenter, while (VHP-1, 33) were able to ferment Mannitol. (VHP-22, 33, 34, 36, 38) were Lactose fermenter. (VHP-1, 22, 29, 36, 38, 51, 53) were mix acid fermenters and gave MR test positive. On the other hand (VHP-33, 34) were able to do butanediol fermentation and gave VP test positive. The 5 isolates (VHP-1, 29, 33, 34, 38) were able to use Citrate as sole carbon source. All the 9 of isolates were able to produce enzymes like Amylase, Protease, Lipase and Gelatinase producers. All the selected isolates could produce these enzymes in presence of 3.5% NaCl and can be considered as moderately halophilic organisms.
Table 4: Biochemical characterization of selected EPS producing isolates
| Isolate→
Biochemical test↓
|
VHP-1 | VHP-22 | VHP-29 | VHP-33 | VHP-34 | VHP-36 | VHP-38 | VHP-51 | VHP-53 |
| Glucose | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) |
| Sucrose | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) | +(A,G) |
| Mannitol | +(A,G) | _ | _ | +(A,G) | _ | _ | _ | _ | _ |
| Lactose | _ | +(A,G) | _ | +(A,G) | +(A,G) | +(A,G) | +(A,G) | _ | _ |
| Methyl Red | + | + | + | _ | _ | + | + | + | + |
| Voges Proskauer | _ | _ | _ | + | + | _ | _ | _ | _ |
| Citrate Permease | + | _ | + | + | + | _ | + | _ | _ |
| Amylase | + | + | + | + | + | + | + | + | + |
| Protease | + | + | + | + | + | + | + | + | + |
| Lipase | + | + | + | + | + | + | + | + | + |
| Gelatinase | + | + | + | + | + | + | + | + | + |
A= Acid Production G= Gas production – Negative + Positive
The secondary screening of these isolates was carried out by quantifying the EPS yield using liquid media. The amount of precipitated EPS produced by various isolates is presented in Graph 2. The EPSs production by isolates ranged from 22.3 to 33.5 mg/ml. Three isolates gave more than 30mg/ml EPS production. The isolate VHP-34 produced gave the highest EPS yield of 33.4mg/ml and was selected for further analysis.
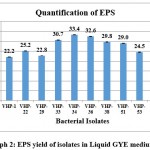 |
Graph 2: EPS yield of isolates in Liquid GYE medium |
Salt Tolerance study of VHP-34
Moderately salt tolerant microbes are able to grow with salt (NaCl) concentration up to 15-20%11.The isolate VHP-34 was grown on modified GYE supplemented with varying salt concentration ranging from 0 to 30% to check its salt tolerance and the results are shown in Table 5. The isolate VHP-34 was able to grow in salt range from 0% to 15% and no growth was obtained at 20% and higher salt concentration. Based on the salt tolerance data isolate VHP 34 can be considered as moderately halotolerant. The effect of increasing salt concentration on EPS yield is illustrated in Graph 3. The isolate VHP 34 gave EPS production in the range of 0-15% salt concentration. Isolate gave good production of EPSs from 2% to 12% salt concentration and the EPS yield varied from 12.6 to 32.4 mg/ml. However, the optimum salt concentration for EPS production was 3.5% and the EPS yield decreased at concentration below 3.5% as well as at salt concentration above 3.5%.
Table 5: Growth of VHP-34 on modified GYEA with different NaCl Concentration.
| Isolate
(VHP-34) |
0% | 1% | 3.5% | 5% | 7.5% | 10% | 15% | 20% | 25% | 30% |
| Growth | ++++ | ++++ | ++++ | +++ | +++ | ++ | + | — | — | — |
++++= Luxurious growth +++/++= Moderate growth += Slight growth —= No growth
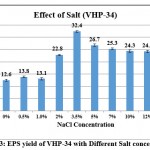 |
Graph 3: EPS yield of VHP-34 with Different Salt concentration. |
Phenotypic and Genotypic characterization of Potent EPS producer VHP-34
The 16srRNA analysis involved 16 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 970 positions in the final dataset. Evolutionary analyses were conducted in MEGA7. From the results of 16srRNA sequencing, the isolate VHP-34 was found to be Enterobacter cloacae (GenBank, Accession No: MW492544). Fig. 2 showed Growth of VHP-34 on modified GYEA plate. Figure 3 showed Capsule staining as well as Gram’s staining of VHP-34 (Enterobacter cloacae). The organism was found to be Gram negative and capsulated. Fig. 4 illustrates the phylogenic analysis of VHP-34.
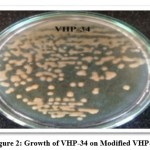 |
Figure 2: Growth of VHP-34 on Modified VHP-34. |
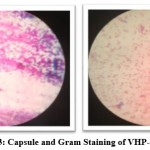 |
Figure 3: Capsule and Gram Staining of VHP-34 |
 |
Figure 4: Phylogenic Analysis of VHP-34 Enterobacter Cloacae |
Discussion
The Soil samples analysis revealed that all samples from 9 different locations have alkaline nature. Sanjay et al have reported that all the coastal soil samples were in the alkaline pH. The coastal region, soils are saline because of addition of salty waters. The soils also have high amounts of soluble salts and the pH is higher than 8.5.21Although all the soils were collected from coastal region each soil has different character as indicated by the soil analysis report. The sample from Jodiya was collected from Mangrove ecosystem and shows highest values for 6 parameters tested and high values for rest of other parameters.
Present study focused on isolation and screening of most novel Exopolysaccharide (EPSs) secreting marine bacteria from different selected coastal sites of Gujarat, India. Related studies on EPS producers from costal area of Gujarat showed the EPS Producing bacterium Bacillus subtilis which was isolated from marine surroundings.22 The microbial diversity of coastal regions of Gujarat for biotechnological application is yet to be fully exploited hence in this context the study was taken up to screen for novel EPS producing isolates from coastal region of Gujarat. Day by day increasing demand of marine microbial EPS for industrial applications has restored the endeavors in the development of quick and efficient techniques for their recovery and purification.6
The EPSs producers were enriched and isolated in modified GYE medium, There are many reports on use of similar media for EPS production The EPS production has been reported various media like Basal salt supplement, Natural seawater medium, Yeast malt glucose agar medium and Zobell marine agar medium.23 The 59 promising isolates were obtained in preliminary screening for EPS producers. The 9 diverse and promising isolates were selected for further study.
Biochemical characterization of 9 isolates was indicated the metabolic diversity of selected isolates. More than 50% of isolates were Lactose fermenters, 80% of isolates were mixed acid fermenter and around 60% of isolates were able to use Citrate as a sole carbon source for their growth. All the selected isolates produced various enzymes like Amylase, Protease, Lipase and Gelatinase indicating their polymer degradation ability.
Based on the Phylogenetic result and on nucleotide homology analysis, the isolate VHP-34 has been identified as Enterobacter cloacae VHP-34. The isolate showed 99.49% sequence similarity with Enterobacter cloacae strain 279-56 (NCBI Accession no. NR028912.1).
The Enterobacter cloacae VHP-34 is moderately halotolerant and can grow and produce EPS over a salt range of 0-15%. Due to Moderately Halotolerant nature of VHP-34 itself and its EPS can be used to combat several environmental applications. To support the EPS production from Halotolerant bacteria research by Diego Chambi et.al stated that EPS production by Halotolerant strain BU-4 isolate was grown under different Glucose as well as NaCl concentrations. When NaCl concentration was increased to 10 to 50 g L-1, it was directly affected on the EPS production. The EPS content (5 mg L-1) was produced at 50 g L-1NaCl concentration.24 Other research regarding EPS from halotolerant bacteria stated that salt tolerant Plant Growth Promoting Rhizobacteria (Enterobacter P23) able to produce an enormous amount of EPS, this EPS can bind to many cations, like Na+ and therefore that affects the amount of Na+ availability for plant uptake and hence by this mechanism plant can manage salt stress.25
The Enterobacter cloacae VHP-34 gave maximum EPS yield of 33.4mg/ml. The EPS yield of Enterobacter cloacae VHP-34 is two to three folds higher than the EPS yield reported for other Enterobacter. The marine isolate Enterobacter sp ACD2 gave 8.6 g/l yield of EPSs.26 Another marine bacterium Enterobacter sp. grown with glycerol gave yield of 12.0 to 18.0 g/l of EPS.27 Enterobacter A47 was able to significantly improved exopolysaccharide synthesis up to 3.43 g L-1.28
Conclusion
The EPSs producing bacteria are extensively distributed in marine surroundings. The novel EPS produced by marine bacteria have numerous environmental roles, bioactive roles as well as extensive biotechnological applications. The microbial diversity from coastal region of Gujarat is largely unexploited in terms of EPS producers and their applications. Present study highlights existence of diverse and promising EPS producers in coastal soils from Gujarat. The 59 EPS producers were isolated from 8 different sites, Maximum EPS producers were obtained from Umargam. The secondary screening identified 9 promising EPS producers out of which isolate VHP 34 was the best EPS producer. The isolate VHP 34 was identified as Enterobacter cloacae VHP-34. The Enterobacter EPS has been used as bioflocculant, bio emulsifier and heavy metal removal agent. Additionally the EPS production in soil can significantly enhance the volume of soil macropores and help in rhizosphere soil aggregation, resulting in increased water availability to plants. Thus EPS produced by Enterobacter cloacae VHP-34 can used for many applications. The EPS yield of Enterobacter cloacae VHP-34 is also much higher than the currently reported yields. The yields of EPS by Enterobacter cloacae VHP-34 can further be improved by media optimization.
Acknowledgement
The research was non-funded. The authors are thankful to the Management of Kadi Sarva Vishwavidyalaya (KSV), Gandhinagar, Gujarat for providing laboratory facility.
Conflict of Interest
The authors disclosed no potential conflicts of interest.
Funding details
The authors disclosed that the research is non funded.
References
- Poli, A., Anzelmo, G., & Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: production, characterization and biological activities. Marine Drugs. 2010; 8(6): 1779-1802.
CrossRef - Gutierrez, T., Morris, G., Green, D. H. Yield and physicochemical properties of EPS from Halomonas sp. strain TG39 identifies a role for protein and anionic residues (sulfate and phosphate) in emulsification of n-hexadecane. Biotechnology Bioengineering. 2009;103(1): 207-216.
CrossRef - Al, N. Characterization of an exopolysaccharide-producing marine bacterium, isolate Pseudoalteromonas sp. AM. African Journal of Microbiology Research. 2011; 5(22).
CrossRef - Devesh Parkar, Rahul Jadhav1, Mukesh Pimpliskar. Marine bacterial extracellular polysaccharides: A review Journal of Coastal Life Medicine.2017; 5(1):29-35.
CrossRef - Upadhyay Kinjal H, Avni M. Vaishnav, Devayani R. Tipre, Shailesh R Dave. Diversity Assessment and Eps Production Potential of Cultivable Bacteria from the Samples of Coastal Site of Alang. Journal of Microbiology, Biotechnology and Food Sciences. 2016; 6(1):661-666.
CrossRef - Dave, S. R., Upadhyay, K. H., Vaishnav, A. M., & Tipre, D. R. Exopolysaccharides from marine bacteria: production, recovery and applications. Environmental Sustainability. 2020; 3(2):139-154.
CrossRef - Nicolaus, B., Kambourova, M., & Oner, E. T. Exopolysaccharides from extremophiles: from fundamentals to biotechnology. Environmental Technology. 2010; 31(10): 1145-1158.
CrossRef - Panosyan, H., Di Donato, P., Poli, A., & Nicolaus, B. Production and characterization of exopolysaccharides by Geobacillus thermodenitrificans ArzA-6 and Geobacillus toebii ArzA-8 strains isolated from an Armenian geothermal spring. Extremophiles. 2018; 22(5):725-737.
CrossRef - Singh, R. P., Shukla, M. K., Mishra, A., Kumari, P., Reddy, C. R. K., & Jha, B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydrate Polymers. 2011; 84(3):1019-1026.
CrossRef - Mohamed, S. S., Amer, S. K., Selim, M. S., & Rifaat, H. M. Characterization and applications of exopolysaccharide produced by marine Bacillus altitudinis MSH2014 from Ras Mohamed, Sinai, Egypt. Egyptian Journal of Basic and Applied Sciences.2019; 5(3): 204-209.
CrossRef - Sandhya G. Nanjani and Harsha P. Soni. Diversity and EPS Production Potential of Halotolerant Bacteria from Veraval and Dwarka. IOSR Journal of Pharmacy and Biological Sciences.2012; 2(2): 20-25.
CrossRef - Brenner DJ, Krieg NR, Staley JT. Bergey’s Manual of Systematic Bacteriology, Volume 2, The Proteobacteria, Part B, The Gamma proteobacteria, Springer, Michigan, USA; 2005.
CrossRef - Vishal Patel, Harsha P Soni, Falguni Patel. Statistical Optimization of Medium for Enhanced Production of Exopolysaccharide by Marine Bacteria Enterobacter cloacae VHP-34. Industrial Biotechnology.2021; 17(6):326-334.
CrossRef - Saitou N. and Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987; 4:406-425.
- Nei M. and Kumar S. Molecular Evolution and Phylogenetics. Oxford University Press, New 2000
- Kumar S., Stecher G., and Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 2016; 33:1870-1874.
CrossRef - Fang L and Catchmark JM. Characterization of cellulose and other exopolysaccharides produced from Gluconacetobacter strains. Carbohydrate polymers. 2015; 115:663-9.
CrossRef - Rühmann B, Schmid J and Sieber V Methods to identify the unexplored diversity of microbial exopolysaccharides. Frontiers in microbiology.2015; 6.
- Mu’minaha, Baharuddinb, Hazarin Subairc, Fahruddin. Isolation and Screening Bacterial Exopolysaccharide (EPS) from Potato Rhizosphere in Highland and the Potential as a ProducerIndole Acetic Acid (IAA) Procedia Food Science. 2015; 3: 74 – 81.
CrossRef - Saad AM Moghannem, Mohamed M.S.Farag, A. M. Shehab, and Mohamed Salah Azab. Media Optimization for Exopolysaccharide Producing Klebsiella oxytoca KY498625 under Varying Cultural Conditions. International Journal of Advance Research in Biological Sciences.2017; 4(3): 16-30
CrossRef - Sanjay Arora, Riddhi Mehta. Characteristics of coastal saline soils inhabiting halophytes and salt tolerant plants. Journal of Soil and Water Conservation.2018; 17(1):107-110.
CrossRef - Rahul Chaudhari, Murtaza Hajoori, Manish Suthar and Sagar Desai. Isolation, Screening and Characterization of Marine Bacteria for Exopolysaccharide Production. Bioscience Discovery. 2017; 8(4): 643-649.
- Prashakha J. Shukla and Bharti P. Dave. Screening and Molecular Identification of Potential Exopolysaccharides (Epss) Producing Marine Bacteria from the Bhavnagar Coast, Gujarat. International Journal of Pharmaceutical Sciences and Research. 2018; 9(7): 2973-2981.
- Chambi, D.; Romero-Soto, L.; Villca, R.; Orozco-Gutiérrez, F.; Vega-Baudrit, J.; Quillaguamán, J.; Hatti-Kaul, R.; Martín, C.; Carrasco, C. Exopolysaccharides Production by Cultivating a Bacterial Isolate from the Hypersaline Environment of Salar de Uyuni (Bolivia) in Pretreatment Liquids of Steam-Exploded Quinoa Stalks and Enzymatic Hydrolysates of Curupaú Sawdust. 2021; 7(33).
CrossRef - Sarkar, P.K. Ghosh, K. Pramanik, S. Mitra, T. Soren, S. Pandey, M.H.Mondal, T.K. Maiti, A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress, Research in Microbiologoy. 2017; doi: 0.1016/j.resmic.2017.08.005.
- Almutairi MH, Helal MMI. Exopolysaccharide production from isolated Enterobacter sp. strain ACD2 from the northwest of Saudi Arabia. J King Saud University – Sci. 2021;33:1013-18
CrossRef - Alves VD, Freitas F, Torres CAV, Cruz M. Rheological and morphological characterization of the culture broth during exopolysaccharide production by Enterobacter sp. Carbohydrate Polymers. 2010;doi:10.1016/j.carbpol.2009.09.006
CrossRef - Antunes, S., Freitas, F., Sevrin, C., Grandfils, C., A. M. Reis, M., Production of FucoPolby Enterobacter A47 using waste tomato paste by-product as sole carbon source, Bioresource Technology. 2016; doi: http://dx.doi.org/10.1016/j.biortech.2016.12.018
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





