How to Cite | Publication History | PlumX Article Matrix
Lipid Nanoparticles: Key Facilitators of mRNA Vaccine Development
Shalmali Shirish Cholkar , Ashwini Ramkrishana Gawade*
, Ashwini Ramkrishana Gawade*  and Ashwin Bhanudas Kuchekar
and Ashwin Bhanudas Kuchekar
School of Pharmacy, Dr. Vishwanath Karad MIT World Peace University, Pune-411038, India,
Corresponding Author E-mail: ashwinigawade@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2979
ABSTRACT:
The current applications of nanomedicine span from the treatment of an infection right up to the treatment of cancer. Lipid nanoparticles (LNPs) have established themselves as reliable delivery systems for delivering therapeutic agents including nucleic acids since they prevent in vivo degradation of nucleic acids and facilitate their target-specific delivery. The mRNA is one such nucleic acid that is delivered by the LNPs for the treatment of infectious diseases. This review provides a detailed insight into the concept of messenger RNA (mRNA) vaccines, their mechanism of action, manufacturing process, critical considerations in the formulation, development, and manufacturing of these vaccines, and explains the vital role of LNPs in the development of these vaccines. Certain shortcomings of the lipid nanoparticle-mRNA vaccine concerning the in vitro stability of the mRNA and the LNP have also been highlighted in this review.
KEYWORDS: Endosomal escape; Lipid nanoparticle-mRNA vaccine; Non-replicating mRNA; Self-adjuvancy; Self-amplifying mRNA; Stealth liposome
Download this article as:| Copy the following to cite this article: Cholkar S. S, Gawade A. R, Kuchekar A. B. Lipid Nanoparticles: Key Facilitators of mRNA Vaccine Development. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Cholkar S. S, Gawade A. R, Kuchekar A. B. Lipid Nanoparticles: Key Facilitators of mRNA Vaccine Development. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3ghuTO2 |
Introduction
Against the backdrop of the ongoing coronavirus pandemic, it is evident that immunization against infection, disease, or disability is of utmost importance to offer protection against it and prevent the incidence of death. Since the beginning of the 21st century, there has been a shift in therapeutic science marked by a switch from traditional drug therapies to nucleic acid therapies to treat the disease at a genetic level.
Nanomedicine is used for targeted therapy in chronic diseases like diabetes, ocular disease, hypertension, atherosclerosis, myocardial ischemia-reperfusion injury, pulmonary tuberculosis, asthma, chronic obstructive pulmonary disease (COPD), Parkinson’s disease, Alzheimer’s disease, and cancer1,2. The research further continues discovering new potential applications of nanomedicine for the treatment of these ailments. Linalool-loaded gold nanoparticles have been studied and proved to be promising treatment alternatives for the treatment of ovarian cancer3. The study carried out on poly-L-lysine-coated superparamagnetic iron oxide nanoparticles (SPIONs-PLL) as carriers for brain-derived neurotrophic factor (BDNF) into neural stem cells under the influence of an external magnetic field suggests their potential use in the treatment of Parkinson’s disease, Alzheimer’s disease, and other neurodegenerative diseases4. The number of lipid nanoparticle patents filed earlier in the year 2021, related to their use as delivery systems for nucleic acids, greatly exceeds the number of such patents published during the entire year’s span of 20205. The development of nucleic acid vaccines was hindered due to the lack of a solution to tackle the problems of intrinsic instability of the nucleic acids resulting from enzymatic degradation and their inability of achieving intracellular access. Hence, there was a need to design carrier systems to deliver the nucleic acid molecules intact from the site of administration to the target cell and facilitate their cellular uptake. Lipid nanoparticles have emerged as successful platforms to facilitate the designing of treatment alternatives for a variety of diseases owing to their ability to enclose nucleic acids in their core and deliver them to the intended site of action. Additionally, the lipid nanoparticles provide a sustained release of the enclosed contents. A substantial number of marketed nanomedicines include lipid nanoparticle formulations6. The therapeutic efficacies of nanoparticle delivery systems and mRNAs have been critically examined during the outbreak of coronavirus pandemic7.
mRNA Vaccines
Overview of mRNA vaccines
Vaccines have indeed improved public health and increased life expectancy. The safety risk associated with the administration of whole-pathogen vaccines and the limited supplies of useful antigens hindered the vaccine development process in the past. However, in current times, the enormous advances in drug delivery systems, genetic engineering, biotechnology, and the ability to acquire numerous synthetic recombinant protein antigens from microbes, have immensely transformed the development course of new-generation vaccines. Vaccines are developed either as live or attenuated vaccines, subunit vaccines, recombinant viral vector vaccines, conjugate vaccines, or nucleic acid vaccines.
mRNA vaccines, which comprise the new-generation vaccines, are capable of addressing the medical needs which are not dealt with efficiently by the conventional vaccine technologies. The potential of mRNA vaccines for the prophylaxis and treatment of a wide spectrum of diseases has been extensively investigated in recent decades. The applications of mRNA in protein replacement therapies, viral vaccines, cancer immunotherapies, genome editing, and cellular reprogramming have been well established to prove the potential of mRNA as a novel form of drug8,9. mRNA vaccines have also established their utility for the treatment of veterinary infectious diseases like rabies, foot and mouse disease virus, and Powassan virus5,10. The sudden upsurge of the coronavirus global pandemic has indeed accelerated the approval of mRNA vaccines for use in humans.
Concept of mRNA vaccine
The active immunization process involves exposing the individual to antigens which causes stimulation and activation of the immune system resulting in the production of antibodies and thereby developing a defensive capability against an infection. mRNA is a single-stranded nucleic acid hydrophilic polyanionic molecule formed by the process of transcription. mRNA comprises a base sequence complementary to one of the strands of the DNA and subsequently causes the production of corresponding protein by the process of translation in the cell cytoplasm. mRNA vaccine consists of a synthetic mRNA molecule comprised of a sequence that specifically encodes for one or more disease-specific antigens11. The expression of this synthetic mRNA sequence produces translated proteins of interest in the cytoplasm of the host cell. The produced protein molecules can be enclosed within a membrane, be secreted extracellularly, or be present intracellularly. mRNA mainly targets the dendritic cells to generate a T cell immune response and holds more capability of targeting the dendritic cells as compared to other vaccines12. The antigens and neutralizing antibodies encoded by the mRNA generate specific immune responses and the proteins with immunostimulatory activity encoded by the mRNA generate innate immune responses13.
Types of mRNA vaccines
Depending upon the type of mRNA sequence incorporated into the vaccine, this vaccine occurs in 2 types: self-amplifying mRNA vaccine or non-replicating mRNA vaccine12,14. The basic components of mRNA incorporated into the vaccine which are common to both the types of mRNA vaccines include a 5’ cap, 5’ and 3’ untranslated regions (UTRs), open reading frame (ORF), and a 3’ poly (A) tail14. The primary aim of the mRNA incorporated into the vaccine is to successfully undergo translation in the cytoplasm of the target cell, resulting in the production of a sufficient quantity of the encoded immunogen, which is conveyed to the immune system, resulting in the generation of an immune response.
Self-amplifying mRNA (SAM)
Self-amplifying mRNA, also known as replicon, is a positive-strand mRNA and is likely to produce an innate immune response. It is derived from the genome of alphaviruses which is a single-stranded RNA virus15. SAM comprises of the same basic elements as mentioned previously (5’ cap, 5’ and 3’ UTRs, 3’ poly (A) tail) along with a coding region. This type of mRNA encodes for the target antigen that is responsible for eliciting an immune response along with additional sequence regions which enable self-replication of the mRNA before the encoded proteins are translated. The coding region (ORF) of SAM consists of sequences encoding for the enzyme RNA-dependant RNA polymerase (RDRP), structural viral proteins (subgenomic sequence), and a promoter for the subgenomic sequence14. The subgenomic sequence can be altered by replacing it with a genomic sequence encoding for a specific protein(s) of interest. SAM, on entry into the cell cytoplasm, brings about the synthesis of RDRP by the process of translation and subsequently undergoes RDRP-mediated replication to form a negative strand. Once RDRP recognizes the promoter in the negative-strand RNA, the subgenomic sequence encoding for structural viral proteins in the RNA undergoes transcription. Thus, a positive-strand viral genome is generated from the negative- strand serving as a template. A huge number of virions are thus produced which justifies the requirement of only a small dose of this type of mRNA vaccine16,17.
Non-replicating mRNA and circular mRNA
Non-replicating mRNA (NRM), also known as non-amplifying mRNA, is a typical mRNA molecule, similar to the endogenous mRNA present in a mammalian cell. NRM comprises a 5’ cap, 5’ and 3’ UTRs, 3’ poly (A) tail, and a coding sequence (ORF). However, the coding sequence of NRM lacks the presence of sequence coding for RDRP which is the self-amplifying machinery of the mRNA construct. Thus, this mRNA gets translated into the cytosol of the cell until it is degraded and eliminated by the body13. Circular mRNA (circRNA) is a single-stranded non-amplifying mRNA having a closed-loop structure. It is synthesized by chemical or enzymatic methods. The circRNA consists of 5’ and 3’ ends of a linear mRNA precursor covalently linked to form a circular loop. This type of mRNA encodes only for the target antigen that is responsible for eliciting an immune response. Since it lacks the presence of free cap and tail regions owing to its circular structure, it is less susceptible to degradation by the enzyme nucleases and has a longer blood circulation time in comparison to linear mRNA. The probability of detachment of the ribosome from the RNA during the step of protein translation is reduced due to the absence of a stop codon in the circular mRNA. As a consequence, translation can proceed at a continuous rate leading to higher protein expression. The immunogenicity of circular mRNA can be reduced to a minimal level by chemically modifying the genomic sequence of the mRNA followed by its purification using chromatographic techniques. This process can also enhance the translation of encoded proteins8. Unlike self-amplifying mRNA vaccines, NRM and circRNA vaccines require repeated administration since a limited number of mRNA molecules are delivered which produce a limited degree of antigen expression8,13.
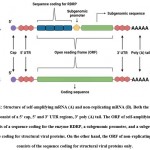 |
Figure 1: Structure of self-amplifying mRNA (A) and non-replicating mRNA (B). Both the types of mRNAs consist of a 5’ cap, 5’ and 3’ UTR regions, 3’ poly (A) tail. |
 |
Figure 2: In vivo expression of self-amplifying mRNA (SAM) and non-replicating mRNA (NRM) constructs |
Advantages of mRNA vaccines
The process of synthesizing an mRNA strand can be accomplished in a few weeks once the target protein antigen and the corresponding genetic sequence have been identified. mRNA vaccines also exhibit a greater level of immunogenicity in early-phase clinical trials. Since the mRNA molecules encoding different antigens have a highly similar chemical and physical nature, the period and cost of resources required for the formulation, design, and manufacture of these vaccines can be efficiently shortened and the scale-up ability along with reproducibility can be greatly enhanced. This is especially important for the viruses which mutate quickly and produce new variants in a very brief period like the severe acute respiratory syndrome corona virus-2 (SARS-CoV-2)18.
mRNA vaccines are more efficacious for the prophylaxis of diseases and infections as compared to viral vectors which lack the ability of replication. Additionally, unlike viral vector-based vaccines, mRNA vaccines do not generate immunity against the carrier. mRNA vaccines do not integrate with the host genome and induce mutagenesis and carcinogenesis which is a faint possibility in the case of DNA vaccines. For eliciting an immune response, the mRNA incorporated into the vaccine needs to enter the host cell cytoplasm for the translation to take place and need not gain access to the host cell nucleus to exert its action, unlike DNA vaccines. This increases the success rate of mRNA vaccines5. In addition to this, the mRNA only needs a brief presence in the host cell cytoplasm to exert its effect. The proteins produced in the host cell serve as a natural presentation to the immune system of the host which enhances the immune response produced. Unlike protein-based vaccines, mRNA offers extended availability of the translated antigenic proteins which profoundly impacts the degree of immune response produced and also leads to affinity maturation of the antibodies produced in response to the antigens. This results in the development of lasting protection against a specific antigen19. The tedious process of developing protein-based drugs or vaccines can certainly be replaced by the development of mRNA vaccine which utilizes the natural machinery of protein production of the host cell to produce its effect. Since the mRNA vaccines are free from infectious agents unlike the vaccines consisting of live or attenuated virus or subunit vaccines, they can be safely administered to immunocompromised patients. In addition to this, the mechanism of mRNA vaccine involves in situ generation of the antigenic proteins which satiates the need for long-term stabilization and protein purification which is much essential in the case of a few antigens13.
Disadvantages and limitations of conventional mRNA vaccines
The translocation of mRNA across the negatively charged host cell membrane is hampered as the mRNA is a negatively charged and hydrophilic molecule20. Shortly after its administration, the naked mRNA can get associated with serum proteins followed by its uptake by the phagocytes and subsequent degradation by the enzyme ribonucleases (RNases) to form small nucleotide units. This poses a barrier to the efficient delivery of mRNA inside the host cell. The degraded mRNA is efficiently excreted by glomerular filtration in less than 10 minutes after its administration. Hence, there is a need for an efficient carrier system to prevent the extracellular degradation of mRNA, enable its intracellular entry and thus deliver the mRNA intact inside the host cell21,22.
mRNA vaccines are capable of producing systemic adverse effects if they are broadly distributed in the body of the host. Hence, the mRNA molecules must be targeted into tissues with abundant immune cells to produce the desired immune response. Once the mRNA enters the cell, its presence is detected by endosomal receptors and nucleic acid sensors present in the cytoplasm and bind to it subsequently producing an innate immune response. This significantly reduces the intracellular stability of mRNA and hinders the translation process. The stability of mRNA is also significantly affected by chemical degradation since it induces modification of bonds in the mRNA molecule. Physical instability results in denaturation of the mRNA structure. Denaturation can also lead to aggregation and precipitation of mRNA which affects the translation process. Hydrolysis and oxidation are the major processes causing in vitro chemical degradation of mRNA. Hydrolysis is catalyzed by the mRNA molecule itself, the enzyme nucleases, and a few other exogenous factors. Hydrolysis causes the mRNA strand to break due to the cleavage of the phosphodiester bonds that form a basic structural framework of the mRNA molecule. Oxidative degradation affects the nucleotide sequence of mRNA as it causes cleavage of the nucleobases but has a lesser impact on the ribose units. Oxidative degradation also causes the mRNA strand to break and alters its secondary structure. However, hydrolysis is the main driving factor for mRNA degradation. Since the motive of in vivo administration of synthetic mRNA molecule as a drug is to bring about the production of the target antigen, even a small alteration in its structure (strand break or oxidation of bases) can halt the translation and markedly impair its efficacy. The techniques used for the evaluation of stability and quality of mRNA include high performance liquid chromatography (HPLC), reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and gel electrophoresis. However, these techniques suffer the disadvantage of variable sensitivity and lack of the ability to distinguish the type of process causing degradation of mRNA and are hence not reliable for determining the cause of instability and extent of damage produced to the mRNA structure. The storage temperatures for current mRNA vaccines vary from 2-8°C to 80°C and the shelf lives vary from days to months. Hence, it is crucial to identify the factors causing this variation that will assist the design of thermostable vaccines. The development of thermostable vaccines will greatly cut down the cost required for maintaining the freezing conditions during vaccine distribution, storage, and handling by the user18.
Manufacturing process of mRNA vaccine
The production of mRNA vaccine begins with the generation of a plasmid DNA (pDNA) molecule consisting of a promoter for the enzyme DNA-dependant RNA polymerase and a sequence for the type of mRNA to be synthesized to be incorporated into the vaccine. This step is followed by the linearization of the plasmid DNA. The enzyme DNA-dependant RNA polymerase then synthesizes the desired mRNA molecule from the linearized plasmid DNA which acts as a template. The residual DNA template is digested by the enzyme DNase in the subsequent incubation step. The synthesized mRNA sequence is sealed at both ends by inserting a cap at its 5’ end and a poly (A) tail at its 3’ end during the process of in vitro transcription. Capping and tailing of mRNA can also be carried out by enzymatic methods employing guanylyl transferase, 2’-O-methyltransferase, poly-A-polymerase after the mRNA is transcribed12. Further, microbeads-based precipitation or high performance liquid chromatography (HPLC) methods are used to remove double-stranded RNA, enzymes, free nucleotides, shortened nucleic acid fragments to purify the mRNA molecule which is then formulated into a drug product8. This product is evaluated for its identity, sterility, purity, and potency before it is released for use. This manufacturing process is carried out following the Good Manufacturing Practice (GMP) facilities which enable to quickly switch from the manufacturing process of one particular vaccine to another vaccine, provided that the GMP facilities available for the manufacturing process remain unchanged14.
Critical considerations in the formulation, development, and manufacturing of mRNA vaccines
The purity of the generated mRNA molecule should be monitored to ensure high protein expression following its release in the host cell cytoplasm and reduced innate immune responses. The enzyme DNA-dependant RNA polymerase used in the in vitro manufacturing process of mRNA has been reported to produce oligonucleotides, double-stranded RNA as impurities that can possibly trigger an inflammatory response. Hence, adapting systematic methods of removal of these contaminants is a crucial step to ensure and enhance the efficacy of the mRNA vaccine. The length of the synthesized mRNA molecule and the regulatory elements of its 3’ and 5’ UTR regions have to be critically monitored and optimized to assure the high efficacy of formulated vaccine product. The 5’cap of mRNA is an essential component for efficient protein expression and it also serves to prevent mRNA degradation by the enzyme 5’-3’exonuclease. Hence, the enzymes used for insertion of the cap in the mRNA molecule and the reaction conditions have to be optimized to allow a maximum percentage of capping to occur. The modifications in the base and sugar moieties in the cap region increase the interaction of mRNA with the ribosome and enhance mRNA stability. In addition to this, various modifications can also be made in the triphosphate bridge within the cap region. The presence of poly (A) tail is a prerequisite for the translation of proteins encoded by the mRNA and it also prevents the degradation of mRNA15. Thus, the manufacturing process must critically monitor and ensure the addition of a tail in the mRNA molecule being synthesized and optimize its length to keep the mRNA protected from degradation and ensure it’s in vivo translation. An increase in the ratio of bases guanine and cytosine (G: C) in the mRNA codon sequence can enhance the protein expression17. Hence, optimization of the codon sequence of mRNA, modification of the nucleotides, and utilization of modified nucleosides like pseudouridine or N-1-methylpseuodouridine results in greater stability, translation efficiency, antigen expression and produces an adaptive immune response and a favorable level of therapeutic index. Since mRNA has a transient nature and is susceptible to enzymatic degradation by ribonucleases which are abundantly present in the extracellular environment, it has to be efficiently protected from the site of its injection till its release in the host cell cytoplasm. Thus, there is a need for an effective mRNA delivery system to tackle this issue.
In preclinical studies, the utilization of unmodified mRNA having an optimized coding sequence and free from inflammatory impurities has been reported to successfully produce the desired proteins, minimize undesirable inflammatory responses and elicit adaptive immune responses. Hence, comparative immunogenicity studies between unmodified and modified mRNA are required to justify immunogenicity differences in the clinical studies, if any. However, unmodified mRNA may be desirable from the view of manufacturing efficiency and precision in transcription. Additionally, studies are required to determine differences between the use of self-amplifying mRNA and non-replicating mRNA.
The general principles mentioned in the guidance documents are referred to for the design and development of mRNA vaccines for human use since there is an absence of regulatory guidelines specifically focused on the clinical development process of these vaccines. However, these guidelines are sufficient to allow the mRNA vaccines to enter into phase I and phase II of the clinical trials. These phases of clinical trials must be appropriately designed to critically monitor undesirable inflammatory reactions and innate immune responses which can potentially be produced by these vaccines. This will assist to determine if the clinical trial needs to be paused or terminated if severe tolerability concerns are raised during the conduct of the trial. Modern techniques like transcriptomics and systems biology along with traditional methods like immune monitoring can be utilized to obtain a detailed insight into immune response generated during treatment and monitor the safety of the trial. The clinical trials have reported that the development of new formulations is essential for optimizing the safety profile. However, these claims still require a confirmation from the trial datasets. Against the backdrop of the established high potency of protein and live-attenuated vaccines, the formulation of mRNA vaccines needs further development to achieve sufficient immunological response while taking the acceptable tolerability into consideration. Additionally, there is insufficient data available concerning repeated administration of these vaccines in humans which necessitates further study in this regard taking into consideration the fact that several vaccines have to be administered in booster doses for fulfilling the intended prophylactic or treatment purpose14.
Lipid Nanoparticles-enabled mRNA Vaccine Delivery
Need of lipid nanoparticles in mRNA vaccines
Currently, it has been established that a wide range of diseases from infections to cancer can be effectively treated by using nucleic acid therapies. However, the negative charge carried by nucleic acids, their in vivo enzymatic degradation, and their uptake by the phagocytes have posed difficulties to the development of nucleic acid therapeutics5,23. Thus, there is a need for an efficient carrier system for delivering the nucleic acid without any alteration to the target site for efficient uptake. Systems for the delivery of genetic drugs should necessarily exhibit high loading capacity, sustainable release of the encapsulated contents, absence of leakage from the delivery system, and simple manufacturing procedures.
A considerable amount of preclinical and clinical studies carried out in the past have successfully established the role of nanoparticles as therapeutic carrier systems. Small size, high surface area, structural flexibility, biodegradability and greater biocompatibility than polymeric and inorganic nanoparticles are the attributes that give lipid nanoparticles an edge over other bulk formulations5. Supplementary to these attributes, ease of manufacturing, low immunogenicity, ability to carry a large amount of load, and the ability to deliver nucleic acids to their desired target site without allowing any alteration makes the lipid nanoparticles advantageous over the rest of the gene therapies and vaccine delivery systems5,24. Lipid nanoparticles are non-viral systems for delivering nucleic acids to their target site and their characteristic structure allows loading of hydrophilic as well as hydrophobic drugs in their structure. This expands the scope of utility of these nanoparticles as a delivery system for delivering a variety of particles25,26. Studies have specifically suggested that charged liposomes are more suitable for vaccine delivery and neutral liposomes are more suitable for cancer chemotherapies. Lipid nanoparticles also provide the advantage of rapid scale-up capability for clinical and commercial applications13. LNPs are suitable delivery systems for mRNA coding antigens. This indeed expands the range of applicability of these nanoparticles for use in anti-cancerous vaccines. They increase the efficacy of cancer therapy by delivering the encapsulated anticancer agent to the target tumor tissue offering enhanced permeability and elevating the retention time of the anticancer agent in the tumor tissues owing to minimal lymphatic drainage of the macromolecular lipid system which is collectively termed as enhanced permeability and retention (EPR) effect3,5.
Concept of lipid nanoparticles
The structure of a lipid molecule primarily consists of 3 domains- polar head, nonpolar tail, and a linker group connecting these 2 domains6. Lipid-based nanoparticles (LNPs), depending upon their design and physicochemical properties of their formulations, are of the following 5 types- liposomes, transfersomes, niosomes, nanostructured lipid carriers, and solid lipid nanoparticles (SLNs)5,22. Liposomes are the early forms of LNPs and are comprised of one or multiple lipid bilayers which enclose an aqueous core and form a structure that mimics the cell membrane structure of an animal cell. Thus, the liposome can spontaneously fuse with the phospholipid bilayered membrane of the animal cell without any inhibition and empty its contents inside the cell. The structure of LNPs typically contains at least one synthetic or natural lipid layer comprising of an ionizable lipid (cationic or anionic), cholesterol, polyethylene glycosylated lipid (PEGylated lipid), neutral lipid, and a core region which can either be oily, aqueous, solid or amorphous. Hence, lipophilic molecules can be integrated into the lipid layer and hydrophilic molecules can be incorporated in the core of LNP. This also allows co-loading of adjuvants in the LNP to enhance the immunogenicity of the mRNA vaccine26. The characteristics like particle size, particle morphology, surface properties, and encapsulation efficiency are largely governed by the lipid composition of LNPs8. Unlike liposomes, the core of lipid nanoparticles contains lipids, which is a characteristic that distinguishes lipid nanoparticles from liposomes. Additionally, some studies also suggest the presence of water in the interior region of LNP18. The components of lipid nanoparticles are described below
The ionizable lipid has a structure similar to the structure of natural lipids except that the polar head domain is comprised of an ionizable group (cationic or anionic). Cationic lipids can electrostatically interact with the polyanionic mRNA molecule and encapsulate it efficiently in an acidic environment during the formation of lipid nanoparticles and are thus preferred over anionic lipids. After administration, the cationic lipids then become neutral species due to a change in pH which prevents their rapid elimination from the bloodstream along with minimizing toxicity. However, a very high magnitude of positive charge if carried by the nanoparticle, may lead to cellular damage and affect the therapeutic efficacy of the vaccine27. The ionizable lipids are hence responsible for the immunogenicity, pharmacokinetics, and tissue tolerability of the LNP26. The structure of the ionizable lipid and its acid dissociation constant determine the efficiency of the LNP to deliver the loaded mRNA inside the host cell13. The acid dissociation constant of the ionizable lipid also impacts the ability of the lipid carrier to bring about the endosomal escape of the loaded mRNA molecules and the degree of protein opsonization of the carrier28.
PEGylated lipid consists of lipid which is covalently bonded to an inert hydrophilic polymer like polyethylene glycol (PEG). The LNPs having PEG molecules incorporated in their structure are referred to as ‘stealth liposomes’26. The presence of these PEG chains prevents the aggregation of lipid nanoparticles. In addition to this, PEG chains also prevent the binding of opsonins on the lipid carrier surface by creating a steric hindrance thereby avoiding the elimination of LNPs by the reticuloendothelial system in the body and extending their circulation half-life in the bloodstream27. This also enables the passive accumulation of LNP in cancer cells and provides a targeted therapy. PEG prevents the retention of LNPs in the extracellular matrices of skin cells and enhances the rate of the removal of LNP from the administration site thereby decreasing tissue irritancy29. According to studies, the incorporation of branched PEG chains in the LNP structure increases the efficiency of targeted delivery of mRNA to the host cell and its intracellular release. However, the incorporation of a large amount of PEG molecules or a long PEG chain can hamper the uptake of LNP by the target cell and also deteriorate its intracellular activity. Thus, PEG chain length is limited to 2000 and its density is kept at less than 2% in the LNP structure26. Stealth liposomes have proven to be promising delivery systems for the delivery of antitumor antibiotic doxorubicin for treating solid tumours22.
Neutral lipids are the helper lipids that help sustain the lipid structure by offering support to the lipid layer. 1, 2 -Distearoyl-sn-glycero-3-phosphocholine (DSPC) is a neutral lipid. The cylindrical geometry of this lipid is due to the presence of saturated acyl chains and a large head group. It promotes the formation of a bilayered structure. It assists in the formation of a stable LNP also due to its high melting point8,26. It has been employed in the currently approved vaccines for coronavirus disease26.
Cholesterol stabilizes the LNP structure and also protects the LNP from interacting with the opsonins. Apart from this, since cholesterol is a natural component of the cell membrane, its presence enhances the biocompatibility of LNP. Cholesterol is also capable of altering the fluidity of the lipid bilayer of the LNP5,24. The presence of cholesterol molecules in the structure of the lipid carrier promotes the fusion of the carrier with the endosomal bilayered membrane and also assists in bringing about the endosomal escape of mRNA. It also facilitates the fusion of lipid carriers with the endosome and promotes endosomal escape of the mRNA. The efficacy of LNP to efficiently deliver the mRNA is affected by the geometry of the cholesterol derivative and can also affect the biodistribution of the lipid carrier8. The use of C-14 alkyl phytosterols has proven to enhance the mRNA delivering efficacy of the lipid nanoparticle8,30. Studies have shown that incorporation of cholesterol in the structure of lipid carriers can significantly impact the pharmacokinetics of the carrier system and also enhance its in vivo residence time24.
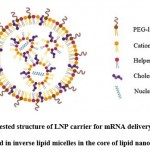 |
Figure 3: Suggested structure of LNP carrier for mRNA delivery: Nucleic acids arranged in inverse lipid micelles in the core of lipid nanoparticle. |
Mechanism of action of lipid nanoparticle-mRNA vaccine
The primary roles of lipid nanoparticles in mRNA delivery are to facilitate the entry of mRNA inside the target cell and to promote the release of mRNA intact into cell cytoplasm bypassing the endosomal destruction of mRNA which thereby increases the availability of the mRNA molecule to the ribosomes for efficient protein translation. The efficiency of the lipid carrier to deliver the mRNA intracellularly is considerably dependant on the type of lipid and the size, degree of PEGylation, and surface charge of the lipid nanoparticle.
Once the vaccine has been administered, the positively charged mRNA molecule which is encapsulated in the core of lipid nanoparticle is carried intact to the target host cell protected from the degradation by ribonucleases. On reaching the target cell, the LNP gets adsorbed on the cell surface due to electrostatic attraction between the LNP which bears a positive surface charge, and the negatively charged cell membrane surface. As a result, the LNP fuses with the cell membrane, and endocytosis occurs31. The endosome, present intracellularly, entraps the LNP shorty after it has entered the cell by endocytosis. The ionizable lipids of LNP become protonated in the acidic environment of the endosome. The formation of ion pairs between the protonated lipids of the carrier system and the negatively charged phospholipids present in the endosomal membrane takes place. This results in disruption of the bilayer membrane structure of the endosome along with the formation of a small non-lamellar structure due to destruction of the lipid carrier structure5,26. This brings about the endosomal escape of the mRNA molecule which is encapsulated by the lipid carrier15. The mRNA molecule is released inside the host cell cytoplasm and utilizes the translation machinery of the host cell to synthesize the target protein(s) followed by the generation of desired immune response14. Additionally, uptake of the lipid carrier loaded with the mRNA by the antigen-presenting cells (APCs) triggers an immune response that imparts the property of self-adjuvancy to the vaccine that further elevates the immunogenicity of the vaccine12.
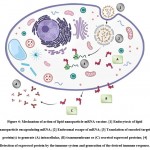 |
Figure 4: Mechanism of action of lipid nanoparticle-mRNA vaccine. [1] Endocytosis of lipid nanoparticle encapsulating mRNA; [2] Endosomal escape of mRNA; |
Manufacturing process of lipid nanoparticle-mRNA vaccine
The methods of manufacturing lipid nanoparticle-mRNA vaccines include solvent injection, thin-film hydration, reverse phase evaporation, detergent removal, heating method, microfluidic hydrodynamic focusing, crossflow injection, and methods using supercritical fluids5. The manufacturing process of the vaccine starts once the purified and tested mRNA molecules are generated. These mRNA molecules are incorporated into lipid nanoparticles by the modified ethanol injection nanoprecipitation method. In this method, the lipid components are added to an ethanol phase and the mRNA molecules are added to an aqueous phase. This step is followed by mixing both these phases in an acidic buffer (pH 4-5) using a pump assembly at an optimum flow rate. The lipid components attain a positive charge due to the acidic pH of the buffer and electrostatically interact with the negatively charged mRNA molecules13. The lipids undergo condensation and readily encapsulate the mRNA molecules into their core. The formulation is now neutralized to physiological pH value which causes loss of the positive charge on the lipid carrier surface. The size of LNP is controlled using ultrasonication, extrusion, or microfluidic method. This technique of rapid mixing reproducibly produces the vaccine product on a large scale with homogenous size distribution and high encapsulation efficiency8,26. The vaccine is subjected to filtration and purification process for removal of the residual solvent ethanol along with other impurities like truncated nascent mRNA sequences which are potentially immunogenic and capable of deteriorating the translation efficiency of the encapsulated mRNA31. This filtered vaccine is ultimately sterilized and stored in frozen conditions. Further, the formulation is subjected to GMP standard tests which evaluate the physical parameters (mRNA encapsulation efficiency, particle size, and surface charge), official tests (particulate matter, sterility, osmolality, bacterial endotoxins), and other quality checks8. The buffer used in the formulation of the vaccine should be free from ribonuclease contaminants to prevent the degradation of the mRNA molecules. Any alteration in the amount and type of lipids, cholesterol to lipid ratio can potentially alter the ability of LNP to efficiently deliver the mRNA molecules intact inside the host cell and also change the pharmacokinetic profile of the vaccine resulting in a requirement of dose adjustments. The sonication amplitude and time are among the various process variables which can significantly impact the particle size and entrapment efficiency of the carrier system32. Hence, the formulation ingredients must be carefully monitored to ensure the formulation of a safe and efficacious vaccine product.
Storage and stability of lipid nanoparticle-mRNA vaccine
The storage conditions (aqueous, freezing, lyophilized storage) along with the cryoprotectants (trehalose, mannitol, sucrose) used in the lipid nanoparticle-mRNA formulation is a crucial parameter for maintaining the long-term stability of these formulations. Cold–chain transportation can maintain the therapeutic activity of the vaccine. However, the development of lipid nanoparticle-mRNA vaccines that do not require cold storage will significantly reduce the production and transportation cost. To achieve this goal, the identification of factors impacting the long-term storage of the vaccine becomes essential8.
From the aforementioned stability concerns concerning mRNA, it is much clear that the mRNA is intrinsically susceptible to physical and chemical degradation and even a minute alteration in its structure and integrity can affect it’s in vivo performance and therapeutic efficacy of the vaccine. Along with taking into consideration the stability of mRNA which is prone to degradation, it is also important to ensure the stability of the lipid carrier as it can undergo physical and chemical degradation. The LNPs can undergo hydrolysis and oxidation. The oxidative impurities can also cause the oxidation of the encapsulated mRNA. Physical degradation of the LNP can cause their aggregation, fusion or can also result in leakage of the encapsulated material. PEG molecules can be incorporated into the LNP structure to help prevent aggregation of the lipid carrier and increase the stability of the carrier during storage. However, there is a need to investigate better alternatives to PEG due to the toxicity concerns associated with the use of PEG in the lipid carrier system. One such alternative investigated is a polysarcosine-modified lipid which minimizes the immunostimulatory response while reducing aggregation of the LNPs. However, additional testing is still needed if such PEG alternatives increase the mRNA stability. Studies have suggested that mRNA, and not the lipid carrier, is the factor governing the stability of the vaccine formulation. The presence of water in the core of the LNP has been suggested in the studies. Thus, the mRNA encapsulated inside the core of the LNP is susceptible to hydrolysis. In such a case, an ionizable lipid-coated mRNA can be placed in the core of the lipid carrier instead of naked mRNA to protect the mRNA from hydrolytic degradation. During the vaccine formulation, the excipients like osmolytes and buffers must be carefully selected taking into consideration their effect on the stability of the vaccine in storage during which there occurs a change in the pH of the product18.
Pre-clinical and clinical trials of lipid nanoparticle-mRNA vaccine
The designing of vaccine formulation has to be carried out considering the biomedical demand since, unlike therapeutic formulations which act on a specific target organ, vaccines exert their effect by interacting with the immune system. The LNP-mRNA vaccines have been extensively investigated which paved a way for the rapid development and successful clinical use of this type of vaccine for the treatment and prevention of COVID-198. mRNA possesses the intrinsic property of being non-infective. In addition to this, mRNA is a non-integrating agent and is capable of encoding a broad range of antigens. The lipid nanoparticle formulation of the mRNA can be effectively utilized to incorporate multiple mRNAs encoding a variety of antigens. Along with this, the LNP-mRNA vaccine can be manufactured as per the GMP standards on a large scale within a short period. All these features have collectively contributed to making LNP-mRNA formulation an on-demand vaccine platform. However, instability, storage conditions and safety concerns have to be taken into consideration while proceeding with their clinical use.
The utility of LNP-mRNA vaccines as vaccines for influenza along with some formulations under clinical study are being investigated. Clinical trials for the use of mRNA vaccines for treating cytomegalovirus, rabies virus, chikungunya virus, metapneumovirus, and respiratory syncytial virus have also been initiated5. The LNP-mRNA vaccine is also under preclinical investigation for human immunodeficiency virus (HIV), dengue, Powassan, Venezuelan, and Ebola virus. This type of vaccine has been reported to protect against parasitical and bacterial infections. Vaccines comprising of gold nanoparticles with loaded mRNA cargo are used for the treatment of cancer33. Clinical trials for treating cancers like leukemia, melanoma, breast cancer, ovarian cancer, glioblastoma, and other solid tumors and preclinical trials on metabolic and hematological disorders are currently in progress5,15. These vaccines are also being clinically investigated for protein replacement therapies to treat cystic fibrosis and propionic acidemia5.
Limitations of lipid nanoparticle-mRNA vaccine
The LNPs are composed of natural lipids and hence possess minimal toxicity. However, in some instances, cationic lipids can cause cytotoxicity by reducing mitosis in cells, forming vacuoles in the cell cytoplasm, and damaging vital cellular proteins like protein kinase C since the cationic lipids are not the natural components of the cell membrane. These detrimental effects of cationic lipids depend on the structure of their polar head moieties. Identification and utilization of natural cationic lipids like ethyl phosphatidylcholines or cationic cholesterols can possibly reduce the cytotoxicity of the LNPs. Lipids with their head portions comprising of quaternary ammonium groups exhibit greater toxicity as compared to the lipids with their head portions comprising of tertiary amine groups. The effect of incorporating hydrophobic chains in the structure of the lipid carrier on the toxicity of these carriers has not been systematically studied which in turn hinders the designing of lipids carriers with reduced toxicity. The PEG molecules incorporated in the lipid carrier can induce hypersensitivity by activating the complement system. In certain cases, the PEG molecules have been proven to generate an undesired immune response by producing antibodies against PEGylated lipid carriers resulting in reduced therapeutic efficiency of these carrier systems5. Administration of PEGylated lipid carriers in subjects having pre-existing anti-PEG antibodies can activate the complement system to a higher degree and also hasten the rate of elimination of the PEGylated lipid carrier from the blood stream19,29,34. This issue can be tackled by incorporating 4 mol % dexamethasone in the LNP to suppress the immune response and antibody production. The critical characteristics including particle size, surface charge, degree of PEGylation, lipid, and cholesterol content of the lipid carrier can significantly impair the therapeutic efficacy of the vaccine and hence need optimisation35.
The lipid nanoparticles currently used in therapeutics exhibit a low degree of endosome escape (<2%) which necessitates finding a solution to this problem. There is a lack of data available to state if the long-term storage renders the vaccine less efficacious. In addition to this, limited information is available regarding the exact structure of the LNPs used to entrap and deliver the mRNA molecule. There is a lack of a detailed study on the susceptibility of the lipid nanoparticle to chemical degradation18. The interaction of biological fluids with the LNP-mRNA system in vivo can lead to the formation of a macromolecular entity due to the adsorption of endogenous biomolecules on the lipid carrier surface. This can potentially lead to aggregation of the lipid carrier particles disturbing the stability of the colloidal system and can also result in the release of mRNA from the lipid carrier core before reaching the target site19.
Conclusion
mRNA vaccine has established itself advantageous over other types of the vaccine due to its high efficacy, accelerated development cycle, and low-cost manufacturing process. The LNP delivery vector has proven to be a vital component of the progression of the science of mRNA vaccines. However, the risk/benefit ratio of the development of lipid carrier enabled nucleic acid vaccines has to be critically analyzed along with assessing their side effect on the kidney, spleen, liver, lymph nodes, and other vital organs of the body. Additionally, designing a systematic regulatory framework for the nanomedicines has to be carried out to assure that these emerging medicines are safe and efficacious. Investigations regarding the incorporation of hybrid nanoparticles like pH-responsive polymeric nanocarriers which can potentially enhance the mRNA delivery potency are needed which can assist the development of systems with a greater level of therapeutic efficacy. Since the interaction with biological fluids can potentially lead to instability of the LNP-mRNA system, there is a need for a detailed study on the colloidal stability of this system to find solutions to maintain the stability of the system and explore other factors which affect the in vitro and in vivo stability of this system. According to study reports which indicate the presence of water in the core of LNP, it is crucial to determine the extent of interaction of water with the mRNA and the effect of this interaction on the mRNA stability. It is also crucial to investigate the exact environment inside the lipid carrier in which the mRNA is encapsulated which will enable a better understanding of the formulation and help modify the formulation aspects to obtain a more stable product. Although lyophilization is a potential option for drying the formulated vaccine preparation, it faces disadvantages of the requirement of high cost, more time, and energy. Thus, there is a need for exploring alternative methods to carry out the procedure18. Investigations of alternatives for PEG which can be incorporated into the formulation to prevent aggregation of LNPs and also increase the mRNA stability are required to be carried out. The choice of delivery system has shifted from traditional liposomes to solid lipid nanoparticles and nanostructured lipid carriers exhibiting enhanced stability, high loading capacity, higher bioavailability of the entrapped contents, the ability to be produced on a large scale by utilizing an organic solvent-free manufacturing process, and better stability to sterilization in comparison to other lipid nanocarriers22,26. The successful use of LNP-mRNA vaccines developed by Pfizer/BioNTech and Moderna in the prophylaxis and treatment of COVID-19 will greatly encourage more research in the field of lipid carrier enabled nucleic acid vaccines5,18.
Acknowledgement
The authors are very grateful to the management and Dean, School of Pharmacy, MIT-World Peace University, Pune for providing an opportunity to carry out the work and extending constant support and encouragement throughout the course of the work.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
There is no funding source.
References
- Singh A. P., Biswas A., Shukla A., Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduction and Targeted Therapy. 2019; 4(1): 33.
- Lu W., Yao J., Zhu X., Qi Y. Nanomedicines: Redefining traditional medicine. Biomedicine and Pharmacotherapy. 2021; 134: 111103.
CrossRef - Jabir M., Sahib U. I., Taqi Z., Taha A., Sulaiman G., Albukhaty S., et al. Linalool-Loaded Glutathione-Modified Gold Nanoparticles Conjugated with CALNN Peptide as Apoptosis Inducer and NF-κB Translocation Inhibitor in SKOV-3 Cell Line. International Journal of Nanomedicine. 2020; 15: 9025-9047.
CrossRef - Albukhaty S. N., Naderi-Manesh H., Taqi T., Jabir M. S. Poly-L-lysine-coated superparamagnetic nanoparticles: a novel method for the transfection of pro-BDNF into neural stem cells. Artificial Cells, Nanomedicine, and Biotechnology. 2018; 46(sup 3): S125-S132.
CrossRef - Tenchov R., Bird R., Curtze A. E., Zhou Q. Lipid Nanoparticles-From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano. 2021;
- Anselmo A. C., Mitragotri S. Nanoparticles in the clinic: An update. Bioengineering and Translational Medicine. 2019; 4(3): e10143.
CrossRef - Shin M. D., Shukla S., Chung Y. H., Beiss V., Chan S. K., Ortega-Rivera O. A., et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nature Nanotechnology. 2020; 15(8): 646-655.
CrossRef - Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nature Reviews Materials. 2021; 1-17.
CrossRef - Meng C., Chen Z., Li G., Welte T., Shen H. Nanoplatforms for mRNA Therapeutics. Advanced Therapeutics. 2021; 4(1): 2000099.
CrossRef - Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA vaccines for infectious diseases. Frontiers in Immunology. 2019; 10: 594
- Linares-Fernández S., Lacroix C., Exposito J. Y., Verrier B. Tailoring mRNA Vaccine to Balance Innate/Adaptive Immune Response. Trends in Molecular Medicine. 2020; 26(3): 311-323.
CrossRef - Okay S., Özcan Ö. Ö., Karahan M. Nanoparticle-based delivery platforms for mRNA vaccine development. AIMS Biophysics. 2020; 7(4): 323-338.
CrossRef - Zeng C., Zhang C., Walker P. G., Dong Y. Formulation and Delivery Technologies for mRNA Vaccines. Current Topics in Microbiology and Immunology. 2020;
CrossRef - Jackson N. A. C., Kester K. .E, Casimiro D., Gurunathan S., DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. npj Vaccines. 2020; 5(1): 11.
CrossRef - Wang Y., Zhang Z., Luo J., Han X., Wei Y., Wei X. mRNA vaccine: a potential therapeutic strategy. Molecular Cancer. 2021; 20: 33.
CrossRef - Li B., Zhang X., Dong Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2019; 11(2): e1530.
CrossRef - Pardi N., Hogan M. J., Porter F. W., Weissman D. mRNA vaccines-a new era in vaccinology. Nature Reviews Drug Discovery. 2018; 17(4): 261-279.
CrossRef - Schoenmaker L., Witzigmann D., Kulkarni J. A., Verbeke R., Kersten G., Jiskoot W., et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. International Journal of Pharmaceutics. 2021; 601: 120586.
CrossRef - Verbeke R., Lentacker I., De Smedt S. C., Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019; 28: 100766.
CrossRef - Kowalski P. S., Rudra A., Miao L., Anderson D. G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Molecular Therapy. 2019; 27(4): 710-728.
CrossRef - Abu Abed O. S. Gene therapy avenues and COVID-19 vaccines. Genes and Immunity. 2021; 22(2): 120-124.
CrossRef - Thi T. T. H., Suys E. J. A., Lee J. S., Nguyen D.H., Park K.D., Truong N.P. Lipid-based nanoparticles in the clinic and clinical trials: From cancer nanomedicine to COVID-19 vaccines. Vaccines. 2021; 9(4): 359.
CrossRef - Pardi N., Hogan M.J., Weissman D. Recent advances in mRNA vaccine technology. Current Opinion in Immunology. 2020; 65: 14-20.
CrossRef - Evers M. J. W., Kulkarni J. A., van der Meel R., Cullis P. R., Vader P., Schiffelers R. M. State-of-the-Art Design and Rapid-Mixing Production Techniques of Lipid Nanoparticles for Nucleic Acid Delivery. Small Methods. 2018; 2(9): 1700375.
CrossRef - Elia U., Ramishetti S., Rosenfeld R., Dammes N., Bar-Haim E., Naidu G. S., et al. Design of SARS-CoV-2 hFc-Conjugated Receptor-Binding Domain mRNA Vaccine Delivered via Lipid Nanoparticles. ACS Nano. 2021; 15(6): 9627-9637.
CrossRef - Gao Y., Yang K., Shelling A. N., Wu Z. Nanotechnology-Enabled COVID-19 mRNA Vaccines. Encyclopedia. 2021; 1(3): 773-780.
CrossRef - Mitchell M. J., Billingsley M. M., Haley R. M., Wechsler M. E., Peppas N. A., Langer R. Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery. 2021; 20(2): 101–124.
CrossRef - Hassett K. J., Benenato K. E., Jacquinet E., Lee A., Woods A., Yuzhakov O., et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Molecular Therapy – Nucleic Acids. 2019; 15: 1-11.
CrossRef - Vu V. P., Gifford G. B., Chen F., Benasutti H., Wang G., Groman E. V., et al. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nature Nanotechnology. 2019; 14(3): 260-268.
CrossRef - Patel S., Ashwanikumar N., Robinson E., Xia Y., Mihai C., Griffith J. P., et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nature Communications. 2020; 11(1): 983.
CrossRef - Gómez-Aguado I., Rodríguez-Castejón J., Vicente-Pascual M., Rodríguez-Gascón A., Solinís M. Á., Del Pozo-Rodríguez A. Nanomedicines to deliver mRNA: State of the art and future perspectives. Nanomaterials. 2020; 10(2): 364.
CrossRef - Kuchekar A., Jathar J., Gawade A., Kuchekar B. Influence of Process Variables on Budesonide Nanoparticles Using Factorial Design. Journal of Pharmaceutical Research International. 2021; 33(4): 58-71.
- Liu J., Miao L., Sui J., Hao Y., Huang G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian Journal of Pharmaceutical Sciences. 2020; 15(5): 576-590.
CrossRef - Grenier P., Viana IM de O., Lima E. M., Bertrand N. Anti-polyethylene glycol antibodies alter the protein corona deposited on nanoparticles and the physiological pathways regulating their fate in vivo. Journal of Controlled Release. 2018; 287: 121-131.
CrossRef - Podutwar A., Polshettiwar S., Gawade A., Baheti A., Wani M., Ghanekar M., Gupta A., Chondorkar P. Recent Advances in Nanoparticle Drug Delivery for Targeting Lymph Node -An Overview in Cancer Immunotherapy. Journal of Pharmaceutical Research International. 2021; 33(43A): 64-74.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






