How to Cite | Publication History | PlumX Article Matrix
Satyabrata Dash, Sabyasachy Parida, Bijayananda Sahoo and Biswajit Rath*
Department of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University, Sriram Chandra Vihar, Takatpur, Baripada-757003, Odisha
Corresponding Author E-mail: brath_2000@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2976
ABSTRACT:
Cyanobacteria as proven source of bioactive secondary metabolites have recently found immense application in pharmaceutical sector. The goal of this study was to find out the antimicrobial property in two species of cyanobacteria, Oscillatoria boryana and Oscillatoria pseudogeminata against different pathogenic microorganisms. Aqueous and different solvent extracts (acetone, methanol, benzene) were prepared and assayed for antimicrobial activity against four bacterial (Staphylococcus aureus, Bacillus subtilis, Vibrio cholerae and Eschercichia coli) and four fungal (Aspergillus niger, Candida albicans, Penicillium verrucosun and Fusarium oxysporum) pathogens. The study results recorded highest antimicrobial activity in methanol extracts of O. boryana showing significant zone of inhibition against V. cholerae (20±0.74 mm) and C. albicans (17±0.80 mm). Least antimicrobial activity was detected in aqueous extract among all the test species. The purified active fractions were analysed using GC-MS, where dominant compounds that are responsible for the inhibitory activity such as n-hexadecanoic (15.42%) 5-Methyl-2-phenyl indolizine (13.3%), Acetic acid (12.47), 5-Nitro-3-cyano-2(1H)-pyridone (10.37 %), 9-Oxo-9,10-dihydro acridine-4-yl (6.8%) were detected.Thus the obtained results suggests that studied species of cyanobacteria can be prospective in biotechnology as an alternative sources of antimicrobial substances.
KEYWORDS: Antimicrobial; Bioactive; Cyanobacteria; Extract; MIC
Download this article as:| Copy the following to cite this article: Dash S, Parida S, Sahoo B, Rath B. Potential Antimicrobial Activity of Cyanobacteria Oscillatoria Boryana and Oscillatoria Pseudogeminata Isolated from Odisha Coast, India.Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Dash S, Parida S, Sahoo B, Rath B. Potential Antimicrobial Activity of Cyanobacteria Oscillatoria Boryana and Oscillatoria Pseudogeminata Isolated from Odisha Coast, India.Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3q8BYX0 |
Introduction
Cyanobacteria are photosynthetic organism found in a variety of ecosystems, including fresh, marine, brackish water and terrestrial environments. Cyanobacteria were found to produce different types of secondary metabolites which inhibit the growth of other microorganisms in their surrounding.1,2 Cyanobacteria possess natural bioactive substances that are difficult to produce by chemical synthesis3. These compounds has a wide range of biological activities such as antimicrobial, antioxidant, anticancer etc4,5-7. The search for antibiotics and other pharmacologically active substances in cyanobacteria has received more attention at present time8. A number of literatures are available on antimicrobial substances produced from microalgae and cyanobacteria9. However, the compounds responsible for antimicrobial properties in cyanobacteria are yet to be identified for its maximum efficacy10,11. In the marine environment cyanobacteria were found to synthesize a large array of chemical compounds due to biotic and abiotic stress12. Antimicrobial substances produced by cyanobacteria are of interest for research not only protective for pathogens, but also as pharmacological important bioactive secondary metabolites13. Moreover, the differential antimicrobial activity of cyanobacteria is correlated to the selected species and the solvents used in experiments14-17. Despite the fact that several work has been carried out on antimicrobial property of cyanobacteria of different habitats but a limited reports were available on the species found across Odisha coast. Thus the current study intends to find out the antimicrobial properties of two selected predominant species (Oscillatoria boryana and Oscillatoria pseudogeminata), obtained from Odisha coast.
Materials and Methods
Collection, culture condition and isolation of cyanobacteria
The test organism (Cyanobacteria) were collected from different sites across Odisha coast (Fig 1A & B) by using a plankton net (2μ) and the samples attached to different substratum using forceps. The samples were isolated to unialgal culture by repeated subculture and serial dilution, observed under trinocular research microscope (Make: Dewinter Model: “SELECT” DGI-1000) and identified by standard procedure18-20. The isolated cyanobacterial species were grown in 1 litre flasks with aeration to obtain large biomass using ASN III medium with illumination of 30 μmol photon m-2 s-1alongwith light/dark cycle of 16 h/ 8h at 25±1°C temperature.
Test Cyanobacteria
Morpho-taxonomic identification
Oscillatoria boryana
Class- Cyanophyceae; Order- Nostocales; Family- Oscillatoriaceae; Genus- Oscillatoria; Species- boryana
Trichome coiled like a screw at the apices or totally, sometimes straight compressed near cross walls, 6-8µ broad, sometimes slight granulation observed adjacent to cross walls, cellsat the apices 4-6µ elongated or rounded or more or less pointed, not capitates, calyptra absent, the breadth of the trichome varies very much between 3.3-5.0µ, 4-6.6µ, 5-7µ and 6-8µ(Fig.1B-i).
Oscillatoria pseudogeminata
Class- Cyanophyceae; Order- Nostocales; Family- Oscillatoriaceae; Genus- Oscillatoria; Species- pseudogeminata
Thallus blue-green in colour, trichomes coiled, ends are not attenuated, cells are 1.3-2.2µ broad, about 2.6µ long, the cross walls are not squeezed but thickened, non granulated and cells are spherical, calyptras absent (Fig.1B-ii).
 |
Figure 1: (A). Sample collection sites across Odisha Coast |
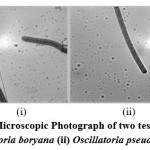 |
Figure 1: (B). Microscopic Photograph of two test cyanobacteria (i) Oscillatoria boryana (ii) Oscillatoria pseudogeminata |
Cyanobacterial extract preparation
The cyanobacterial cultures (10-14days old) were harvested by centrifugation (3500 rpm for 15min) and dried at 45 °C in a hot air oven. The dried samples were grounded using a sterile mortar and pestle. Then, 0.5 g of grounded sample was mixed with 10 ml of solvents (acetone, methanol, benzene and aqueous) used and kept overnight at room temperature in a rotary shaker for complete extraction. At 4000rpm for 15minutes the extracts were centrifuged and concentrated at 50°C under reduced pressure. The dried residue was dissolved in DMSO to obtain final concentrations of 1mg/ml, and used for antimicrobial activity21.
Pathogenic strains
Four pathogenic bacteria i.e two Gram +ve (Staphylococcus aureus MTCC-96, Bacillus subtilis MTCC-441) and two Gram -ve (Vibrio Cholerae MTCC-3906, Eschercichia coli MTCC-443) and four pathogenic fungi (Candida albicans MTCC 183, Aspergillus niger MTCC-1344, Penicillium verrucosun MTCC-1758, Fusarium oxysporum MTCC-284) were used for the experimental purpose. These microorganisms were procured from Institute of Microbial Technology, Chandigarh and kept in the Department of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University, Takatpur, Mayurbhanj, Odisha.
Antibacterial assay
The agar cup method22is used for the antibacterial study, using Mueller-Hinton agar (MHA) media for bacteria. Petriplates with 20ml of sterilised solid media was inoculated with 20μl of test pathogens (106 CFU/ml) were uniformly swabbed. 6 mm diameter wells were formed and 50 μl of cyanobacterial extracts (DMSO, 1 mg/ml) were put into the wells and placed for incubation at 37 ± 1 ºC for 24 hours. The inhibition zone formed around the wells was measured after incubation period. The average value of diameter of three plates was recorded. Ampicillin at a concentration of 100µg/ml are used as positive control.
Antifungal assay
The antifungal activity study was carried out on potato dextrose agar medium (PDA). 22 Sterilized potato dextrose agar (PDA) was poured into sterile petriplates (20ml), solidified and inoculated with 20μl of fungal test pathogens (106 CFU/ml) and swabbed uniformly. 6 mm diameter wells were formed and 50 μl of cyanobacterial extracts (DMSO, 1 mg/ml) were put into the wells and placed for incubation at 25 ± 2 ºC for 72 hours. The inhibition zone formed around the wells was measured after incubation period. The average value of diameter of three plates was recorded. Clotrimazole at a concentration of 100µg/ml are used as positive control.
Study of Minimum inhibitory concentration (MIC)
MIC experiment was done using 96 well micro titer plates containing broth medium. The first series of well was given 50 µl of Mueller-Hinton broth (MHB) and 50 µl bacteria culture as negative control. Starting from second series of wells until eighth row were embedded with various concentration of extract(15.62, 31.25,62.5,125, 250,500 and 1000µg/ml) by two fold dilution in MHB media with pathogenic microorganisms as control and kept at 37º C for 24 h and subsequently were analysed with microplate reader (make-TECAN, model-M200PRO)and interpreted.23-25
Gas Chromatography/Mass Spectrometry (GC-MS) analysis
The GC-MS evaluation was determined at Analytical Chemistry, Triyat Scientific Co., Nagpur, Maharashtra, India. The GC-MS analysis was done using a Agilent 7890A Gas Chromatography system equipped with a HP-5 column (30ml x 250µm ID, 0.25µm thick film) and Agilent 5975C inert Mass Spectrometer Detector with Helium as carrier gas. The sample extract was injected into the injector port and the Helium gas expel the sample down the column where different chemical constituents get separated. The injection port was maintained at 80 ºC for 4 minutes. The run time was continued for 40 minutes. The flow rate of 1.50ml/min; injector and column oven temperature of 280ºC and 80ºC; injector split ratio of 20:1 was set. GC-MS mass spectrum interpretation was conducted and compared with the reference data base from NIST.
Statistical analysis
Statistical analysis of results is performed. The values are expressed as mean with standard deviations (±SD) and were subjected to one way ANOVA, and the results were the average of three replicates (p ≤ 0.05).
Results
Antibacterial Activity
The results obtained in the evaluation of the antibacterial activity of different solvent extracts of two marine cyanobacteria Oscillatoria boryana and Oscillatoria pseudogeminata against four pathogenic bacteria (S. aureus, B. Subtilis, V. cholerae and E. coli) strains using the agar well diffusion method was shown in Table 1(A&B). Differential antimicrobial activity was observed in separate solvent extracts against particular pathogen. The maximum zone of inhibition of 20mm was detected against V. cholerae using methanol extracts of O. boryana, followed by 18 mm and 17 mm with B. Subtilis and S. aureus respectively (Table 1A). A Smaller zone of inhibition of 9mm was found in aqueous extract of O. Pseudogeminata against B. Subtilis and E. coli (Table 1B). Further, larger inhibition zone was observed in methanol extract than by acetone and benzene whereas minimum zone of inhibition was found in aqueous extract in both the test species of marine cyanobacteria. The study result reveals that the methanolic extract of O. boryana found to have significant antibacterial activity in V. cholerae and in contrary neglible activity was marked in aqueous solvent in agar well diffusion assay.
Antifungal Activity
The results exhibiting the in-vitro antifungal activity of different solvent extracts of two marine cyanobacteria O. boryana and O. pseudogeminata against four fungal pathogens (A. niger, C. albicans, P. verrucosun and F. oxysporum) strains using the agar cup method was shown in Table 1A&B. Maximum zone of inhibition of 17mm against C. albicans and 14mm against A. niger in O. boryana and O. pseudogeminata respectively in methanol extract and the minimum zone of inhibition of 9mm was found in aqueous extract of O. boryana against A. niger and 8mm in O. pseudogeminata against F. oxysporum (Table 1A& B).
Table 1: (A). Antibacterial and antifungal activity of different solvent extract of Oscillatoria boryana with bacterial and fungal pathogens.
| Bacterial and Fungal Pathogens | Inhibition zone diameter (mm) | |||
| Acetone | Methanol | Benzene | Aqueous | |
| S. aureus | 16±0.82 | 17 ±0.73 | 13±0.75 | 11±0.76 |
| B. subtilis | 15±0.77 | 18±0.76 | 14±0.79 | 10±0.83 |
| V. cholerae | 16 ±0.79 | 20±0.74 | 14±0.78 | 10±0.80 |
| E. coli | 14±0.81 | 15 ±0.78 | 13 ±0.76 | 10±0.75 |
| A. niger | 14±0.76 | 16±0.81 | 10±0.75 | 09±0.77 |
| C. albicans | 12±0.79 | 17±0.80 | 13±0.77 | 10±0.81 |
| P. verrucosun | 13 ±0.84 | 15±0.76 | 12±0.80 | 10±0.77 |
| F. oxysporum | 12±0.81 | 15 ±0.76 | 11 ±0.73 | 10±0.82 |
Table 1: (B). Antibacterial and antifungal activity of different solvent extracts of Oscillatoria pseudogeminata with bacterial fungal pathogens.
| Bacterial and Fungal Pathogens | Inhibition zone diameter (mm) | |||
| Acetone | Methanol | Benzene | Aqueous | |
| S. aureus | 12±0.73 | 13±0.81 | 11±0.74 | 10±0.79 |
| B. subtilis | 10±0.75 | 12±0.76 | 10±0.77 | 09±0.77 |
| V. cholerae | 11±0.82 | 11±0.79 | 10±0.79 | 11±0.75 |
| E. coli | 10±0.77 | 11±0.84 | 11±0.81 | 09±0.77 |
| A. niger | 12±0.82 | 14±0.80 | 12±0.79 | 11±0.75 |
| C. albicans | 11±0.83 | 13±0.84 | 10±0.76 | 11±0.77 |
| P. verrucosun | 10±0.75 | 11±0.82 | 10±0.78 | 09±0.76 |
| F. oxysporum | 12±0.77 | 12±0.79 | 09±0.82 | 08±0.72 |
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration was estimated at a concentrations range of 1000µg/ml to 15.62µg/ml and optimum dilution showing inhibition was observed as MIC value (Table 2).The MICs of the extracts were measured using serial dilutions up to 1000µg/ml of extracts in DMSO. The MIC detected for O. boryana was 31.2 µg/ml and was 125µg/ml for O. pseudogeminata. The MIC result thus reveals that O. boryana has significant antimicrobial property as compared to O. Pseudogeminata.
Table 2: Minimum inhibitory concentration (MIC) of O. boryana and O. pseudogeminata against bacterial and fungal pathogens.
| Pathogens | Minimum inhibitory concentration (µg/ml) | |||||||
| Oscillatoria boryana | Oscillatoria pseudogeminata | |||||||
| Acetone | Methanol | Benzene | Aqueous | Acetone | Methanol | Benzene | Aqueous | |
| S. aureus | 125 | 125 | 500 | 500 | 250 | 250 | 500 | 500 |
| B. subtilis | 250 | 62.5 | 250 | 250 | 500 | 125 | 500 | 500 |
| V. cholerae | 125 | 31.2 | 250 | 500 | 500 | 500 | 250 | 250 |
| E. coli | 250 | 125 | 500 | 500 | 500 | 500 | 500 | 1000 |
| A. niger | 125 | 125 | 500 | 500 | 250 | 125 | 500 | 250 |
| C. albicans | 250 | 62.5 | 250 | 250 | 250 | 125 | 500 | 500 |
| P. verrucosun | 125 | 125 | 250 | 500 | 500 | 500 | 500 | 500 |
| F. oxysporum | 500 | 125 | 500 | 500 | 250 | 250 | 1000 | 1000 |
GC-MS Analysis of methanolic extract of O. boryana
The chemical constituents of methanol extract of O. boryana detected by GC-MS was presented in Fig. 2, Table 3. Major chemical components present in the methanol extract of O. boryana indicated the occurence of many novel bioactive compounds. Thirteen compounds are detected in methanolic extract of O. boryana with the highest value of n-hexadecanoic acid (15.42 %) followed by 5-Methyl-2-phenyl indolizine (13.3%). The other prevailing compounds were Acetic acid (12.47%), 5-Nitro-3-cyano-2(1H)-pyridone(10.37%), 9-Oxo-9,10-dihydro acridine-4-yl (6.8%), Hexadecanoic acid and Silicic acid 5.02% each. The analysis also revealed the presence of few biologically active compounds in low concentration such as Benzenemethanol (1.9%), Undecanoic acid (1.73 %), Phytol(1.58%), Propane,1,1,3-triethoxy (0.92 %), Erucic acid (0.87%) and Benzoic acid (0.78 %). The study results infers that possibly these chemical compounds are important in terms of their antimicrobial activity 27,28.
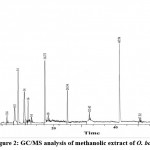 |
Figure 2: GC/MS analysis of methanolic extract of O. boryana |
Table 3: Compounds identified in methanol extract of O. boryana as revealed by GC-MS analysis.
| No | Compounds name | Mol. Formula | Mol. weight (g/mol) | Ret. Time RT
(mins) |
Peak area
(%) |
| 1 | Propane,1,1,3-triethoxy- | C9H20O3 | 176.25 | 4.3 | 0.92 |
| 2 | Benzoic acid | C7H6O2 | 126.12 | 5.6 | 0.78 |
| 3 | Undecanoic acid | C11H22O2 | 186.29 | 9.5 | 1.73 |
| 4 | 5-Nitro-3-cyano-2(1H)-pyridone | C5H4NH(O) | 165.11 | 14 | 10.37 |
| 5 | Acetic acid | C2H4O2 | 60.05 | 14 | 12.47 |
| 6 | 9-Oxo-9,10-dihydro acridine-4-yl | C13H9N | 179.13 | 16 | 6.8 |
| 7 | Hexadecanoicacid | C18H36O2 | 284.48 | 16 | 5.02 |
| 8 | 5-Methyl-2-phenyl indolizine | C15H13N | 207.27 | 16.75 | 13.3 |
| 9 | Phytol | C20H40O | 128.17 | 18 | 1.58 |
| 10 | Silicic acid | Si (OH)4 | 96.11 | 26.54 | 5.02 |
| 11 | Benzenemethanol | C7H8O | 108.14 | 32.40 | 1.9 |
| 12 | n-hexadecanoic acid | C16H32O2 | 256.4 | 40.94 | 15.42 |
| 13 | Erucic acid | C22H42O2 | 338.57 | 47.52 | 0.87 |
Discussion
Unregulated use of antibiotics gives rise antibiotic resistance in bacteria, fungs and thus raises an issue of health hazards. The alarming increase in microbial contamination has boosted the search for new, novel and secure candidate to fight against infection. Thus more specific research on identification of bioactive compounds derived from various microalgae and cyanobacteria is of interest at present. Cyanobacteria are potential organism for producing a variety of bioactive secondary metabolites. These compounds derived from cyanobacteria were found to possess antimicrobial activity. Based on this fact; two species of marine cyanobacteria Oscillatoria boryana and Oscillatoria pseudogeminata were studied for antimicrobial activity. The findings of present work explained that among the two test cyanobacteria, methanol extract of O. boryana exhibited significant antimicrobial activity than O. pseudogeminata. In the similar pattern, 29also reported that Oscillatoria brevis has antibacterial activity against S. aureus(ATCC 13565);B. cereus(EMCC 1080); S. typhi (ATCC25566); P. aeruginosa (NRRL B-272) and K. Pneumonia (LMD 7726). Earlier study by Sethubathi and Prabu30 which is in agreement to our result revealed that the crude extracts of Oscillatoria sp.; Phormidium sp. and Lyngbya majuscule were found to possess antimicrobial activity against pathogens such as S. mutants; S. aureus; P. aeruginosa; B. Subtilis and K. pneumonia. The MIC value as recorded in the present piece of work was promising and the cyanobacterial extracts showed significant antimicrobial potency against V. cholerae; B. subtilis and C. albicans which match to the work carried out by Wijesekara and Manage 31 with Oscillatoria boryana extract showing maximum antimicrobial activity against S. aureus as compared to S. typhi. Moreover, the findings of the present investigation on GC-MS analysis showed the presence of n-hexadecanoic acid, Acetic acid, 5-Nitro-3-cyano-2(1H)-pyridoneetcas predominant compounds which might be responsible for antimicrobial activity in Oscillatoria boryana, which matches to the work carried out by Mundt et al.32 and Shanab33,34. Similarly, El-Syad et al.35 detected presence of hexadecanoic acid and bis(2-ethylhexyl) phthalate in the phenolic extract of Spirulina and in addition, phytol was previously found in a marine cyanobacteria, N. muscorum and in the S. platensis 36showing antimicrobial property. Recently, Hobby et al.37 stated that modification of phospholipid composition by fatty acids suppress the antimicrobial compounds. However, there is no any specific reason established between chemical classes and their role in bioactive properties such as antimicrobial activity38. The saturated fatty acids might be binding the cell membrane and alter the cellular construct thereby enhancing in antimicrobial activity. Thus, deeper study is required to focus on the bioactivity of metabolites synthesise in cyanobacteria for the production of novel antimicrobial compounds that maybe useful for commercial purpose.
Conclusion
This study demonstrates that both the cyanobacteria have the potential to produce potent antimicrobial compounds that act against human pathogens. Among different solvents used, the methanolic extract of both the species of Oscillatoria showed better activity, inhibiting the growth of test pathogens. The present research has shown that the antimicrobial activity of different species of cyanobacteria is dependent on the solvents used to make the extracts and the effect of those solvents. It is thought that the findings obtained from this investigation can be used for future research and for the production of antibacterial drugs of cyanobacterial origin. Moreover, this study has paved the way towards demonstrating the presence of potential high-value pharmaceutical antimicrobials having ample scope for further investigation towards drug development in pharmaceutical and health care industry.
Acknowledgment
The authors are thankful to Head, Department of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University for providing facilities for conducting the experiments.
Conflict of Interest
The authors declares no conflict of Interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Ali A.H., Moustafa E.E., Abdelkader S.A., Hafez S.S., Abdallah S.A. Antibacterial potential of macro and microalgae extracts against pathogens relevant to human health.Plant Archives. 2020; 20(2): 9629-9642.
- Bhuyar P., Sathyavathi S., Math R.K. Production of bioethanol from starchy tuber (Amorphophallus commutatus) and antimicrobial activity study of its extracts. Afr J BiolSci.2020; 2:70– 76.https://doi.org/10.33472/AFJBS.2.2.2020.70-76.
CrossRef - Goud M.L., Seshika A.D., Charya M.A.S. Antibacterial activity and Bimolecular composition of certain freshwater micro-algae from river Godavari (India).Sci. World J.2007; 2: 19-23.
CrossRef - Estela Silva-Stenico, Maria, Ramon Kaneno, Fabiana Albani Zambuzi, Marcelo GMV Vaz, Danillo O Alvarenga, and Marli Fatima Fiore. “Natural products from cyanobacteria with antimicrobial and antitumor activity.” Current pharmaceutical biotechnology 14, no. 9 (2013): 820-828.
CrossRef - Shishido T. K., Popin R. V., Jokela J., Wahlsten M., Fiore M. F., Fewer D. P., Sivonen K. Dereplication of natural products with antimicrobial and anticancer activity from Brazilian cyanobacteria. Toxins. 2020; 12(1):12.
CrossRef - Chakraborty K., Maneesh A., Makkar F. Antioxidant activity of Brown seaweeds. Aquat. Food Prod. Technol. 2017; 26:406–419.
CrossRef - Liu , Gao T., Yang Y., Meng F., Zhan F., Jiang Q., Sun X.Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules. 2019;24(4286) :1–14.
CrossRef - Rimsha R., Richa J., Sheela K., Shrivastava P.N., Manju J. Bioactive substances of cyanobacteria (Nostocmuscorum): a review.Int J PharmaSci Res.2014;5:320-322.
- Ming L., Yixiang M.J., Cao L., Guang-Ming Q., Chen L., Sun H., Chen H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. CarbohydratePolymers. 2017;172: 294-305. https://doi.org/10.1016/ j.carbpol.2017.05.060
CrossRef - Amaro H.M., Guedes A.C., Malcata F.X. Science against microbial pathogens: communicating current research and technological advances A. Mendez-Vilas (Ed.), Antimicrobial activities of microalgae: an invited review. 2011;1272-1280.
- Pina-Perez M.C., Rivas A., Martinez A., Rodrigo D. Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chemistry.2017. https://doi.org/10.1016/j.foodchem.2017.05. 033.
CrossRef - Bhagavathy S., Sumathi P., Jancy Sherene Bell I. Green algae Chlorococcum humicola: a new source of bioactive compounds with antimicrobial activity. Asian Pac J Trop Biomed.https://doi.org/10.1016/S2221-1691(11)60111-1.
CrossRef - Oliveira D. T. D., Costa A. A. F. D., Costa F. F., Rocha Filho G. N. D., Nascimento L. A. S. D. Advances in the biotechnological potential of Brazilian marine microalgae and cyanobacteria. Molecules. 2020; 25(12): 2908.
CrossRef - Prakash J.W., Johnson M., Solomon J. Antimicrobial activity of certain freshwater microalgae from Thamirabarani. Asian Pac J Trop Biomed. 2011;1: 170-173.
CrossRef - Rath B., Thatoi H.N., Priyadarshani I. Antibacterial properties of extracts in cyanobacteria isolated from coastal region of Orissa, India. Int J current research and review.2011;3(3):81-87.
- Radhika D., Veerabahu C., Priya R. Antibacterial activity of some selected seaweeds from Gulf of Mannar Coast, South India. Asian J Pharmaceut Clin Res.2012;5: 89-90.
- Dash S., Parida S., Rath B. Antimicrobial Activities of Some Selected Marine Cyanobacteria Isolated from Bay of Bengal of Odisha Coast. International Journal of Pharmacy and Biological Sciences. 2019;9 (3):196-200.
- Desikachary T.V. Cyanophyta. Indian Council of Agricultural Research, New Delhi. 1959; 686.
- Anand N. Culture studies and taxonomy of blue-green algae certain identification problems. Hydrobiol. Suppl. 1988; 80:141–147.
- Anagnostidis K., Komárek J. Modern approach to the classification system of cyanophytes 3 – oscillatoriales. Algol. Stud. 1988; 50:327–472.
- Jelodarian S., Abdolrasoul Haghir E., FereshtehJ.K.Evaluation of antimicrobial activity of Malusdomestica fruit extract from Kashan area. Avicenna Journal of Phytomedicine.2013;3(1): 1-6.
- Abedin R.M.A., Taha H.M. Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of medium components by Plackett-Burman design for antimicrobial activity of Spirulina platensis. Global Journal of Biotechnology and Biochemistry. 2008;3(1): 22-31.
- Skov R., Frimodt-Moller N., Blomstrom A., Espersen F. Comparison of different MIC methods and establishment of zone diameter breakpoints for mecillinam, using NCCLS methodology. In Program and abstracts of the 40thInterscience Conference on Antimicrobial Agents and Chemotherapy (Toronto), Canada.2019.
- Prasannabalaji N., Muralitharan G., Sivanandan R.N., Kumaran S., Pugazhvendan S.R. Antibacterial activities of some Indian traditional plant extracts.Asian Pacific Journal of Tropical Disease.2012; 2: 291-295.
CrossRef - Singh R.K., Tiwari S.P., Rai A.K., Mohapatra T.M. Cyanobacteria: an emerging source for drug discovery. Antibiot.2011; 64 (6): 401–412.
CrossRef - Phytochemical and Ethnobotanical Databases. 2013.www.arsgov/ cgi-bin/duke.
- Mitrović T., Stamenković S., Cvetković V., Tošić S., Stanković M., Radojević I. Antioxidant, antimicrobial and antiproliferative activities of five lichen species. International Journal of Molecular Sciences.2011; 12(8):5428-5448.
CrossRef - Saxena S., Rao P.B. GC-MS Screening of Bioactive Constituents and Antioxidant Profiling in an Invasive Weed. Malvastrum coromandelianum (L.)Garcke. The Pharma Innovation Journal.2018;7 (4):738-746.
- Marrez D.A., Sultan Y.Y., Embaby M.A. Biological activity of the cyanobacterium Oscillatoria brevis extracts as a source of nutraceutical and bio-preservative agents. Int. J. Pharmacol.2017;13 (8): 1010–1019.
CrossRef - Sethubathi G.V.B., Prabu V.A. Antibacterial activity of cyanobacterial species from adirampattinam coast, southeast coast of palk bay. Current Research Journal of Biological Sciences .2010;2(1): 24-26.
- Wijesekara W.A.M.A., Manage. P.M. Isolation, purification and structure elucidation of antimicrobial and bio- active compounds of Lyngbya 22 st International Forestry and Environment Symposium Proceeding.2017.
CrossRef - Munda S., Kreitlow S., Jansen R. Fatty acids with antibacterial activity from the cyanobacterium Oscillatoria redekei HUB 051. Appl. Phycol.2003;15: 263–267. https:// doi.org/10.1023/A:1023889813697
CrossRef - Shanab S.M.M. Bioactive allelo-chemical compounds from Oscillatoria species (Egyptian isolates).Int. J. Agric. Biol. 2007; 9 (4): 617–621.
- Marrez D. A., Sultan Y. Y., Naguib M. M., Higazy A. M. Antimicrobial Activity, Cytotoxicity and Chemical Constituents of the Freshwater Microalga Oscillatoria princeps. Biointerface Research In Applied Chemistry. 2021; 12 (1): 961-977.
CrossRef - El-Sayed S.T., Ali A.M., El-Sayed M., Shousha W.G., Omar N.I . Characterization and potential antimicrobial effect of novel chitooligosaccharides against pathogenic microorganisms. Journal of Applied Pharmaceutical Science. 2017;7:6-12.
- Armstrong L., Vaz M. G. M. V., Genuário D. B., Fiore M. F., Debonsi H. M. Volatile compounds produced by cyanobacteria isolated from mangrove environment. Current microbiology. 2019; 76(5):575-582.
CrossRef - Hobby C.R., Herndon J.L., Morrow C.A., Peters R.E., Symes S.J., Giles D.K. Exogenous fatty acids alter phospholipid composition, membrane permeability, capacity for biofilm formation, and antimicrobial peptide susceptibility in Klebsiella pneumoniae.
Microbiology Open.2019;8(2):635. https://doi.org/10.1002/mbo3.635 e00635.
CrossRef - Demay J., Bernard C., Reinhardt A., Marie B. Natural products from cyanobacteria: focus on beneficial activities. Drugs.2019; 17 (6):320. https://doi. org/10.3390/md17060320.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





