Recent Upgradation in Bioanalytical Studies
Department of Pharmaceutical Chemistry, Mumbai Education Trust’s, Institute of Pharmacy, BKCs Adgaon, Nashik, Maharashtra, (M.S.), India-422003
Corresponding Author E-mail: rshelke07@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2972
ABSTRACT:Analytical methodologies are critical throughout the medicine development process, including marketing and post-marketing studies. The advancement of bio-analytical techniques has resulted in a dynamic field with many exciting potentials for further advancement in the future. Bio-analysis is commonly utilised in the pharmaceutical drug development of drug's and its metabolites' quantitative levels. The goal is to undertake pharmacokinetic and pharmacodynamic studies, as well as kinetics, toxicokinetics, bioequivalence, and exposure studies. Bioanalytical research employs a variety of bioanalytical techniques, including new instrumental techniques, separation techniques, and ligand-Indused test. This study emphasizes the importance of bio-analytical techniques and hyphenated devices in evaluating drug bio-analysis and the role of several current bio-analytical techniques such as LC-Mass, HPLC-PDA, UPLC-Mass spectroscopy, HPTLC, LC-Tandem, AAS, ICP-Mass.etc., and their recent modernization in drug analytical and bio-analysis investigations
KEYWORDS:Analytical techniques; Analytical testing; Bioanalysis; Bioanalytical methods
Introduction
Bio-analysis is a important step in the development and research of medications. Pharmaceutical research organizations must construct thorough bio-analytical techniques during the medicine discovery and development process. During the creation of novel pharmaceuticals, bioanalysis is now commonly used in toxicological testing, as well as pharmacokinetic and pharmacodynamic research. Bioanalytical method validation can also be used to detect a range of analytes quantitatively in biological matrices. In current bioanalytical procedures, a robust sample preparation and modern instrument methods are required. The bioanalytical technique procedure includes selection, processing conditions, testing, standardization, data evaluation, and reporting. In regulatory bioanalysis, Method validation is also required to ensure the accuracy of the used technique and to assist the licencing of novel drugs or biologic drugs.
Why plasma?
In bioanalysis, plasma is the most important and often utilised biological fluid. Compared to other biological matrices, plasma has various advantages. including the fact that it’s easily accessible, inexpensive, and provides an accurate indicator of drug concentration in the blood. If the drug concentration in the blood is low, plasma is favored above any other matrix. Plasma has the highest rate of recovery of any matrix. One of the best matrices is plasma.to employ if the treatment has a strong protein binding capability. Because the sample size in animal studies is so small, assessing plasma drug concentration is the preferred method. Plasma has a significant edge versus serum in this regard a sample is less likely to be lost in plasma. Blood is centrifuged with anticoagulant and the supernatant is collected to obtain plasma. When entire blood gets collected in anticoagulant-treated tubes, plasma is formed. 1 For estimating and measuring pharmaceutical substances in various biological matrices, a number of detection techniques have been published. Plasma, urine, serum, cerebrospinal fluid (CSF), tissue, and other biological matrices all have their own criticality when it comes to sample preparation in Bioanalysis. The goal of this study is to assess drug sample analysis in plasma and develop practical methodologies for research in pharmacokinetics, toxicology, availability of drug in blood stream, and biopharmaceutical studies For the assessment of pharmacological compounds in various biological matrices, a variety of detection approaches have been reported. When it comes to sample preparation in Bioanalysis, plasma, urine, serum, cerebrospinal fluid (CSF), tissue, and other biological matrices all have their own criticality.
Modern analytical techniques in bioanalysis
Separating a pure component from a mixture are among the most basic analytical methods. is chromatography. There are so many separation techniques each has its own set of uses, as well as advantages and disadvantages. Amoung that HPLC is is the most extensively used chromatographic process. Initially, detection was achieved using liquid chromatography with an ultraviolet detector (LC-UV), but mass spectrometry was subsequently used to upgrade the method (MS). 2 Because pharmaceutical medications are non-volatile, thermolabile, and polar, they are not suited for gas chromatography (GC). UV, fluorescence, and electrochemical detection are the most prevalent LC detection methods. Although fluorescent and electrochemical detections are more sensitive and selective than UV detections, they are limited to molecules containing fluorescent or electroactive groups or need derivatization. 3 Mass spectrometry (MS) and off-line LC-MS have been used in qualitative analysis for many years. Sample preparation, chromatography, and detection are the three main components of bioanalytical, quantitative LC-MS investigations. The factors described above have an impact on the analytical method’s reliability, reproducibility. On-line LC-MS for quantification was difficult prior to the 1980s, when atmospheric pressure ionization (API) techniques (e.g., atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI) were established. Ion-spray (ISP; pneumatically assisted ESI with an additional hot drying gas perpendicular to the spray), turbo ion-spray (TISP; pneumatically assisted ESI with an additional hot drying gas perpendicular to the spray), and heated nebulizer (HN)-APCI have all improved LC-MS interfaces significantly in recent years (pneumatically aided APCI). LC-MS is a well-known, commonly utilized, and well-recognized bench-top technology in the field of pharmaceutical analysis. 4 Liquid chromatography (LC) and tandem mass spectrometry (MS/MS) have been used in drug bioanalysis for a long time. In bioanalytical, LC MS/MS is employed. The fundamental purpose of chromatography is to separate analytes from matrix components rather than other analytes, internal standards, or potential metabolites. Co-eluting peaks do not interfere with analyte identification because of the MS’s selectivity, but they can cause ion suppression. For this separation, short LC columns are usually sufficient, resulting in much reduced run times. The most common positive ionization LC-MS/MS additions are ammonium acetate, ammonium format, acetic acid, and formic acid. Negative ionization studies can benefit from the use of ammonium hydroxide. 4 In chromatography, additives are used to help the process, but they are also required for ionization. HPTLC is a type of analytical techniques TLC that uses high-performance polymer surfaces and development chambers. It usually needs systematic procedure invention, optimization, documentation, and deployment. Both qualitative and quantitative modalities are available, the HPTLC technique is used to separate compounds in mixtures, with the method of quantification working in a standardized protocol manner, making it suitable for compound assays in samples.5 Atomic spectrometry and atomic mass spectrometry (MS) are the most powerful element detectors (e.g., ICP-MS). However, because materials are added for atomization, excitation, and even ionisation, no molecular information can be recovered Several groups involved in the field of atomic spectrometry and atomic mass spectrometry have recently focused their efforts on developing new bioanalysis approaches, either directly or indirectly. Researchers are still fascinated by aspects of atomic spectrometry and atomic mass spectrometry for determining total metal concentration in biological samples. Some of the other techniques are CE-UV (capillary electrophoresis with an ultraviolet detector), CE-LIF (capillary electrophoresis with a laser-induced fluorescence detector), GC-MS (gas chromatography–mass spectrometry), IR (infrared spectroscopy), and Raman spectroscopy 6. Analytical methodology advancements would improve in the knowledge of basic biological processes. The research was published in peer-reviewed journals in a variety of fields, medicine, pharmacy, and chemistry are only a few examples. This page contains publications that look at quantitative bioanalysis of pharmaceutical drugs utilising a variety of bioanalytical techniques, such as HPLC-UV, LC-MS, LC-MS/MS, HPTLC, AAS, and ICP-MS, to name a few. (see fig.1).
 |
Figure 1: Analytical techniques commonly used in bioanalytical studies |
Sample Treatment
By increasing the concentration of the analyte in the sample, procedure’s sensitivity is improved by sample treatment. The physicochemical properties of the drug, the type of matrix used, and the detecting technology used all influence the therapeutic method. For various matrices, such as blood, peripheral blood mononuclear cells (PBMC), cells, and tissues, a variety of sample preparation processes are used. A scientist must remove matrix and other interferences from a sample. The most usual sample preparation methods are protein precipitation (PPT), liquid–liquid extraction (LLE), and solid phase extraction (SPE) (SPE). In each of the three therapies, different recovery procedures are used. Analyte recovery in the matrix is determined by the distinction between the two areas of extracted samples and unextracted standard. SPE is more effective than the other two procedures in terms of recovery, but it is also more expensive. Because it can extract non-polar medicines from the matrix utilizing organic immiscible solvents such ethyl acetate, diethyl ether, tertiary butyl methyl ether, and hexane, LLE is effective for extracting non-polar pharmaceuticals from the matrix. When the analyte of interest is detected in urine, PPT is typically employed. The sample preparation techniques were chosen because they were simple to use, inexpensive, and long-lasting. Clean-up is crucial when only a little amount of a material needs to be detected since it enhances recovery and sensitivity. The efficacy of clean-up influences the effects of interferences and matrices. 7
Liquid–liquid extraction
For extracting analytes from biological materials, the LLE method is well-known. It distinguishes between polar and non-polar liquids in a matrix. LLE employs two immiscible stages. The sample instantly distributes between the polar and non-polar phases based on its affinity. The phases are separated after mixing by centrifugation, freezing, or a semi-permeable barrier. The non-polar phase is chosen based on the solubility and polarity of the sample. The extraction capability of a solvent can be modified by combining it with a different lipophilicity solvent. One of LLE’s main weaknesses is the lack of automation. A semi-automated process with automation in liquid handlers is being utilised to separate the two stages. It’s trustworthy and produces a pure sample. LLE frequently achieves more than 80% recovery when an effective extracting solvent is used. 8
Protein precipitation
PPT is a process that occurs when a protein entity reacts with a solvent. Exposing a protein to acidic or alkaline conditions can change its ionisation state. Concentrated salts can reduce a protein’s solubility by modifying its hydration state. The hydration state of proteins is altered by a variety of detergents, as well as non-polar solvents and salts. PPT is used to remove bigger protein groups from biological matrices before testing medicines. Using a water-miscible organic solvent with the PPT method is one way to decrease analyte loss. How much protein was extracted is determined by the amount of solvent utilised. Protein precipitates can occur when food is refrigerated. 9
Solid phase extraction
In comparison to LLE and PPT, SPE is a more advanced approach. Small laboratory-made columns were utilized in the past, but the technology was not widely accepted. As full cartridges became available, this method gained popularity. In these cartridges, silica is filled with stationary phase, just like in chromatographic columns. This approach determines the affinity of an analyte for the stationary phase in the same manner as chromatography determines the affinity of an analyte for the stationary phase. SPE provides a number of advantages over other extraction processes. Automation, Increased extraction efficiency, the use of less solvent quantities, the availability of a variety of stationary phases for different types of analytes, and simultaneous clean-up are just a few of the benefits. When it comes to selecting the correct SPE equipment, the volume of sample, contaminants, matrix complexity, and analyte concentration all play a factor. 10
Bioanalytical method development and validation
In regular sample analysis, there are two phases for creating, validating, and applying a specific bioanalytical method.
Developing a bioanalytical method and an assay protocol, as well as calculating analytical run and/or batch requirements and using a validated bioanalytical method for routine drug analysis are all tasks that need to be completed. 11
Terminology
Validation
The concise definition of bioanalytical method is likely to change multiple times over the standard drug development procedure. Multiple levels of validation are necessary to verify that an assay’s performance is still valid after evolutionary changes [e.g., adding a metabolite, reducing the quantification’s lower limit] (LLOQ). Full validation, partial validation, and cross-validation are the three levels/types of method validations. 12
Full validation
Validation in its entirety complete validation is essential when developing and deploying a bioanalytical method for the first time for a novel medicinal drug. If metabolites are added to an existing mixture for quantification, the test must be adequately validated for all analytes.
Partial validation
Partial validation, according to the researchers, is the demonstration of assay reliability after modifying an existing bio-analytical method that has already been thoroughly validated. The level of validation required will be determined by the nature of the modification.
Cross-validation
The brief definition of bioanalytical method is likely to change multiple times during the standard medication development process. Multiple levels of validation are required to demonstrate that an assay’s performance remains valid after evolutionary changes [e.g., adding a metabolite, lowering the quantification’s lower limit] (LLOQ). There are three levels/types of method validations: full validation, partial validation, and cross-validation. 12
Table 1: Validation of bioanalytical methods in the United States is governed by FDA rules.13
| Bioanalytical validation methods US FDA guidelines | |
| Selectivity (specificity) | At least six sources should be employed to obtain blank samples of the suitable biological fluid. At the LLOQ, each blank will be verified for contamination and selectivity. |
| Accuracy | At least six concentration measurements should be conducted. For accurate concentration determination, at least three levels in the predicted range are advised. With the exception of LLOQ, where it should not differ by more than 20%, An average must be 15% of the total amount. The accuracy is determined by taking the mean of the true values. |
| Precision | A minimum of five measurements per concentration has to be used to estimate precision. At least three concentrations should be tested within the predicted range. With the exception of the LLOQ, where it should not exceed 20% of the CV, At any concentration level, the precision determined must n’t reach 15% of the CV. |
| Recovery | Recovery experiments should be carried out at three concentrations low, medium, and high levels with 100% recovery. |
| Calibration curve | The LLOQ, a blank sample (without internal standard), a zero sample (with internal standard), and eight non-zero samples encompassing the expected range should be given. |
| LLOQ | The analyte response should be five times the blank response as compared to a blank response. |
| Freeze–thaw stability | The stability of the analyte should be tested after 3 freeze–thaw cycles. After being held at the appropriate storage temperature for 24 hours, at least three aliquots of each of the low and high concentrations should be frozen and thawed at room temperature. Refreeze for 12–24 hours under the same circumstances once completely thawed. This process should be performed twice more, after which the 3rd cycle should be analysed. The standard deviation of error, according to experts, should be around 15%. If the analyte is unstable, freeze it three times at -60°C. |
| Short-term stability | Defrost 3 aliquots of each low and high dose and keep them at room temperature for 4–24 hours before testing. The difference in percentage should be less than 15%. |
| Long-term stability | Under the same conditions as the study samples, at least 3 aliquots of low and high concentrations were prepared. Three different analyses should be performed. The time interval between the first and last sample collection should be longer than the time interval between them. |
| Stock-solution stability | Drug stock solutions and internal standards should be tested for at least 6 hours at room temperature. The difference in percentage should be less than 15%. |
| QC samples | Each assay run should comprise Quality control samples in triplicate at 3 concentration levels. A minimum of four out of every six numbers must be within 15% of the nominal value. At any given time, two of the six might be outside the 15% range, but not both. [14] |
Analysis of Pharmaceutical Drugs Using Different bioanalytical techniques
HPLC-UV
Table 2 summarizes the various chromatographic settings utilized in HPLC-UV bio-analytical procedures. S.H. Gan et al. devised an HPLC-UV technique for detecting tramadol in spiked plasma, using the LLE extraction procedure and a Lichrosorb RP-18 column for separation. The mobile phase in isocratic mode is a 30:70 (v/v) mixture of acetonitrile and phosphate buffer. With a detection range of 10 to 2000 ng/ml and a 98.63 percent recovery rate, the approach is linear. The intra-day accuracy was 87.55 percent to 105.99 percent, while the inter-day accuracy was 93.44 percent to 98.43 percent. 15 N. Lindegardha et al. created a technique for simultaneously identifying lumefantrine and desbutyl-lumefantrine in spik plasma using SPE. The SB-CN column was used to separate the samples. The mobile phase was a 55:45 (percent v/v) mixture of acetonitrile–phosphate buffer and sodium perchlorate 0.05 M. The quantification limits for lumefantrine and desbutyl-lumefantrine had 0.024 and 0.021 gram/ml, respectively.16 Akhlaghi et al. devised a method for quantifying iohexol in human plasma. The analyte was extracted from plasma samples using Protein Precipitation. A Bondapak C18 was kept at 30°C was used to for chromatographic separation. In Gradient mode, the mobile phase was a combination of Acetonitrile and water. A dual wavelength UV detector set to 254 nm was used to detect iohexol. 17 Kong et al. developed a method for detecting dibenzoylmethane in rat plasma using LLE pretreatment. A Phenomenex Gemini C18column was used to separate the materials. The mobile phase in gradiant mode was made up of water, methanol, and 0.1 percent Trifluoroacetic acid.18 Srinubabu and his colleagues employed LLE as an extraction method and a zorbax octadecylsilane column for separation to develop a method for quantifying Efavirenz in plasma. The mobile phase is isocratic elution must be acetonitrile–phosphate buffer (pH 3.5). The reaction was linear in concentration of 0.1 –10 ng/ml.19 Zzaman et al. devised a method for quantifying Fenofibrate in spiking human plasma. On a Lithosphere 60 RP C18 silica column, the isocratic mobile phase comprised of buffer (pH 6.0) and ACN 70:30 (% v/v). The method response was linear in spiking human plasma over a concentration range of 0.095 to 19.924 g/ml.20 Fortuna et al. found carbamazepine, oxcarbazepine, eslicarazepine, and their metabolites in human plasma. SPE was used for extraction, and a LiChroCART C18 column was used for separation. The elution mobile phase was 62:32:6 water–methanol–acetonitrile (%v/v/v). Carbamazepine, oxcarbazepine, and eslicarazepine are all types of carbamazepine. The linearity of CBZ-E, transdiol, and licarbazepine was studied Using six different concentrations of calibration standards within the prescribed plasma concentration. Pal et al. Using the LLE methodology as a pretreatment, they created a method for determining the quantities of zidovudine, lamivudine, and nevirapine in human plasma. 21 A Hypersil BDS C18 column was used to separate the samples. The mobile phase was a 40:60 (percent, v/v) mixture of 1 % buffer, acetic acid and methanol. For zidovudine, lamivudine, and nevirapine, the method was linear over concentration ranges of 50–3000, 50–2000, and 10–3000 ng/ ml, with lower limit of quantifications (LLOQ) of 50, 50, and 10 ng ml-1, respectively. The established and verified method was used to effectively complete pharmacokinetic research with 12 healthy human subjects. 22 In 2012, Sonawane et al. devised an LLE extraction method to measure eplerenone in human plasma. a 50:50 (% v/v) acetonitrile: water mobile phase was employed for chromatographic separation. In the range of 100–3200 ng/ml, the calibration curve was linear. 23 Zheng et al. devised a way for figuring out erlotinib and vemurafenib concentrations in cancer patients’ plasma. A C8 Xterra MS column was used to achieve chromatographic separation. In the mobile phase, glycine buffer and acetonitrile were combined 45:55 (percent, v/v). Erlotinib and vemurafenib were identified using a spectrophotometry with two wavelengths of 240 nm and 328 nm, respectively. 24 Aatif et al. used LLE as the extraction method to establish a method for quantifying Glipizide in spiking human plasma. With an isocratic mobile phase of buffer and CAN 65:35 (% v/v) adjusted to pH 4.25, a ZORBAX ODS C18 silica column was employed. The method response in spiking human plasma was linear over a range of 50- 1600 ng/ml. Precision and accuracy scores were less than 15% intra-day and inter-day.25 Ahmadkhaniha et al. used LLE as an extraction technique to create a method for quantifying captopril in human plasma. Captopril and 2-propene-1-thiol (internal standard) are derivatized in the pre-column using the new reagent 2-naphthyl propiolate. The chromatographic separation was carried out on a C-18 column with a mobile phase of 75:25 (percent, v/v) Methanol: phosphate buffer (pH 8.0). Throughout the calibration range, RSDs of 5.9–12.4 percent (accuracy from 97.5 to 93.6 percent) and 6.4–12.8 percent (accuracy from 97.3 to 95.2 percent) for intra- and inter-assay, respectively, were satisfactory. 26 Mannemala et al. used PPT to develop a method for assessing Aliskiren and Amlodipine in plasma at the same time. The Hibar C18 column is used to separate the samples. A 60:40 (percent v/v) acetonitrile–phosphate buffer combination served as the mobile phase (pH 6.0). 27 Charbe et al. devised a method to assess an antivirals in HIV-positive patients’ in 2016. SPE was used for extraction, and an XBridge C18 column was used for separation. In the mobile phase, ACN and acetate buffer at pH 5.4 were utilised at a flow rate of 1 ml/min. A gradient elution was used. The PDA detector was used to detect at 260 nm. 28 Loregiana developed a SPE-based approach for measuring Daclatasvir in spiked human plasma. On an XTerra RP18 silicon column, the isocratic mobile phase was a 44:56 (% v/v) mixture of ACN and acetate buffer (pH 5.0, 10 mM). Test results ranged from 2.6 to 5.5 percent intraday and 3.8 to 8.9 percent interday, respectively. Precision and accuracy scores were less than 15% intra-day and inter-day. 29 Hidau et al. devised a method for quantifying talazoparib in rat plasma using a Luna C18 column as an extraction method and PPT as a separation method. In the mobile phase, isocratic elution of methanol: water in a 60:40 ratio and acetonitrile: water in a 65:35 ratio occurred. A UV detector with a 227 nm wavelength was used. The entire chromatographic runtime was ten minutes. 30 Mei et al. developed a method for measuring lamotrigine, oxcarbazepine, and its metabolite 10,11-dihydro-10-hydroxycarbazepine in plasma using PPT in 2019. The Acclaim C18 Column is used to separate the samples. Potassium dihydrogen phosphate buffer (50 mM) and methanol 61:39 (percent v/v) make up the mobile phase. The calibration range for lamotrigine, oxcarbazepine, and its metabolite was 2.4 to 120 mg/L. 31 Ameeduzzafar et al. developed an LC research for dapagliflozin in rat plasma using PPT as the extraction procedure. An isocratic mobile phase of 70:30 (percent, v/v) acetonitrile and water was used with an RP C18 silica column. The technique response is in the concentration range of 10–1200 ng/ml. It was discovered that the extraction recovery rate was between 95.09 and 98.98 percent. 32
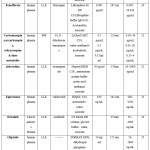 |
Table 2: Pharmaceuticals in plasma can be analysed using HPLC-UV bioanalytical techniques. |
For detecting the enantiomers of warfarin (WAR) in human EDTA plasma, Naidong et al. devised an LC-MS/MS technique (R-WAR and S-WAR). In the extraction process, LLE was used, and in the column, -cyclodextrin was used. A 1000:3:2.5 combination of acetonitrile, acetic acid, and triethylamine served as the mobile phase (v:v:v). In the mass spectrometric analysis, ionisation was done in negative mode with ESI and detection was done with MRM. 33 In human plasma, Souppart et al. developed an LC-MS method to detect artemether and its active dihydroartemisinin metabolite. A LLE is used in this method. The mobile phase was acetonitrile glacial acetic acid 0.1 percent 66:34 (percent, v/v) for chromatography on a C reversed-phase column. The technique response was linear over the concentration range of 5–200 ng/ml. APCI was utilised for ionisation and SIM was employed for detection in the mass spectrometric analysis. 34 Woodet al. developed an LC-MS/MS method for amphetamine, methamphetamine, and ephedrine in human plasma and oral fluids. PPT was used for extraction, and a Hypersil BDS C18 column was used for separation. A premixed mobile phase with 10mM ammonium acetate and 75:25 (% v/v) acetonitrile was used for elution. 35 Alali et al. developed a liquid chromatography-mass spectrometry method for determining the total quantity of ketotifen in human plasma (unchanged and conjugated). LLE was used for extraction, and reverse phase gradients with switching columns were used for chromatography. In gradient mode, the mobile phase is a 0.01M ammonium acetate and methanol combination. The technique response was linear for the concentration range of 0.5–20 ng/ml. Liang et al. devised a method for detecting Telmisartan in human plasma using LC-MS. The extraction technique used in this operation is a PPT. The chromatography was done on a Hypersil-K’eystone C18 reversed-phase column with a mobile phase of acetonitrile–10mM ammonium acetate 42:58 (percent, v/v). The technique’s response was linear at a concentration range of 1–2000 ng/ml. In the mass spectrometric analysis, ESI was used for ionisation and SIM was used for detection. 37
Lianget al. developed an LC-MS method for quantifying indapamide in human plasma that utilised the LLE method of pretreatment. A Shim-pack C18 column was used to separate the samples. In gradient mode, a 0.1M acetate and methanol combination was utilized as the mobile phase. The mass analysis was performed using a quadrupole mass analyzer in SIM mode and the ESI interface in negative ionization mode.38 Yang devised an LC-MS/MS method for determining the concentration of Daunorubicin in rat plasma. The separation column was a Thermo Finnigan Beta Basic phenyl C18 column, and the extraction process was PPT. The mobile phases in gradient elution mode were acetonitrile and water, each of which contained 0.1 percent formic acid. The technique response was linear in 0.25 – 100 ng/ml concentration. For mass analysis, ESI in positive mode was used. 39 For Deoxyschizandrin measurement in rat plasma, Kaishun Bi et al. established an LC-MS technique employing LLE and separation with an Elite Hypersil C18 column with a mobile phase of methanol and water 84:16 (percent v/v) in isocratic mode. The mass spectrometer employed APCI in positive ion mode by selecting ion monitoring (SIM) mode.40
Mullangi et al. used LC-Tandem to detect Pramipexole in human plasma. In this approach, a SPE is used as an extraction technique. The mobile phase was 0.01M ammonium acetate (pH 4.4) – acetonitrile 30:70 (percent, v/v) and chromatography was performed on a Discovery CN column. The technique response was linear for the concentration range of 20–3540 pg/ml.41 In 2010, Silvestro et al. developed an LC–MS/MS technique to detect clopidogrel in human plasma using PPT. The chromatographic separations were performed using Ascentis RP-Amide, Ascentis Express C8, and Ascentis Express RP amide reversed phase columns. For this 3 method, the gradient elution mobile phase is made up of methanol and water, both of which contain 0.1 percent formic acid. The technique is linear at values of 5–1800 pg/ml.42
Macwan et al. established an LC-Mass Mass method for measurement of atorvastatin and its acid and lactone metabolites in human plasma, in which the sample was pre-treated with 0.1 percent acetic acid in acetonitrile to precipitate proteins, then separated using a Zorbax-SB Phenyl column. Glacial acetic acid, acetonitrile, and methanol make composed the mobile phase. A quadrupole mass analyzer in MRM mode and an ESI interface in positive ion mode were used for the mass analysis.43 Sparidans et al. established a method for measuring crizotinib in mouse plasma using the PPT method of extraction. The samples were separated using a ODS-18 column. The mobile phase was made up of 0.1 percent ammonium hydroxide and methanol. In a calibration range of 10–10,000 ng/ml, the test was validated.44
Yang et al. created an LC–MS approach for detecting Entecavir in human plasma using SHLLE in sample pre-processing in 2012. A Hanbon Lichrospher RP C18 reversed phase column was used for the chromatographic separations. In gradient elution mode, the mobile phase for this method contains 0.1 percent acetic acid, acetonitrile, and methanol. The approach is linear for concentration ranges of 0.5–30 ng/ml. A triple quadrupole mass analyzer in SIM mode and the ESI interface in positive ionisation mode were used for the mass analysis. 45 Bourget et al. devised a method for quantifying midostaurin using PPT as the extraction technique. In gradient mode, a ODS silica column was utilised with a premixed mobile phase consisting of 10 mM ammonium formate in water and acetonitrile with 0.1 percent formic acid on a Sunfire C18 silica column kept at 30°C. For concentrations between 75 and 2500 ng/ml, the method is linear. 46 Wani et al. established a UPLC-Tandem method for neratinib quantification in human plasma utilising a PPT extraction procedure and a UPLC BEH C18 column for separation. The mobile phase was made up of a 70:30:0.1 (v/v) of methanol, water. 47 Rood et al. developed a method for quantifying ibrutinib in human and mouse plasma that included PPT pretreatment. The samples were separated using an XBridge BEH300 column. The mobile phase consisted of 1% formic acid in water and methanol.48
In Human Plasma, Van Nuland et al. developed an LC-MS/MS method for the simultaneous detection of abiraterone, enzalutamide, Ndesmethyl enzalutamide, enzalutamide carboxylic acid, abiraterone N-oxide sulphate, and abiraterone sulphate. For mass analysis, an ESI in positive mode ionisation source was used, and detection was done using a triple quadrupole detector in MRM mode. 49
In 2018, M. van Nuland and colleagues developed an ultra-sensitive LC–MS/MS method for quantifying gemcitabine and its metabolite. The extraction was done with SPE, and the separation was done with an Acquity UPLC HSS T3 column. In gradient elution, an ammonium acetate, water, and acetonitrile mobile phase was utilised.50 The mass analysis used a turbo ion spray (TIS) in positive mode as an ionisation source, with detection using a triple quadrupole detector in MRM mode. S. Guan et al developed LC-MS/MS to determine gefitinib and its major metabolites in human plasma. The chromatographic separation was achieved using an X-TerraMS column. The mobile phase was made up of 35:65 (percent v/v) water and acetonitrile with 0.1 percent formic acid51
Table 3 summarises the different chromatographic settings used in bioanalytical methods using LC-MS or LC-MS/MS.
Table 3: Bioanalytical methods for the analysis of drugs in plasma by LC-MS or LC-MS/MS
| Compound | Analytical Technique | Sample
Pretreatment |
IS | Column, mobile phase, elution mode | LLOQ
|
Detection Techniques | Quantification
Range |
Ref
|
| Warfarin | LC-MS/MS | LLE | p-chlorowarfarin | β-cyclodextrin column, acetonitrile–acetic acid–triethylamine, Isocratic | 1 ng/ml | ESI
MRM mode |
1–100 ng /ml | 33 |
| Artemether | LC-MS | LLE | Artemisinin | Alltima C18 column,
acetonitrile– glacial acetic acid, Isocratic |
5 ng/ml | APCI technique,SIM mode | 5–200 ng/ml | 34 |
| Amphetamines | LC-MS/MS | PPT | Deuterated internal standards | Hypersil BDS, ammonium acetate – acetonitrile, Isocratic | 0.5 ng/ml | ESI
MRM mode |
0.5-500 ng/ml | 35 |
| Ketotifen | LC-MS | LLE | Pizotifen | Hypersil BDS and a Symmetry C18, ammonium acetate – methanol, gradient | 0.5 ng/ml | single quadrupole with ESI, SIM mode
|
0.5-20 ng/ml | 36 |
| Telmisartan | LC-MS | PPT | Valsartan | Hypersil-Keystone C18, ammonium acetate – acetonitrile, Isocratic | 1 ng/ml | single quadrupole with ESI, SIM mode
|
1–2000 ng/ml | 37 |
| Indapamide | LC-MS | LLE | Chlorpropamide | Shim-pack C18 , ammonium acetate – methanol, gradient | 0.5 ng/ml | single quadrupole with ESI, SIM mode
|
0.5–100.0 ng/mL | 38 |
| Daunorubicin | LC-MS/MS | PPT | Doxorubicin | ThermoFinnigan BetaBasic Phenyl C18 column, acetonitrile- water, gradient
|
0.25 ng/ml | triple quadrupole mass spectrometer | 0.250–100 ng/ml | 39 |
| Deoxyschizandrin | LC–MS | LLE | DDB | Elite Hypersil C18, methanol-water, isocratic. | 1 ng/ml | APCI technique,SIM mode | 1 –50 ng/ml | 40 |
| Pramipexole | LC-MS/MS | SPE | Memantine | Discovery CN ammonium acetate – acetonitrile, isocratic
|
20 pg/ml | Triple quadrupole ESI
MRM mode |
20–3540
pg/ml |
41 |
| Clopidogrel | LC-MS/MS | PPT | d3-clopidogrel | Ascentis RP-Amide, Ascentis Express C8, Ascentis Express RP Amide, methanol-water, gradient | 5 pg/ml | Triple quadrupole ESI MRM mode | 5–1800
pg/ml |
42 |
| Atorvastatin | LC-MS/MS | PPT | d5- Atorvastatin | Zorbax-SB Phenyl column, glacial acetic acid – acetonitrile – methanol, gradient | 0.05 ng/ml | Triple quadrupole ESI MRM mode | 0.05-100 ng/ml | 43 |
| Crizotinib | LC-MS/MS | PPT | crizotinib-13C2-2H5 | BEH C18 column, ammonium hydroxide- methanol, gradient
|
10 ng/ml | Triple quadrupole — in SRM mode | 10–10000 ng/ml | 44 |
| Entecavir | LC–MS | SHLLE | acyclovir | Hanbon Lichrospher RP C18, acetic
acid–ammonium acetate – acetonitrile, gradient |
0.05 ng/ml | Triple quadrupole ESI SIM mode | 0.05–20 ng/ml | 45 |
| Midostaurin | LC-MS/MS | PPT | midostaurin-d5 | SunFire C18 silica column, ammonium formate- acetonitrile -formic acid, gradient | 75 ng/ml | Triple quadrupole ESI SIM mode | 75- 2500 ng/ml | 46 |
| Neratinib | UPLC-MS/MS | PPT | Crizotinib | UPLC BEH C18 column, methanol- water- formic acid, isocratic | 4 ng/ml | Triple quadrupole in MRM mode | 4 – 500 ng/ml
|
47 |
| Ibrutinib | LC-MS/MS | PPT | dihydrodiol‐ibrutinib | XBridge BEH300 column, methanol- water- formic acid, gradient | 5 ng/ml | Quadrupole ESI in SIM mode | 5 -4000 ng/mL | 48 |
| Abiraterone | LC-MS/MS | PPT | H4-abiraterone | Kinetex C18 column,
formic acid–water- methanol, isocratic |
1 ng/ml | Quadrupole ESI in MRM mode | 1–100 ng/ml | 49 |
| Gemcitabine | LC-MS/MS | SPE | 13C,15N2-gemcitabine | Acquity UPLC HSS T3 column ammonium acetate – water-acetonitrile, gradient
|
2.5 pg/ml | Quadrupole TSI in MRM mode | 2.5–500 pg/ml | 50 |
| Gefitinib | LC-MS/MS | PPT | Vatalanib | X-Terra RP18 column, water-acetonitrile – formic acid, isocratic | 0.5 ng/ml | Triple quadrupole ESI SRM mode | 0.5–1000 ng/ml | 51 |
HPTLC
With the advancement of technology, high performance thin layer chromatography (HPTLC) has become a significant tool in drug analysis. HPTLC is a fast separation technology that can be used to examine a variety of materials. This process is beneficial in a number of aspects, including its convenience of use and the fact that analysing the complex or crude sample cleanup requires only a short amount of time. HPTLC uses a variety of parameters to analyse the entire chromatogram in real time. Additionally, on each plate, many samples and standards are generated simultaneously yet separately, resulting in higher results reliability [52]. The details of bioanalytical techniques for HPTLC analysis of pharmaceuticals in plasma are listed in Table 4. A. Mirfazalian et al. developed an HPTLC method for measuring theophyline in human plasma, employing the LLE method to extract theophylline and silica gel 60F254 HPTLC plates to separate it. The mobile phase in isocratic mode was a mixture of acetic acid, isopropanol, and tolune 01:12:06. With a range of 500–10000 ng/ml, the technique is linear. A camag TLC scanner II coupled with a deuterium lamp is adjusted to 277 nm for detection in reflection mode. [53] Milijkovicet al. developed a method for measuring Nimesulide in plasma using LLE. The samples were separated using 60F254 silica gel plates. For ascending chromatography, Tolune and acetone 90:10 (percent v/v) were used. Using a camag TLC scanner II, the resulting TLC plates were densitometrically assessed at 310nm. The quantitative limit of nimesulide is 0.5 g/ml. [54]. A method for quantifying Sparfloxacin in human plasma was developed by Shah et al. PPT was used to extract the analyte from human plasma samples. The samples were separated using 60F254 HPTLC plates. The mobile phase contained 4:4:1.5:0.2 (v/v) chloroform, toluene, methanol, and diethylamine, with densitometric analysis performed at 301 nm. The calibration curve was linear across a concentration range of 80–200ng/spot. [55] A. Jamshidi et al. developed a method for quantifying atorvastatin in human plasma using PPT for extraction and silica gel 60F254 HPTLC plates for separation. The mobile phase is made up of 70:30 (percent v/v) toluene and methanol in a two-step isocratic development. In the range of 101–353.5 ng/ zone, the analyte’s calibration function was linear. [56]. Using LLE as the extraction method, Jainet al. devised a method for measuring Minocycline in human plasma. A silica gel 60F254 HPTLC plate was used with an isocratic mobile phase is used. The technique response was linear over a concentration range of 100–1200 ng per zone in spiking human plasma. [57] Rote et al. established reverse phase-HPLC and HPTLC methods to quantify gemifloxacin mesylate in human plasma. For extraction, PPT was used, and for separation, silica gel 60F254 HPTLC plates were used. For elution, a premixed mobile phase was utilised. In the stated plasma concentration ranges of 0.5–6 g/ml, the method’s linearity was verified using seven different concentrations of calibration standards. The obtained TLC plates were densitometrically analysed at a wavelength of 254 nm. [58] In 2010, Ramadanet al. proposed a method for measuring Amlodipine besilate and valsartan in spiked human plasma, employing the PPT method for pretreatment. The samples were separated using Silica gel 60F254 HPTLC plates. Ethyl acetate, methanol, and ammonium hydroxide were mixed in a 55:45:5 (percent, v/v/v) mobile phase. For amlodipine besilate and valsartan, the method was linear over concentration ranges of 0.5–4 g/spot and 2–12 g/spot, with lower limit of quantifications (LLOQ) of 0.5 and 2 g/spot, respectively. With a scanning wavelength of 237 nm, the absorbance scan mode was used. [59]. In the same year, S. R. Tambe et al. developed an extraction method for Olmesartan quantification in human plasma utilising LLE and SPE. The samples were separated using Silica gel 60F254 HPTLC plates. At 269 nm, ethyl acetate, methanol, and acetic acid were combined in a ratio of 8.0:2.0:0.05 (v/v/v). The calibration curve was plotted across a concentration range of 80 ng to 600 ng. The average recovery rates for LLE and SPE were 90.12 percent and 79.64 percent, respectively. 60 Faiyazuddinet al. devised a method for quantifying Terbutaline sulphate in spiked human plasma using PPT as an extraction method. the calibration curve was linear. At a limit of quantification (LOQ) of 18.35 ng/spot, selectivity was explored.61 In the same year, Roteet al. developed a method to measure cefpodoxime proxetil and ambroxol hydrochloride in human plasma. LLE was used to extract the analyte from plasma samples. To achieve chromatographic separation, a silica gel 60F254 HPTLC plate was employed. Chloroform and methanol are mixed 9:1 (v/v) in the mobile phase. Densitometric analysis was utilised to detect the presence of bacteria at a wavelength of 240 nm. 62. Rote et al. used LLE to develop a method for simultaneously assessing Telmisartan and hydrochlorothiazide in plasma. The samples were separated using Silica gel 60F254 HPTLC plates. The mobile phase is made up of chloroform, methanol, and toluene 8:2:4 (v/v/v). Densitometric analysis (percent v/v) was performed at a wavelength of 278 nm. Concentrations of 200, 400, 600, 800, 1000, and 1200 ng/spots were utilised in the calibration curves for hydrochlorothiazide and telmisartan, respectively. 63 Khanvilkar et al. used PPT as the extraction method to develop an HPTLC method for measuring Eprosartan Mesylate in spiked human plasma. The separation was carried out using an isocratic mobile phase of ethyl acetate, acetonitrile, and glacial acetic acid6:4:0.2 (v/v/v) on a silica gel 60F254 HPTLC plate. The linear regression analysis data from the calibration plots demonstrated an acceptable linear relationship in the concentration range of 0.7-32 g/ml. 64 Roteet al. devised a method for measuring Metoprolol tartrate and hydrochlorothiazide in spiking human plasma using LLE as an extraction technique. The chromatographic separation was performed on a silica gel 60F254 HPTLC plate with a mobile phase of chloroform, methanol, and ammonia in the ratio of 9:1:0.5 (v/v/v). Densitometric analysis was carried out at a wavelength of 239 nm.65 Bokka Ramesh et al. used PPT as the extraction technique to develop a high-performance thin-layer chromatography coupled with electrospray ionisation mass spectrometry (HPTLC-ESI-MS) approach for Darunavir in spiked rat plasma. The mobile phase was a saturated 6:2:2 (v/v/v) mixture of toluene, acetone, and methanol. At 262 nm, densitometric quantification was performed using reflectance scanning. The approach was linear throughout a concentration range of 5–150 ng/ml. 66 El-Koussiet al. used fluorescence detection to create a method for quantifying Gemifloxacin mesylate in plasma. On a silica gel 60F254 HPTLC plate, this HPTLC approach was used to avoid preparation of the plasma sample and separation. The mobile phase was made up of 8:4.5:3 (v/v/v) ethyl acetate, methanol, and ammonia. Fluorescence detection was used to perform HPTLC plate scanning. After excitation at 342 nm, the emission intensity was measured using optical filter K400. In the range of 3–180 ng/band, the calibration curve was linear. 67. S. Shrivastav et al. established an HPTLC method for determining aliskiren and hydrochlorothiazide in a fixed dose tablet formulation and human plasma in 2014. 68 Abdelaleem et al. developed an HPLC method for detecting paracetamol, 4-aminophenol, pseudoephedrine hydrochloride, and loratidine in human plasma. HPTLC aluminium plates precoated with silica gel 60GF254 and a developing system of acetone, hexane, and ammonia 4:5:0.1 (v/v/v) were used to create the chromatographic method. Densitometric scanning was done with a CAMAG TLC scanner in the reflectance absorbance mode at 254 nm for paracetamol, 4-aminophenol, and loratidine, and at 208 nm for pseudoephedrine hydrochloride. The paracetamol calibration curve showed a strong relationship over concentration ranges of 0.1–6 g/band, 0.2–3.5 g/band for 4- aminophenol, and 0.1–6 g/band for 4- aminophenol. Loratidine has a concentration of 0.1–2 g/band, while pseudoephedrine hydrochloride has a concentration of 1.6–12 g/band. 69 SM Gosavi and colleagues developed a method for measuring Esomeprazole Magnesium in human plasma. LLE was used to extract the analyte from plasma samples. To achieve chromatographic separation, 60GF254 silica gel plates were employed. 9:1:0.5 (v/v) ethyl acetate, methanol, and ammonia make up the mobile phase. A densiometric analysis was carried out at a wavelength of 301 nm. The calibration curve was created using an esomeprazole magnesium concentration range of 200-700 ng/spot.70 Al Alamein et al. used LLE to develop a method for determining Sacubitril and Valsartan in spiked human plasma at the same time. Silica gel 60F254 HPTLC plates were used to separate the samples. 71. In the year 2019, Gadallah et al. used PPT to develop a method for determining ertapenem and paracetamol in rabbit plasma simultaneously. Silica gel 60F254 HPTLC plates were used to separate the samples. The developed approach used a simple planar chromatographic separation using a 35:15(v/v) mixture of acetonitrile and water, as well as dual-wavelength detection at the 294 and 247 nm wavelengths of ertapenem and paracetamol, respectively. Ertapenem had a calibration range of 40-600 ng/band, while paracetamol had a range of 15-200 ng/band. 72 Iqbalet al. developed an HPTLC method for quantification of Delafloxacin in human plasma using PPT as the extraction technique. On 60F254 silica gel plates, chromatography was performed with a mobile phase of 5:4:2 (v/v/v) methanol,ethyl acetate and ammonia solution. Over the concentration range of 16–400 ng/band, the method response was linear. 73
Table 4: Bioanalytical methods for the analysis of drugs in plasma by HPTLC
| Compound | Sample
Pretreatment |
IS | Column, mobile phase, elution mode | LLOQ | Detection wavelength | Quantification
Range |
Ref |
| Theophyline | LLE | Acetaminophen | silica gel 60F254 ,acetic acid-isopropanol-tolune.
|
200 ng/ml | 277 nm | 500–10000 ng/ml | 53 |
| Nimesulide | LLE | Not used | silica gel 60F254,
tolune- acetone. |
0.5 µg/ml | 310 nm | 0.5 – 10µg/ml | 54 |
| Sparfloxacin | PPT | Not used | silica gel 60F254,
chloroform-toluene–methanol-diethylamine |
80 ng/spot | 301 nm | 80–200 ng/spot | 55 |
| Atorvastatin | PPT | diclofenac
sodium |
silica gel 60F254 ,toluene–methanol. | 101 ng/ zone | 280 nm | 101–353.5ng/ zone | 56 |
| Minocycline | LLE | Not used | silica gel 60F254 methanol–acetonitrile–isopropanol–water | 15.4 ng/zone | 345 nm | 100–1200 ng /zone | 57 |
| Gemifloxacin
Mesylate |
PPT | linezolide | silica gel 60F254 ,
ethyl acetate – methanol-ammonia |
0.5 µl/ml | 254 nm | 0.5–6µl/ml | 58 |
| Amlodipine
besilate and valsartan |
PPT | Not used | silica gel 60F254 ,
Ethyl acetate-methanol -ammonium hydroxide |
0.5 and
2µl/ml |
237 nm | 0.5–4 µg/spot and 2–12 µg/ spot | 59 |
| Olmesartan | LLE & SPE | Zidovudine | silica gel 60F254 , ethyl acetate-methanol-acetic acid | 80 ng/ml | 269 nm | 80–600 ng/ml | 60 |
| Terbutaline sulfate | PPT | Not used | silica gel 60F254 ,
chloroform–methanol |
18.35 ng /spot | 366 nm | 100–1000 ng/spot | 61 |
| cefpodoxime proxetil and
ambroxol hydrochloride |
LLE | Paracetamol | silica gel 60F254 ,
Chloroform-methanol |
500 and 1000 ng/spot | 240 nm | 500-3500 and 1000-7000 ng/spot | 62 |
| Telmisartan and Hydrochlorothiazide | LLE | Paracetamol | silica gel 60F254,
Chloroform-methanol- toluene |
2 μl/ml. | 278 nm | 200-1200 ng/spot | 63 |
| Eprosartan Mesylate | PPT | losartan | silica gel 60F254,
ethyl acetate-acetonitrile- glacial acetic acid |
0.7 μg/ml | 238 nm | 0.7-32 μg/ml | 64 |
| Metoprolol tartarate and hydrochlorothiazide | PPT | Paracetamol | silica gel 60F254,
Chloroform-methanol- ammonia . |
2000 and 200 ng/ml | 239 nm | 2000-12000 and 200-1200 ng/ml | 65 |
| Darunavir | PPT | Not used | TLC plates, Toluene-acetone- methanol | 3.85 ng/ml | 262 nm | 5–150 ng/ml | 66 |
| gemifloxacin mesylate | Not Performed | Montelukast | silica gel 60F254,
ethyl acetate-methanol- ammonia |
1.5 ng/band | After excitation at 342 nm. | 3–180 ng/band | 67 |
| Aliskiren and Hydrochlorothiazide | SPE | Nebivolol | silica gel 60GF254,
methanol-chloroform |
1.0 and 0.1 μg/band | 225 nm | 1.00-10.0 and 0.10-1.00 μg /band | 68 |
| Paracetamol, Pseudoephedrine and Loratidine | PPT | Not used | silica gel 60F254,
acetone–hexane–ammonia |
0.5, 1.6 and 0.4 μg/band | 208 nm | 0.5–6 , 1.6–12 and 0.4–2 μg/band | 69 |
| Esomeprazole Magnesium | LLE | Not used | silica gel 60F254,
ethyl acetate: methanol: ammonia |
200 ng/ml | 301 nm | 200-700 ng/spot | 70 |
|
Sacubitril and Valsartan |
LLE | Not used | silica gel 60F254,
ethyl acetate – methanol- glacial acid |
6.13 and 7.42 ng/spot | 260 nm | 9-75 ng/spot | 71 |
| Ertapenem and paracetamol | PPT | Not used | silica gel 60F254,
acetonitrile – water |
39.64 and 10.81 ng/ band | 294 and 247 nm | 40-600
and 15-200 ng/ band |
72 |
| Delafloxacin | PPT | gatifloxacin | silica gel 60F254,
Ethylacetate-methanol-ammonia solution |
16 ng/band | 344 nm | 16–400 ng/band |
73 |
AAS
Table 5: Bioanalytical methods for the analysis of drugs in plasma by AAS
| Compound | Analytical Technique | LLOQ
|
hollow cathode
lamp wavelength |
Calibration
Range |
Ref
|
| NAMI-A | AAS | 0.85 μM | 349.9 nm | 1.1 – 220 μM | 74 |
| JM216
|
AAS | 10 ng Pt/ml | 265.9 nm | 10-150 ng pt/ml | 75 |
| AP5280 | AAS | 0.250 μmol/l | 265.9 nm | 0.250–5.00 μmol/l | 76 |
| Oxaliplatin | AAS | 0.5 μmol/l | 265.9 nm | 0.5–400 μmol/l | 77 |
| Cisplatin, carboplatin, and oxaliplatin | ICP-MS | 75.0 ng/l | ———— | 75.0- 10000 ng/l | 78 |
| Strontium | AAS | 0.2 μg/ml | 460.7 nm | 0.2 to 10 μg/ml | 79 |
| Cisplatin | AAS | 10 ng Pt/ml | 265.9 nm | 10–160 ng Pt/ml | 80 |
| Cisplatin | ICP-AES | 0.375 μg/ml | 195 nm | 0.375 – 15 μg/ml | 81 |
M. Crulet et al. developed a method for detecting NAMI-A (ImH[trans-RuCl4(DMSO)Im] in human plasma using GF AAS. The standard line was created using plasma and a 1: 10 (v/v) buffer. The validated range of determination for plasma was 1.1–220 M. NAMI-A. 60F254 silica gel plates were used to separate the samples. Tolune and acetone 90:10 (percent v/v) were employed for ascending chromatography. Using a camag TLC scanner II, the resulting TLC plates were densitometrically assessed at 310nm. The quantitative limit of nimesulide is 0.5 g/ml. 74 L.A. Decosterd et al. developed an AAS method for quantifying JM216 bis-acetato-ammine dichlorocyclohexylamine platinum (IV) in human plasma, which requires the application of the matrix modifier 5 percent Triton directly onto the graphite furnace before adding plasma samples. With a range of 10-150 ng Pt/ml, the technique is linear. 75 Tibben et al. devised an AAS Determination of total platinum in human plasma using a dosage of By spiking plasma with AP5280 at concentrations of 0.25, 1.0, 50, and 250 mol/l, four tiers of quality control samples were created. Over a concentration range of 0.250–5.00 mol/l, the calibration curve was linear. At a wavelength of 265.9 nm, platinum concentrations were measured.76 Brouwers et al. used AAS to develop a method for measuring platinum in human plasma derived from oxaliplatin. Five replicates of each sample in plasma were analysed using QC samples at concentrations of 0.05, 0.15, 0.497, 1.799, and 9.942 mol/l. Seven non-zero calibration standards were linear in the range of 0.5–400 mol/l. Absorbances were measured at a wavelength of 265.9 nm. 77 Brouwers et al developed an ICP-MS technique for measuring platinum in human plasma ultrafiltrate produced from cisplatin, carboplatin, and oxaliplatin. As a sample pretreatment, materials are diluted with 1 percent HNO3. Drug-free plasma ultrafiltrate was spiked at four concentration levels to obtain quality control samples: 75, 225, 1000, and 7000 ng/l. Seven nonzero calibration standards in a dynamic range of 75.0 to 10000 ng/l of platinum in plasma ultrafiltrate were used to create the calibration curve. 78 For evaluating total strontium concentrations in rat plasma, Qi Zhanget al. designed an assay method. Intra-day variance ranged from 95.46 to 102.24 percent, while inter-day variance ranged from 101.74 to 102.15 percent (inter-day variation). The intraday precision ranged from 1.86 to 8.24 percent, while the interday precision ranged from 1.27 to 11.20 percent. The technique response was linear for the concentration range of 0.2 to 10 g/ml. Beinneret al. tested the stability and pH sensitivity of pH-sensitive stealth liposomes delivering cisplatin in mouse plasma. To determine the accuracy, mouse plasma was spiked at each concentration of 10, 20, 40, 80, and 160 ng Pt/ml in five duplicates. For the five concentrations, the intra- and inter-assay accuracy was less than 15%. For evaluating total strontium concentrations in rat plasma, Qi Zhanget al. designed an assay method. Intra-day variance ranged from 95.46 to 102.24 percent, while inter-day variance ranged from 101.74 to 102.15 percent (inter-day variation). The intraday precision ranged from 1.86 to 8.24 percent, while the interday precision ranged from 1.27 to 11.20 percent. For concentrations ranging from 0.2 to 10 g/ml, the technique response was linear. 79
Conclusion and Future Prospective
The primary goal of pharmacological drugs is to assist humans in becoming disease-free or preventing disease. To accomplish its intended purpose, the medicine must be devoid of impurities and any interference that could harm humans. This study focuses on the role of various analytical instruments in plasma and gives a detailed literature overview of bioanalytical research approaches. The review also demonstrates how the approaches have evolved, beginning with the older method and progressing to sophisticated hyphenated technique phases. The effective hyphenation of chromatographic and spectroscopic has had a significant influence in the field pharmaceutical drug quantification from biological materials. The majority of the articles in this page were written within the last 20 years.
Acknowledgement
I thank my mentor Dr. Dinesh D. Rishipathak for their assistance and I would like to thank Respected Principal Dr. S. J. Kshirsagar, MET’s Institute of Pharmacy, Adgaon, Nashik for their Support. Special thanks to dear Colleague Prof. Shubham Jagdish Khairnar and Prof. Abhijit Sainath Deore for their technical assistance.
Conflict of Interest
The authors’ Rakesh U. Shelke and Dr. Dinesh D. Rishipathak declare no conflict of interest, financially or otherwise.
Funding Source
There is no funding or financial support for this research work.
References
- Jain, S., Jadav, T., Sahu, A.K., Kalia, K. and Sengupta, P., 2019. An exploration of advancement in analytical methodology for quantification of anticancer drugs in biomatrices. Analytical Sciences, p.19R002.
CrossRef - Otsuki, Y., Kotani, A. and Kusu, F., 2012. Capillary Liquid Chromatography with UV Detection Using N, N-Diethyl Dithiocarbamate for Determining Platinum-Based Antitumor Drugs in Plasma. Chemical and Pharmaceutical Bulletin, 60(5), pp.665-669.
CrossRef - Stokvis, E., Rosing, H. and Beijnen, J.H., 2005. Liquid chromatography‐mass spectrometry for the quantitative bioanalysis of anticancer drugs. Mass spectrometry reviews, 24(6), pp.887-917.
CrossRef - Moein, M.M., El Beqqali, A. and Abdel-Rehim, M., 2017. Bioanalytical method development and validation: Critical concepts and strategies. Journal of Chromatography B, 1043, pp.3-11.
CrossRef - Shewiyo, D.H., Kaale, E.A.K.K., Risha, P.G., Dejaegher, B., Smeyers-Verbeke, J. and Vander Heyden, Y., 2012. HPTLC methods to assay active ingredients in pharmaceutical formulations: A review of the method development and validation steps. Journal of pharmaceutical and biomedical analysis, 66, pp.11-23.
CrossRef - Hu, J., Yang, P. and Hou, X., 2019. Atomic spectrometry and atomic mass spectrometry in bioanalytical chemistry. Applied Spectroscopy Reviews, 54(3), pp.180-203.
CrossRef - Nishino, T., 2018. Surface-enhanced Raman Spectroscopy. Analytical Sciences, 34(9), pp.1061-1062.
CrossRef - Chang, M.S., Ji, Q., Zhang, J. and El‐Shourbagy, T.A., 2007. Historical review of sample preparation for chromatographic bioanalysis: pros and cons. Drug Development Research, 68(3), pp.107-133.
CrossRef - Cantwell, F.F. and Losier, M., 2002. Liquid—liquid extraction. In Comprehensive Analytical Chemistry(Vol. 37, pp. 297-340). Elsevier.
CrossRef - Burgess, R.R. and Deutscher, M.P. eds., 2009. Guide to protein purification. Academic Press.
- Haixia, Z., Hua, D., Mancang, L. and Pengling, Z., 2001. Octyl, Phenyl, Amino and Cyano Bonded Phases Containing Polyglycol Chains Used in Solid Phase Extraction [J]. Chinese Journal of Analytieal Chemistry, 9.
- Tiwari, G. and Tiwari, R., 2010. Bioanalytical method validation: An updated review. Pharmaceutical methods, 1(1), pp.25-38.
CrossRef - McDowall, R.D., 1999. The role of laboratory information management systems (LIMS) in analytical method validation. Analytica chimica acta, 391(2), pp.149-158.
CrossRef - Bioanalytical Method Validation Guidance for Industry, US Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Veterinary Medicine (CVM) May 2018 Silver Spring, Maryland. USA.
- Gan, S.H., Ismail, R., Adnan, W.W. and Wan, Z., 2002. Method development and validation of a high-performance liquid chromatographic method for tramadol in human plasma using liquid–liquid extraction. Journal of Chromatography B, 772(1), pp.123-129.
CrossRef - Lindegårdh, N., Annerberg, A., Blessborn, D., Bergqvist, Y., Day, N. and White, N.J., 2005. Development and validation of a bioanalytical method using automated solid-phase extraction and LC-UV for the simultaneous determination of lumefantrine and its desbutyl metabolite in plasma. Journal of pharmaceutical and biomedical analysis, 37(5), pp.1081-1088.
CrossRef - Rudrapal, M., Khairnar, S.J., Chutia, D., Bhattacharya, S., Sarwa, K.K. and Hussain, N., 2021. Development and Validation of Reverse-phase High-performance Liquid Chromatography Method for Simultaneous Estimation of Riluzole and Levodopa in Tablet Dosage Form. Asian Journal of Pharmaceutics, 15(1), p.172.
- Shen, G., Hong, J.L. and Kong, A.N.T., 2007. Development and validation of an HPLC method for the determination of dibenzoylmethane in rat plasma and its application to the pharmacokinetic study. Journal of Chromatography B, 852(1-2), pp.56-61.
CrossRef - Sailaja, A.L., Kumar, K.K., Kumar, D.R., Kumar, C.M., Yugandhar, N.M. and Srinubabu, G., 2007. Development and validation of a liquid chromatographic method for determination of efavirenz in human plasma. Chromatographia, 65(5), pp.359-361.
CrossRef - Zzaman, M.T., Khan, S.A., Arora, A. and Ahmad, O., 2009. Method development and validation of fenofibrate by hplc using human plasma. Rev Electron Biomed/Electron J Biomed, 3, pp.41-54.
- Fortuna, A., Sousa, J., Alves, G., Falcão, A. and Soares-da-Silva, P., 2010. Development and validation of an HPLC-UV method for the simultaneous quantification of carbamazepine, oxcarbazepine, eslicarbazepine acetate and their main metabolites in human plasma. Analytical and bioanalytical chemistry, 397(4), pp.1605-1615.
CrossRef - Nandi, U., Das, A., Roy, B., Choudhury, H., Gorain, B. and Pal, T.K., 2013. Development and validation of an HPLC‐UV method for simultaneous determination of zidovudine, lamivudine, and nevirapine in human plasma and its application to pharmacokinetic study in human volunteers. Drug testing and analysis, 5(6), pp.485-491.
CrossRef - Gide, P., Sonawane, S. and Chitnis, A., 2012. Development and validation of RP-HPLC method for estimation of eplerenone in spiked human plasma. Journal of pharmaceutical analysis, 2(5), pp.390-393.
CrossRef - Zhen, Y., Thomas-Schoemann, A., Sakji, L., Boudou-Rouquette, P., Dupin, N., Mortier, L., Vidal, M., Goldwasser, F. and Blanchet, B., 2013. An HPLC-UV method for the simultaneous quantification of vemurafenib and erlotinib in plasma from cancer patients. Journal of Chromatography B, 928, pp.93-97.
CrossRef - Atif, M., Khalid, S.H., Kit, G.O., Sulaiman, S.A.S., Asif, M. and Chandersekaran, A., 2013. Development and validation of RP-HPLC-UV method for the determination of Glipizide in human plasma. Journal of Young Pharmacists, 5(1), pp.26-29.
CrossRef - Rastkari, N., Khoobi, M., Shafiee, A., Khoshayand, M.R. and Ahmadkhaniha, R., 2013. Development and validation of a simple and sensitive HPLC–UV method for the determination of captopril in human plasma using a new derivatizing reagent 2-naphthyl propiolate. Journal of Chromatography B, 932, pp.144-151.
CrossRef - Mannemala, S.S. and Nagarajan, J.S.K., 2015. Development and validation of a HPLC‐PDA bioanalytical method for the simultaneous estimation of Aliskiren and Amlodipine in human plasma. Biomedical chromatography, 29(3), pp.346-352.
CrossRef - Charbe, N., Baldelli, S., Cozzi, V., Castoldi, S., Cattaneo, D. and Clementi, E., 2016. Development of an HPLC–UV assay method for the simultaneous quantification of nine antiretroviral agents in the plasma of HIV-infected patients. Journal of Pharmaceutical Analysis, 6(6), pp.396-403.
CrossRef - Nannetti, G., Messa, L., Celegato, M., Pagni, S., Basso, M., Parisi, S.G., Palù, G. and Loregian, A., 2017. Development and validation of a simple and robust HPLC method with UV detection for quantification of the hepatitis C virus inhibitor daclatasvir in human plasma. Journal of pharmaceutical and biomedical analysis, 134, pp.275-281.
CrossRef - Hidau, M.K., Kolluru, S. and Palakurthi, S., 2018. Development and validation of a high‐performance liquid chromatography method for the quantification of talazoparib in rat plasma: Application to plasma protein binding studies. Biomedical Chromatography, 32(2), p.e4046.
CrossRef - Jin, S., Zhao, Q., Zhang, D., Zhao, Z. and Mei, S., 2019. Development and validation of an improved HPLC-UV method for simultaneous determination of lamotrigine and oxcarbazepine and its active metabolite 10, 11-dihydro-10-hydroxycarbazepine in human blood plasma and comparison with an UHPLC-MS/MS method. Journal of Analytical Science and Technology, 10(1), pp.1-10.
CrossRef - Ameeduzzafar, El-Bagory, I., Alruwaili, N.K., Imam, S.S., Alomar, F.A., Elkomy, M.H., Ahmad, N. and Elmowafy, M., 2020. Quality by design (QbD) based development and validation of bioanalytical RP-HPLC method for dapagliflozin: Forced degradation and preclinical pharmacokinetic study. Journal of Liquid Chromatography & Related Technologies, 43(1-2), pp.53-65.
CrossRef - Naidong, W., Ring, P.R., Midtlien, C. and Jiang, X., 2001. Development and validation of a sensitive and robust LC–tandem MS method for the analysis of warfarin enantiomers in human plasma. Journal of pharmaceutical and biomedical analysis, 25(2), pp.219-226.
CrossRef - Souppart, C., Gauducheau, N., Sandrenan, N. and Richard, F., 2002. Development and validation of a high-performance liquid chromatography–mass spectrometry assay for the determination of artemether and its metabolite dihydroartemisinin in human plasma. Journal of Chromatography B, 774(2), pp.195-203.
CrossRef - Wood, M., De Boeck, G., Samyn, N., Morris, M., Cooper, D.P., Maes, R.A.A. and De Bruijn, E.A., 2003. Development of a rapid and sensitive method for the quantitation of amphetamines in human plasma and oral fluid by LC-MS-MS. Journal of analytical toxicology, 27(2), pp.78-87.
CrossRef - Alali, F.Q., Tashtoush, B.M. and Najib, N.M., 2004. Determination of ketotifen in human plasma by LC–MS. Journal of pharmaceutical and biomedical analysis, 34(1), pp.87-94.
CrossRef - Chen, B.M., Liang, Y.Z., Wang, Y.L., Deng, F.L., Zhou, P., Guo, F.Q. and Huang, L.F., 2005. Development and validation of liquid chromatography–mass spectrometry method for the determination of telmisartan in human plasma. Analytica Chimica Acta, 540(2), pp.367-373.
CrossRef - Chen, B.M., Liang, Y.Z., Wang, Y.L., Deng, F.L., Zhou, P., Guo, F.Q. and Huang, L.F., 2005. Development and validation of liquid chromatography–mass spectrometry method for the determination of telmisartan in human plasma. Analytica Chimica Acta, 540(2), pp.367-373.
CrossRef - Yang, Y., 2007. Development and validation of a high-performance liquid chromatography–tandem mass spectrometric method for quantification of daunorubicin in rat plasma. Talanta, 71(2), pp.596-604.
CrossRef - Deng, X., Chen, X., Yin, R., Shen, Z., Qiao, L. and Bi, K., 2008. Determination of deoxyschizandrin in rat plasma by LC–MS. Journal of pharmaceutical and biomedical analysis, 46(1), pp.121-126.
CrossRef - Bharathi, D.V., Hotha, K.K., Sagar, P.V., Kumar, S.S., Naidu, A. and Mullangi, R., 2009. Development and validation of a sensitive LC‐MS/MS method with electrospray ionization for quantitation of pramipexole in human plasma: application to a clinical pharmacokinetic study. Biomedical Chromatography, 23(2), pp.212-218.
CrossRef - Silvestro, L., Gheorghe, M.C., Tarcomnicu, I., Savu, S., Savu, S.R., Iordachescu, A. and Dulea, C., 2010. Development and validation of an HPLC–MS/MS method to determine clopidogrel in human plasma. Use of incurred samples to test back-conversion. Journal of Chromatography B, 878(30), pp.3134-3142.
CrossRef - Macwan, J.S., Ionita, I.A., Dostalek, M. and Akhlaghi, F., 2011. Development and validation of a sensitive, simple, and rapid method for simultaneous quantitation of atorvastatin and its acid and lactone metabolites by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Analytical and bioanalytical chemistry, 400(2), pp.423-433.
CrossRef - Sparidans, R.W., Tang, S.C., Nguyen, L.N., Schinkel, A.H., Schellens, J.H. and Beijnen, J.H., 2012. Liquid chromatography–tandem mass spectrometric assay for the ALK inhibitor crizotinib in mouse plasma. Journal of Chromatography B, 905, pp.150-154.
CrossRef - Zhao, F.J., Tang, H., Zhang, Q.H., Yang, J., Davey, A.K. and Wang, J.P., 2012. Salting-out homogeneous liquid–liquid extraction approach applied in sample pre-processing for the quantitative determination of entecavir in human plasma by LC–MS. Journal of chromatography B, 881, pp.119-125.
CrossRef - Bourget, P., Amin, A., Chandesris, M.O., Vidal, F., Merlette, C., Hirsch, I., Cabaret, L., Carvalhosa, A., Mogenet, A., Frenzel, L. and Damaj, G., 2014. Liquid chromatography–tandem mass spectrometry assay for therapeutic drug monitoring of the tyrosine kinase inhibitor, midostaurin, in plasma from patients with advanced systemic mastocytosis. Journal of Chromatography B, 944, pp.175-181.
CrossRef - Wani, T.A., Zarger, S. and Ahmad, A., 2015. Ultra performance liquid chromatography tandem mass spectrometric method development and validation for determination of neratinib in human plasma. South African Journal of Chemistry, 68, pp.93-98.
CrossRef - Rood, Johannes JM, Stephanie van Hoppe, Alfred H. Schinkel, Jan HM Schellens, Jos H. Beijnen, and Rolf W. Sparidans. “Liquid chromatography–tandem mass spectrometric assay for the simultaneous determination of the irreversible BTK inhibitor ibrutinib and its dihydrodiol-metabolite in plasma and its application in mouse pharmacokinetic studies.” Journal of pharmaceutical and biomedical analysis118 (2016): 123-131.
CrossRef - van Nuland, M., Hillebrand, M.J., Rosing, H., Schellens, J.H. and Beijnen, J.H., 2017. Development and validation of an LC-MS/MS method for the simultaneous quantification of abiraterone, enzalutamide, and their major metabolites in human plasma. Therapeutic drug monitoring, 39(3), pp.243-251.
CrossRef - van Nuland, M., Hillebrand, M.J., Rosing, H., Burgers, J.A., Schellens, J.H. and Beijnen, J.H., 2018. Ultra-sensitive LC–MS/MS method for the quantification of gemcitabine and its metabolite 2′, 2′-difluorodeoxyuridine in human plasma for a microdose clinical trial. Journal of pharmaceutical and biomedical analysis, 151, pp.25-31.
CrossRef - Guan, S., Chen, X., Wang, F., Xin, S., Feng, W., Zhu, X., Liu, S., Zhuang, W., Zhou, S., Huang, M. and Wang, X., 2019. Development and validation of a sensitive LC–MS/MS method for determination of gefitinib and its major metabolites in human plasma and its application in non-small cell lung cancer patients. Journal of pharmaceutical and biomedical analysis, 172, pp.364-371.
CrossRef - Sonia, K. and Lakshmi, K.S., 2017. HPTLC method development and validation: An overview. Journal of Pharmaceutical Sciences and Research, 9(5), p.652.
- Mirfazaelian, A., Goudarzi, M., Tabatabaiefar, M. and Mahmoudian, M., 2002. A quantitative thin layer chromatography method for determination of theophylline in plasma. Pharm. Pharm. Sci, 5(2), pp.131-134.
- Branislava Miljkovic, Quantitative analysis of nimesulide in plasma by thin-layer chromatography: Application to pharmacokinetic studies in man, JPC – Journal of Planar Chromatography – Modern TLC · June 2003, DOI: 10.1556/JPC.16.2003.3.8.
CrossRef - Shah, R.R., Suhagia, B.N., Rathod, I.S., Shah, S.A. and Patel, D.M., 2005. HPTLC Method Development For Pharmacokinetic Study Of Sparflaxacin In Plasma. Indian journal of pharmaceutical sciences, 67(6), p.687.
- Jamshidi, A. and Nateghi, A.R., 2007. HPTLC determination of atorvastatin in plasma. Chromatographia, 65(11), pp.763-766.
CrossRef - Jain, G.K., Jain, N., Iqbal, Z., Talegaonkar, S., Ahmad, F.J. and Khar, R.K., 2007. Development and validation of an HPTLC method for determination of minocycline in human plasma. Acta Chromatographica, 19, p.197.
- Rote, A.R. and Pingle, S.P., 2009. Reverse phase-HPLC and HPTLC methods for determination of gemifloxacin mesylate in human plasma. Journal of Chromatography B, 877(29), pp.3719-3723.
CrossRef - Ramadan, N.K., Mohamed, H.M. and Moustafa, A.A., 2010. Rapid and highly sensitive HPLC and TLC methods for quantitation of amlodipine besilate and valsartan in bulk powder and in pharmaceutical dosage forms and in human plasma. Analytical Letters, 43(4), pp.570-581.
CrossRef - Tambe, S.R., Shinde, R.H., Gupta, L.R., Pareek, V. and Bhalerao, S.B., 2010. Development of LLE and SPE procedures and its applications for determination of olmesartan in human plasma using RP-HPLC and HPTLC. Journal of liquid chromatography & related technologies, 33(4), pp.423-430.
CrossRef - Faiyazuddin, M., Rauf, A., Ahmad, N., Ahmad, S., Iqbal, Z., Talegaonkar, S., Bhatnagar, A., Khar, R.K. and Ahmad, F.J., 2011. A validated HPTLC method for determination of terbutaline sulfate in biological samples: Application to pharmacokinetic study. Saudi Pharmaceutical Journal, 19(3), pp.185-191.
CrossRef - Rote, A.R. and Kande, S.K., 2011. Development of HPTLC method for determination of cefpodoxime proxetil and ambroxol hydrochloride in human plasma by liquid–liquid extraction. Pharmaceutical methods, 2(4), pp.242-246.
CrossRef - Rote, A.R. and Sonavane, P.R., 2012. Development and validation of bioanalytical method for determination of telmisartan and hydrochlorothiazide using HPTLC in human plasma.
CrossRef - Khanvilkar, V., Dalvi, V., Tambe, A., Parmar, D. and Kadam, V., 2013. HPTLC method for determination of eprosartan mesylate in human plasma. Indo American Journal of Pharm Research, 3(10).
- Rote, A.R. and Sonavane, P.R., 2013. Bioanalytical method development and validation for determination of metoprolol tartarate and hydrochlorothiazide using HPTLC in human plasma. Brazilian Journal of Pharmaceutical Sciences, 49, pp.845-851.
CrossRef - Ramesh, B., Ramakrishna, S., Reddy, R.K.K., Babu, K.H., Sarma, V.U.M. and Devi, P.S., 2013. HPTLC method for determination of darunavir in rat plasma and its application in pharmacokinetic studies. Journal of Liquid Chromatography & Related Technologies, 36(2), pp.167-179.
CrossRef - El-Koussi, W.M., Atia, N.N., Mahmoud, A.M. and El-Shabouri, S.R., 2014. HPTLC method for direct determination of gemifloxacin mesylate in human plasma. Journal of Chromatography B, 967, pp.98-101.
CrossRef - Pandya, J.J., Bhatt, N.M., Chavada, V.D., Sharma, P., Sanyal, M. and Shrivastav, P.S., 2016. Simultaneous Analysis of Aliskiren and Hydrochlorothiazide in their Combined Dosage Form and Spiked Human Plasma by HPTLC. Journal of Taibah University for Science.
CrossRef - Farid, N.F. and Abdelaleem, E.A., 2016. HPTLC method for the determination of paracetamol, pseudoephedrine and loratidine in tablets and human plasma. Journal of chromatographic science, 54(4), pp.647-652.
CrossRef - Gosavi, S.M. and Tayade, M.A., 2017. Development and Validation of High Performance Thin Layer Chromatography for Determination of Esomeprazole Magnesium in Human Plasma. J Chromatogr Sep Tech, 8(360), p.2.
- Abou Al Alamein, A.M., 2018. Validated eco-friendly chromatographic methods for simultaneous determination of sacubitril and valsartan in spiked human plasma and in pharmaceutical formulation. JAPS, 8(2), pp.011-017.
- Gadallah, M.I., Ali, H.R.H., Askal, H.F. and Saleh, G.A., 2019. Facile HPTLC-densitometric determination of ertapenem and paracetamol in pharmaceuticals and rabbit plasma with pharmacokinetic insights. Microchemical Journal, 150, p.104093.
CrossRef - Alam, P., Iqbal, M., Ezzeldin, E., Khalil, N.Y., Foudah, A.I., Alqarni, M.H. and Shakeel, F., 2020. Simple and accurate HPTLC-densitometric method for quantification of delafloxacin (a novel fluoroquinolone antibiotic) in plasma samples: Application to pharmacokinetic study in rats. Antibiotics, 9(3), p.134.
CrossRef - Crul, M., Van Den Bongard, H.J.G.D., Tibben, M.M., Van Tellingen, O., Sava, G., Schellens, J.H.M. and Beijnen, J.H., 2001. Validated method for the determination of the novel organo-ruthenium anticancer drug NAMI-A in human biological fluids by Zeeman atomic absorption spectrometry. Fresenius’ journal of analytical chemistry, 369(5), pp.442-445.
CrossRef - Vouillamoz-Lorenz, S., Bauer, J., Lejeune, F. and Decosterd, L.A., 2001. Validation of an AAS method for the determination of platinum in biological fluids from patients receiving the oral platinum derivative JM216. Journal of pharmaceutical and biomedical analysis, 25(3-4), pp.465-475.
CrossRef - Tibben, M., Rademaker-Lakhai, J., Rice, J., Stewart, D., Schellens, J. and Beijnen, J., 2002. Determination of total platinum in plasma and plasma ultrafiltrate, from subjects dosed with the platinum-containing N-(2-hydroxypropyl) methacrylamide copolymer AP5280, by use of graphite-furnace Zeeman atomic-absorption spectrometry. Analytical and bioanalytical chemistry, 373(4), pp.233-236.
CrossRef - Brouwers, E.E., Tibben, M.M., Joerger, M., van Tellingen, O., Rosing, H., Schellens, J.H. and Beijnen, J.H., 2005. Determination of oxaliplatin in human plasma and plasma ultrafiltrate by graphite-furnace atomic-absorption spectrometry. Analytical and bioanalytical chemistry, 382(7), pp.1484-1490.
CrossRef - Brouwers, E.E., Tibben, M.M., Rosing, H., Hillebrand, M.J., Joerger, M., Schellens, J.H. and Beijnen, J.H., 2006. Sensitive inductively coupled plasma mass spectrometry assay for the determination of platinum originating from cisplatin, carboplatin, and oxaliplatin in human plasma ultrafiltrate. Journal of mass spectrometry, 41(9), pp.1186-1194.
CrossRef - Ma, B., Chen, M., Zhang, Q., Yang, Q., Yang, Z., Wu, Z., Cheng, Y., Wang, Y. and Ying, H., 2011. Determination of strontium in rat plasma and plasma ultrafiltrate by Zeeman Furnace atomic absorption spectroscopy and its application to a pharmacokinetic study. African Journal of Biotechnology, 10(78), pp.18039-18045.
CrossRef
Accepted on: 14-03-2022
Second Review by: Dr. Sarwar Hossain
Final Approval by: Dr. rer. nat.Elsayed Ahmed









