How to Cite | Publication History | PlumX Article Matrix
Said M.R. Kewedar * and Khaleel Adel Ahmed Abulamoun
* and Khaleel Adel Ahmed Abulamoun
Princess Tharwat University College, affiliated to Balqa Applied University, Muhawish Yusuf, Amman, Jordan.
DOI : http://dx.doi.org/10.13005/bbra/2967
ABSTRACT:
COVID-19 is an infectious disease caused by a new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus originated from Wuhan city, which spread rapidly throughout the world after it hit China in December 2019 and became a global pandemic. There are two significant classes of the Coronavirus affecting human beings: SARS and MERS. Coronavirus is a positive-sense virus, an RNA virus with a single strand of RNA. And gets its title from the crown-like spikes on their surface. Presently, testing for COVID-19 is done by taking a nasal swab, tracheal aspirate, or bronchoalveolar samples and there are different conventional techniques are available for the detection like CT-scan, PCR, Sequencing, CRISPR, ELISA, LFA, LAMP, RT-PCR, and Rapid Antigen Test. COVID-19 treatment generally depends on the severity and/or the health status of the infected patient. The treatment procedure, at the beginning of the pandemic, includes the use of antiviral drugs which have limited availability. And clinical trials of vaccines are going on by different companies and some are released at the beginning of the COVID-19 currently, 10 vaccines are approved by the WHO. As more clinical examinations continue to be done, the availability of antivirals increases as well as vaccines. Preventive measures are social/physical distancing, masking, and isolation of infected individuals. There is an immense need to consider elective available resources to boost one’s immune system along with probiotics. Along with diminishing the pressure by expanding exercise and meditation. Although educational systems have used different learning management systems, there are concerns about the online teaching system in comparison to the traditional classroom teaching system. Our objective has been to examine the effects of COVID-19 on health systems globally and various aspects of human life as well.
KEYWORDS: COVID-19; Educational Systems; Global Health; Probiotics; real-time PCR; Vaccine
Download this article as:| Copy the following to cite this article: Kewedar S. M. R, Abulamoun K. A. A. The Effects of Coronavirus on Human Health and Their Influence on Other Aspects of Life: A Scoping Review. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Kewedar S. M. R, Abulamoun K. A. A. The Effects of Coronavirus on Human Health and Their Influence on Other Aspects of Life: A Scoping Review. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3weH2fE |
Introduction
One-stranded, enveloped, positive-sense RNA viruses are known as Coronaviruses that are responsible for infectious diseases affecting the respiratory system. It is a common virus affecting members of the animal kingdom, including humans, birds, and other mammals. Recently, the serovar of coronavirus i.e., (SARS-CoV-2) (Figure 1) was discovered in Wuhan city, apparently transmits this disease from animals to humans. At the Huangan Seafood Wholesale Market in Wuhan, Hubei Province, China 1-3. There are many assumptions about the theory of the natural host of this virus, which is still unknown and is believed to have an animal origin e.g., pangolins, bats, etc. It is assumed humans acquired infection from these animals, but research is still going on. The first report of COVID-19 was published at the end of 2019. Since then, the distribution and occurrence of the virus have been noted and monitored, it has spread all over the world, causing a pandemic situation and thus a healthcare emergency 4-5.
It may differ from country to country or content to others, but it is very clear that the most affected countries are the USA, India, Brazil, Russia, Italy, China, Iran, Egypt, Israel, all gulf countries, and others. The study indicates that the infection and outbreak of (SARS-CoV-2) are widespread throughout the globe. (SARS-CoV-2) was first reported in Kerala, India. Most numbers of the positive case reported states to date in India are Maharashtra, Kerala, Uttar Pradesh, and also spread to almost all states in India. COVID-19 has resulted in a ‘once-in-a-century pandemic’ with about 404,910,528 confirmed cases, including 5,783,776 deaths globally by 11 February 2022, reported to WHO. As of 6 February 2022, a total of 10,095,615,243 vaccine doses have been administered. 6-12.
It turned out that the Coronavirus Phylum is Incertae Sedis, the Order is Nidovirales, the Family is Coronaviridae, the Subfamily is Ortho coronavirinae, and the Genus is Beta coronavirus. Four genera Alpha, beta, gamma, and delta coronaviruses make up the coronavirus subfamily. The coronavirus subfamily is further classified into four genera: alpha, beta, gamma, and delta coronaviruses. (1Alpha-coronavirus (alpha CoV), (2) Beta-coronavirus (betaCoV), (3) Delta-coronavirus (delta IV) and (4) Gamma-coronavirus (gammaCoV). Out of these, the Alpha and Beta coronaviruses cause human infections. (SARS-CoV) virus, Middle East Respiratory Syndrome (MERS-CoV), virus and severe acute respiratory syndrome virus all belong to the Beta coronavirus (betaCoV) family. Coronaviruses inherit their name from the Latin corona, which means crown or wreath, based on their morphological characteristics. 13-15.
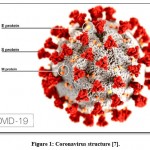 |
Figure 1: Coronavirus structure [7]. |
It has a large fringe, bulbous surface projections, and viral spike peplomers, which are proteins on the outside of the virus. The external viral envelope comprises a lipid bilayer where the layer, envelope, and spike auxiliary proteins are connected 7.
Problem of the research
This study focused on analyzing and evaluating the influence/effect of covid-19 on overall prosperity, education, economy, security, management, and other angles of human life. These aspects are the backbone of human life. Best methods are required to minimize the negative impact on them by controlling the pandemic (taking care of/protecting methods). Clinical studies should continue on a greater scale, to increase the availability of antiviral drugs and evaluate the level of toxicity of these medications [14]. And also accelerate the study of vaccinations. All these processes will lead to a normal life before COVID-19 is discovered.
Significance of the research
COVID-19 has disrupted many aspects of life and the lives of people around the world. The current investigation is basically aimed to understand most of the aspects of COVID-19 from day one to date. There are numerous points and goals which assessed, for the illustrated history of pandemics, SARS- CoV2, Extreme Intense Respiratory Disorder (SARS), Center Eastern Respiratory Infection (MERS), Developmental beginning, Transmission of the infection, Pathophysiology, Clinical introduction, Testing, Treatment and Suggestions for investigating 16-19.
The study might have an impact on treatment COVID-19. This study focused and analyze the influence of covid-19 on health, education, economy, security, management, and other aspects of human life, which is required to concentrate on minimizing the negative effects of this pandemic by protecting ourselves from the virus by social distancing, masking, and isolation of infected individuals as well as taking care of our immune system. Scientists should do more clinical studies, to increase the availability of antiviral drugs and evaluate the level of toxicity of these medications. This is considered an attempt to analyze the impact of COVID-19 on the prosperity structures around the world and different perspectives of human life.
Scope of the research
The research scope of Infectious disease (SARS-CoV2) spread primarily from person to person. It has spread between people most frequently when they are physically nearby. It transmits exceptionally effectively and economically through the discussion, essentially through beads containing little particles of an infection, such as mist concentrates, created after a contaminated individual breathes, hacks, wheezes, talks or sings.
The current examination is fundamentally pointed to the impacts of the COVID-19 on global health, education, economy, security, management, and other aspects of human life by focus, analysis, and evaluation to minimize the negative impact on them by different methods of controlling the pandemic. Scientists should do more clinical studies, to increase the availability of antiviral drugs and evaluate the level of toxicity of these medications. And also accelerate the study of vaccinations and also boost one’s immune system along with preventive measures of social distancing, masking, and isolation of infected individuals. All these processes will lead to a normal life before COVID-19 is discovered.
Objectives of the research
This manuscript highlights potential areas like current health system challenges, education, security, management, global business, culture, and other aspects of human life, which get influenced/impacted by COVID-19. The goals of this paper are to supply mindfulness and distinguish the investigation zones related to COVID-19 by identifying the best methods to minimize the negative impact on these aspects of controlling the pandemic COVID-19. (Taking care of/protecting methods). Clinical studies should continue on a bigger scale, to increase the availability of antiviral drugs and evaluate the level of toxicity of these medications. And also accelerate the study of vaccinations. It may offer assistance to make strides in the understanding of this infection, which will have positive impacts on this pandemic scenario of change as the disease spreads. The study of the disease can also lead to the development of life-saving drugs or vaccines. Better health is central to human happiness and well-being.
Limitation of the research
The main drawback of the study of a pandemic of COVID-19 is zoonotic and started in China. There are many theories about the originality of the virus, which means identifying the creature source of the irresistible operator and have not decided whether a diligent creature store of the irresistible operator exists. In case the infection may be a regular infection that would have retreated on its claim.
Scientists have not yet been able to answer some questions regarding COVID-19. The other, the treatment, limited availability of antivirals at the beginning of the pandemic and no vaccine; a lot of medications were initially repurposed to treat COVID-19 but due to the acute side effects of these drugs treatment is an unsuccessful story, that is why the entire world is suffering. There are more clinical studies continuing to be done to help for full control of this pandemic.
Crevices within the information of Coronavirus and its impacts.
It shows that Coronavirus is an irresistible disease, zoonotic, and began in China. There are many theories about the source of the infection but have not yet confirmed the main. So many questions are still not answered by the Scientists. The answers to these questions would without a doubt progress the world’s capacity to anticipate and get ready for a resurgence of COVID-19.
COVID-19 has had a tremendous effect on medical research, among other things. All organizations, including healthcare organizations, universities, and research centers were influenced by COVID-19 and started bleeding cash. The SARS-CoV-2 infection has altogether influenced the wellbeing, economy, and socio-economic texture of the worldwide society.
The study has carried out the effect of covid-19 on overall prosperity and other perspectives of human life. Coronavirus (COVID-19) is widespread in developing exponentially within the entirety of the world (all-inclusive). It was, to begin with, recognition in December 2019 in Wuhan, Hubei, China, and has brought about a widespread. The overall affirmed Coronavirus Cases is 282 by 20th January2020. It has been detailed from four nations counting China (278 cases), Thailand (2 cases), Japan (1 case), and the Republic of Korea (1 case). COVID-19 has brought about in a ‘once-in-a-century pandemic’ with around, 274,409,082 coronavirus cases, more than 5,365,363 deaths and 246,273,643 recuperated all-inclusive by Moment-December 18, 2021 20-25.
An overwhelming amount of literature started pouring out of countries, impacted the most at the onset of the pandemic. Almost every journal has provided free access to articles on COVID-19. Analysts, technologists, specialists, and other wellbeing care laborers are working day and night to bridge the hole from day one till nowadays, for the development of vaccines and medicines (antiviral properties and immunomodulatory properties) with little known side effects can be used in the fight with COVID-19 22
Data collection
In arranging to realize the objectives of the study, it is exceptionally vital to extricate the data from the best logical assets on a coronavirus-related investigation to complete the assignments of the term paper. These are distinctive classes of solid sources for COVID-19, which executed in this term paper such as investigate on COVID-19, one of them, the World Health Organization (WHO) site, specialists give a worldwide viewpoint of the COVID-19 infection, whereas too sharing individual best practices on subjects all information and updates about COVID-19, (Scientific / medical journals), best websites (Scientific / medical websites), such as the Clarivate Web of Science (WoS), Elsevier Scopus, and PMC-sourced materials drawn from CORD-19 (COVID-19 Open Research Dataset), Health and Medical News, Coronavirus and Higher Education like Search engines (e.g. Google), and Business and the Economy.
Comparative analysis
Any overlap between articles found over the distinctive source materials was evacuated.
History of Pandemics
A pandemic has been historically described as “a scourge happening around the world, or over an awfully wide region, crossing universal boundaries and as a rule, influencing an expansive number of people”.
There are several types of secondary data that are applied in the research paper.
Spanish flu (H1N1-influenza virus) in 1918-1919 caused about 20-50 million victims. And also, the virus-infected 500 million people worldwide.
Asian flu (H2N2-influenza virus) originated in Guizhou, China, in 1957-1958 causing about 1-4 million victims.
Hong Kong flu (H3N2-influenza virus) in 1968 caused about 1-4 million victims. It is suspected that this virus evolved from the strain of influenza 9.
Current pandemics
A pandemic has been historically described as an outbreak of a disease occurring over a wide geographic area (spanning countries) and affecting a significantly higher proportion of people. COVID-19 is a pandemic that continues to cause unimaginable losses. The World Health Organization has declared the novel coronavirus (SARS-Co-V-2) a global pandemic.
HIV (Human Immunodeficiency Virus) /AIDS (Acquired Immunodeficiency Syndrome)
As described by some as pandemic and by WHO as a global epidemic, HIV is believed to have originated in Africa and started with the primary case within the Democratic Republic of Congo in 1976, and in2006, the HIV predominance rate among pregnant ladies in South Africa was 29%. Education in African substances plays an awfully critical part in diminishing the rate of contamination rates, by instructing them around more secure sexual hones and Bloodborne disease safety measures preparing.
Since the point has influenced more than 37.9 million individuals with approximately 770,000 deaths in 2018 from HIV-related illnesses.
Swine flu began in 2009 could be a respiratory infection caused by influenza (H1N1 infections) widespread. It may be a generally modern strain of an influenza infection that causes indications compared to the customary flu. Swine flu began in pigs, but is spread/ transmission essentially from individual to individual. Begun in 2009 could be a respiratory infection caused by influenza (H1N1 infections) widespread. It may be a generally modern strain of an influenza infection that causes indications compared to the customary flu. Swine flu began in pigs, but is spread/transmission essentially from individual to individual. It is affirmed at a research facility that 18,500 casualties; but analysts gauge that almost 200,000 respiratory deaths and around 80,000 cardiovascular deaths were related to this pandemic.
COVID-19 (coronavirus disease), which is covered by this research paper 23.
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2)
On its revelation, the researchers named it ‘2019 novel coronavirus (2019-nCoV)’. In any case, the World Health Organization renamed the infection ‘Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)’. The disease caused by (SARS-CoV-2) is called ‘Coronavirus Disease 2019 (COVID-19) [6]’. Molecular and Genome information of coronavirus studies shows that after the genome analysis study of nCoV-2019 revealed that two viruses may have been combined 24.
It is an RNA molecule containing a positive-sense, single-stranded RNA genome with a genome measure extending for 30 to 34 kilobases (largest among RNA viruses). It contains at least 15 genes, including the S gene, which codes for surface proteins. The molecular phylogenetic investigation appeared nCoV is 88% similar to bat derived SAR- like coronavirus 79% close to SAR-Cov and50% similar to MERS‐Cov (Middle East Respiratory Syndrome Coronavirus). The following sign and symptoms report from the person who has received the (SARS-CoV-2) infection. A Transmission electron micrograph of (SARS-CoV-2) virus particles is represented in (Figure2).
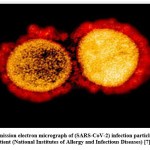 |
Figure 2: Transmission electron micrograph of (SARS-CoV-2) infection particles, disconnected from a patient (National Institutes of Allergy and Infectious Diseases) [7]. |
Severe Acute Respiratory Syndrome (SARS)
SARS (severe acute respiratory syndrome) could be a viral respiratory illness of zoonotic root caused by extreme intense respiratory disorder coronavirus (SARS-CoV or SARS-CoV-1). Coronaviruses commonly cause contaminations in both people and creatures. SARS originated in China in Nov 2002 with the outbreak rapidly spreading and resulting in about 8000 cases and an estimated 800 deaths.
Cases of SARS were reported in about 30 countries and the outbreak was controlled by epidemiological surveillance, identification, and isolation of patients, contact tracing, and quarantining of contacts, thereby interrupting person-to-person transmission of the virus. Treatment includes strong care. There are no particular antiviral medications or antibodies accessible 24-26. There has been no known transmission of SARS anywhere within the world since 2004.
Testing /Diagnosis
Testing for Coronavirus plays a very significant part in containing the spread of the pandemic by distinguishing positive cases, that can then be confined to prevent the spread of sickness. Inadequate research facility testing is a vital reason for the continuing with the spread of Coronavirus in numerous countries. The Research facility Determination recognizes and tests coronavirus (SARS-CoV-2) by distinctive strategies or procedures classified into Antigen tests, Molecular/PCR tests, and Antibody tests. For example, Polymerase chain response (PCR) tests, Rt polymerase chain response (RT¬-PCR), and Serological methods. Real-time RT-PCR tests are suggested for the determination of COVID-19. The examination is done by taking a nasopharyngeal swab from patients in arranging to identify (SARS-CoV-2) Positives that come about are shown based on RT-PCR of over tests as it were. Few cases have detailed a positive RT-PCR for nasopharyngeal swab, but a negative RT-PCR for urine and stool tests of patients. Since the respiratory framework is influenced in COVID-19, the occurrences of nasopharyngeal swabs are considered substantial as against any other obsessive tests. RT-PCR is a greatly delicate strategy and plays a really vital part in affirming the determination of COVID-19 7-9
Middle Eastern Respiratory Virus
The Middle East Respiratory Syndrome (MERS) is one more kind of coronavirus. It enters its host cell by binding to the DPP4 receptor. Typically, the host of MERS coronavirus includes humans, bats, and camels. The first instance of this virus was recorded in Saudi Arabia in June 2012, when the patient died of pneumonia and renal failure. MERS was also responsible for a pandemic spreading in over 27 countries. Statistically, WHO has estimated about 2500 cases with more than 800 deaths from MERS in 2020 10-11.
Transmission of Coronavirus
The Mode of Transmission of coronavirus, it can be possible by these methods, like Human-to-human contact (personal contact), cough and sneeze through the air, and contaminated objects.
Pathophysiology
Pathophysiology (comprising the Greek beginning words “pathos” = suffering; “physis” = nature, origin; and “logos” = “the study of”). The investigation of unusual changes in body works that are the causes, outcomes, or concomitants of Coronavirus forms.
COVID-19 can influence the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs). SARS-CoV-2 enters have cells through interaction of its spike protein with the section receptor the angiotensin-converting protein sort 2 (ACE2) within the proximity of Transmembrane protease, serine 2 (TMPRSS2). Proposed instruments for COVID-19 caused by contamination with SARS-CoV-2 incorporate (1) coordinate virus-mediated cell harm; (2) dysregulation of the renin-angiotensin-aldosterone framework (RAAS) as a result of deregulation of ACE2 related to viral section, which leads to diminished cleavage of angiotensin I and angiotensin II; (3) endothelial cell harm and thromboinflammation; And (4) dysregulation of the safe reaction and hyper inflammation caused by restraint of intergalactic signaling by the infection, T cell lymphodepletion, and the generation of proinflammatory cytokines, especially IL-6 and TNFα 26
Clinical Presentation
According to scientists, there are three classes of medical conditions when the virus infects the respiratory system. First is a mild, second moderate and lastly, a severe condition is reported. Both first- and second-degree cases do not require special care since the immune system effectively helps in overcoming the symptoms of the disease. However, the third scenario requires special treatment, failing which full recovery of patients is not possible. The patients may require a respiratory support system, nursing support, and a heavy dose of medicines 12-15. Table 1 represents the common symptoms associated with COVID-19.
Prevention
The Covid infection 2019 (Coronavirus) worldwide pandemic brought about by extreme intense respiratory condition Covid 2 (SARS-CoV-2) is a significant general wellbeing event. The government bodies and health officials in China have been working extremely hard to slow down the spread of COVID-19 [1-5]. The motivation behind ‘social, separating’ is to diminish the transmission of this disease. It is advised for people to maintain a 2-meter distance from each other. This is considered a safe distance by WHO. Other preventative ways, such as washing your hand regularly with Sanitizers or soap or 70% alcohol, wear a face mask, and always covering your mouth while coughing or sneezing. The mortality rate is very low, i.e., 2% to 8%, and the highest is observed in older persons. Other measures include curfews and lockdowns to discourage large gatherings. It is a very important step to minimize the spread of this disease.
Jordan was the first Arab country to implement these rules and regulations and excellent results of the above measures were observed. WHO ordered the nations into Areawide premise on the seriousness of the sicknesses/disease, chances: (a) High-hazard (alleged “red zone” or level 1 danger zone; (b) mean danger (level 2 danger zone); (c) residual region, which was considered at okay (level 3 danger zone). In the resulting stages, it makes the methodologies for these spaces effective by taking significant assurances and security 27-36.
The Exponential Moving Average (EMA) has recommended that patients do not interrupt their treatment with Angiotensin Changing over Enzyme (ACE) inhibitors or Angiotensin Receptor Blockers (ARBs). They advance suggest that switching over to other medicines is not required in case of COVID-19 disease. According to international scientists and epidemiologists, diseases like hypertension, heart failure, or renal diseases could worsen the condition of patients suffering from COVID-19. This may occur due to decreased immunity. Hence it is recommended that in such cases patients continue to use prescribed medications. It is also supported by clinical evidence 26.
To ensure effective prevention and control should be implemented early of these processes (detection, reporting, diagnosis, isolation, and treatment) and also full supports of centralized responses for these processes such as (centralized coordination by specialists, concentrated distribution of assets, incorporated situation of patients, and brought together arrangement of treatment) with all of these efforts which may lead to avoiding the complication for (COVID-19) cases/patients. Incorporation of portable innovation, enormous information, and man-made brainpower into Coronavirus reacting expanded admittance to wellbeing administrations, decreased falsehood, and limited the effect of phony news. The entirety of the past advances plays a vital principle to change the situation of (Coronavirus) by controlling flare-ups and commitment to the treatment of pandemic infection 37-43.
As a unique populace, kids have exceptional respiratory lot structure attributes, youthful resistant framework, and weakness to respiratory infectious diseases. In this way, clinicians must treat the tainted kids mindfully in spite of most pediatric patients having milder side effects and better forecasts contrasted with the grown-up patients. Exceptionally extraordinary consideration is suggested for kids during the course of avoidance, conclusion, and treatment of kids with Coronavirus summed up the study of disease transmission, clinical attributes, analytic rules, clinical groupings, differential analysis, and medicines 44-48.
Table 1: Comparison of normal indications between normal cold, roughage fever and Coronavirus 1, 4-7,15
| Symptoms | Cold | Flu | Allergies | COVID-19 |
| Fever | Rare | Common and high (above 100 degrees); lasts for 3 to 4 days. | No | Common, one of the first sign; start weak and become worse (above 100 degrees) |
| Headaches | Rare | Prominent | Common | Sometimes |
| Body Aches | Sometimes | Common and usually severe | Never | Sometimes |
| Fatigue | Very mild | Common, may last for 2 to 3 weeks | Common | Sometimes |
| Extra Exhaustion | No | Early Prominent | Unusual | Sometimes |
| Stuffy Nose | Common | Sometimes | Common | Sometimes |
| Sneezing | Unusual | Sometimes | Common | Rare |
| Sore throat | Common | Sometimes | Sometimes | Sometimes |
| Shortness of breath | No | No | No | Sometimes |
| Chest discomfort,
cough |
Mild to moderate, hacking cough | Common and can be severe | Sometimes | Usual, dry cough |
| Chills | Rarely | Common | No | Sometimes |
| Treatment | Pain relievers, cough syrup, decongestants | Annual vaccine, Antiviral drugs 1 to 2 days after symptoms start | Allergy Medications and controlling environment | No known vaccines or treatment |
*This is not a complete list of symptoms and is only a guide, anyone who is concerned should contact a medical provider.
The Sign and manifestations of Covid incorporate Higher Fever, Dry Hack, Weariness, Sore throat, Windedness, and Sputum creation. The brooding period for SARS-CoV-2 is typically 5 to 6 days, however, may go from 2 to 14 days. The World Health Organization (WHO) announced the Covid episode, a pandemic and Public Health Emergency of International Concern (PHEIC).
Treatment
The treatment strategy depends upon the severity of illness per NIH guidelines 50-54.
Asymptomatic illness
When a patient is a carrier for a disease/ infection of (COVID-19) by having a positive test of molecular diagnostic (polymerase chain response) or antigen test for (SARS-CoV-2) yet encounters no manifestations. There are reports of loss of feeling, of smell in individuals, who in any case have no side effects, it is considered under this case. The best strategy for treatment ought to be holed up at home. It doesn’t need serious testing or extra treatment and on the off chance that they stay asymptomatic, they can stop detachment 10 days from the day of trial. Follow physical removal directions to try not to spread the infection since no signs of the infection. On the off chance that the clinical condition falls apart, they should check with doctors.
Mild illness
This gathering incorporates patients with different signs and indications of COVID 19 such as (fever, dry hack, tiredness, feeling somewhat breathless, muscle torments, cerebral pain, sore throat, loose bowels). Patients at moment level [pre-symptomatic (SARS-CoV-2) contamination] ought to self-isolate at home. No extra research facility testing and no particular treatment.
Moderate illness
Individuals who have proven lower respiratory infection by clinical evaluation or Imaging or immersion of oxygen (SpO2) ≥94% on room discuss at ocean level. Tend to have an expanded heart rate, especially on the off chance that they’re moving around and this can be caused by the aggravation of the lungs, so side effects like hacking and breathlessness may be more regrettable.
Patients should be monitored closely (hospital), isolation, limiting provider exposure; laboratory tests, including complete blood counts, metabolic profile, renal and liver function studies; ECG, imaging. The treatment should refer to Antiviral Therapy, Immune-Based Therapy. Otherwise, their condition may become worse.
Severe illness
Severe illness Patients who have respiratory recurrence >30 breaths each moment, SpO2 <94% on room Individuals who have respiratory disappointment, septic shock, as well as numerous organ brokenness. The contrast, among serious and basic ailments, is tiny, just a single medical clinic medical services proficient will make. Both seriousness levels should be critical in an emergency clinic. Treatment of Coronavirus ought to apply Antiviral Treatment, Insusceptible Based Therapy.
Critical illness
People who have respiratory disappointment, septic shock, or potentially numerous organ brokenness. The contrast, among serious and basic diseases, is tiny, just a single emergency clinic medical care proficient will make. Both seriousness levels should be in the emergency clinic urgently.
Treatment of COVID-19 should apply Antiviral Therapy, Immune-Based Therapy. The dedicated intensive care unit (ICU) plays a pivotal role in the treatment of COVID-19 especially for Severe illness and Critical illness.
There is a study about a series of basically sick patients with research facility affirmed Coronavirus conceded to ICUs in Lombardy, Italy, the larger part was more old men, an enormous extent required mechanical ventilation and high PEEP values and ICU mortality were significant 49.
The ICU is separated into green, yellow, and red regions. Every ICU bed is furnished with a full checking of essential boundaries and a mechanical ventilator. Each screen is copied in the unified control unit furnished with amplifiers and glasses to permit the correspondence between the staff. In the ICU, there is a research facility area, including two committed ultrasound machines, expendable fiberoptic bronchoscopes, video laryngoscope, mark-of-care blood vessel blood gas, coagulation investigations, transport ventilator, and crisis truck with a defibrillator. Clinical group (specialists and attendants) accessible 24 hours ×7 days for unique Coronavirus patients. This is standard by WHO. Spray Box, A Working Room Safety effort in Coronavirus Pandemic plays an effective role in preventing direct infections for medical staff by minimizing the risk and decreasing significantly the stress factor on them, which leads to Efficient job for treatment of COVID-19 patients 50-54.
Treatment For COVID-19
Probiotics
Probiotics are live microorganisms that are planned to have medical advantages when burned through or applied to the body. Probiotics might give novel ways to deal with both infection counteraction and treatment by boosting one’s immune system. There is a study that showed the protective effects of probiotics against viral infections 55.
Immune-based therapy
This kind of treatment portrays the treatment of sickness by initiating or stifling the resistant framework. It’s intended to evoke or enhance an invulnerable reaction are called initiation immunotherapy through immunotherapies that decrease or smother are called concealment immunotherapies. These phenomena can be called Immunotherapy or biological therapy.
This type is used for the treatment of Coronavirus. COVID-19 recuperating plasma or (SARS-CoV-2) immune globulins are acquired from people who have recuperated from contamination and have created an insusceptible reaction against the tainting microorganism. Killing antibodies are believed to be the real dynamic part; other safe arbiters may likewise contribute. It is strongly recommended to follow local/regional medical society guidelines for its use. The National Institutes of Health (NIH) summarized that no complete data/research evidence is available to go with or against this type of treatment COVID-19.
Therapeutic options
In extreme instances of Coronavirus contamination, the infection can enter the circulation system and taint endothelial and other objective cells in the kidneys, throat, bladder, ileum, heart tissues, and focal sensory system. What’s more, basically sick patients with Coronavirus disease frequently present indications of high oxidative pressure and fundamental aggravation – the main sources of mortality. Atomic factor E2-related factor 2 (Nrf2) is a record calculated that people are encoded by the NFE2L2 quality. (Nrf2) assumes a vital part to lessen responsive oxygen species (ROS) along with glutathione biosynthesis antecedents, e.g., N acetylcysteine. This treatment can be utilized to decrease harm to cells and tissues, to forestall respiratory disappointment and intense respiratory misery condition (ARDS) for Coronavirus patients. (Nrf2) action fundamentally diminishes with age, older patients because of vulnerability to oxidative pressure interceded sicknesses, which incorporate sort 2 diabetes, ongoing aggravation, and viral diseases 53.
Treatments of coronavirus are the main core of this epidemic/ problem, so far, no permanent treatment is available, however on a trial basis antiviral drugs have been used. Antivirals are drugs that are utilized for treating viral infections. A few antivirals target explicit infections, while others neutralize various infections. These medications can work in various ways, for example, keeping the infection from entering host cells, repeating, or delivering viral particles to infect other cells. Antiviral drugs such as Remdesivir, Chloroquine, Hydroxychloroquine, Favipiravir, Ritonavir/lopinavir. Moreover, different kinds of Coronavirus. Immunizations are a work in progress. Notwithstanding, suspected ADRs to these medications, are being accounted for to Vigibase, the WHO worldwide data set of individual case security reports (ICSRs) which are overseen by the Uppsala Checking Center in Sweden 18. A clinical preliminary of immunization improvement has been begun by various organizations across the globe.
In the course of Covid Infection 2019(Coronavirus) – related intense hypoxemic respiratory disappointment (AHRF), which is extreme blood vessel hypoxemia that is headstrong to supplemental oxygen. Nasal high stream (NHF) addresses another technique to help relax. These gadgets can deliver a warmed and humidified wind current applied by an enormous bore nasal prong [50]. The use of NHF as first-line ventilatory help during Coronavirus-related AHRF might have blocked the requirement for intubation a may have forestalled the requirement for intubation in up to 33% of cases. In the present situation, the ROX record estimated inside the initial 4 h after NHF commencement could be a simple two-utilized marker of early ventilatory response.
Drug–Drug Interactions and Side Effects
These medicines have their own limitations due to the toxicity of these drugs as well as are not particular or specific (COVID-19). It may be playing a role, but not a very important one. In the USA, the treatment of Coronavirus patients involves the utilization of drugs like chloroquine & hydroxychloroquine, which needs to be monitored carefully. This is due to the incidental effects associated with these medicines, indicated by the advisory of the USFDA. These drugs were used extensively either individually or in combination with other medicines (to improve their efficiency and minimize the harmful effects). The US Medical scholars monitored the general health of the COVID-19 patients during the treatment and evaluated the level of toxicity of these drugs. They reported toxic effects, in contrast to the expected benefits, with the use of these drugs 16. The European Medicines Agency (EMA) has also started a high risk associated with the utilization of chloroquine or hydroxychloroquine treatment of COVID-19. Further, they encourage close monitoring of patients. Moreover, Denmark had stopped trials immediately due to the negative impact of these drugs on COVID-19 patients 23-24. A study announced the treatment of Coronavirus patients with Hydroxychloroquine, Azithromycin, Lopinavir/Ritonavir, and Chloroquine. These patients were additionally getting different medications related to QTc prolongation. The examination, completed in France, showed the relationship of these medications with heart Antagonistic Medication Responses (ADRs). They showed the danger of cardiotoxicity can be tried not to break down the positive advantage to hazard equilibrium of any picked drug 17. Another examination further affirmed that a high-portion Chloroquine ought not to be suggested in patients with a serious Coronavirus condition since it prompts extreme intricacies. These examinations were completed by a randomized clinical preliminary. JAMA Organization Open, further showed that the light – portion sought to be checked 33-34. Analysts from Ireland have additionally affirmed that the utilization of Hydroxychloroquine for the treatment of Coronavirus patients might bring about expanding the cardiotoxicity, which might be deadly 35. An investigation of the benefit-risk assessment team (BRAT), for Remdesivir in COVID-19 (Mechanism of action, Pharmacokinetics uses, and Clinical toxicities), concluded that this drug is not effective at all forCOVID-19 treatment [19]. Nonsteroidal mitigating drugs (NSAIDs) are generally accessible for the treatment of torment. In certain nations, they are likewise utilized for the treatment of fever. The NSAID, for example, ibuprofen is accessible by solution just as over-the-counter. In any case, there have been mindlessly made through news reports (especially of online media) Referencing that both WHO and Rascal have demonstrated deteriorating Coronavirus indications with the utilization of NSAIDs (counting ibuprofen) 20. A new letter distributed in ‘The Lancet’ was accounted for by the USFDA. This review theorized an increment in the centralization of a particular protein (a particle that helps the cell biochemical responses) with the utilization of NSAIDs. The enzyme, in turn, may further aggravate the symptoms of COVID-19. An overview conducted by the ‘French National Agency for Medicines and Health Products Safety (ANSM)’ in May 2019, has also suggested the worsening of symptoms in the case of certain bacterial infections and chickenpox (varicella) with the use of NSAIDs like ibuprofen and ketoprofen 21-22. Despite the reports of harmful effects of NSAIDs in aggravating the symptoms of COVID-19, there has been no scientific evidence reported by US FDA and EMA to confirm the same 27-28. Hence, the patients should keep on utilizing the NSAIDs as coordinated by their PCP or drug specialist, medical care proficient as per naming guidelines 27-28. Scientists in Italy indicated that the patients suffering from COVID-19 have an increased chance of acquiring the Guillain-Barré syndrome (GBS) 25. The discoveries of an imminent French review distributed Available for use: Arrhythmia and Electrophysiology proposed the utilization of lopinavir/ritonavir (LPV/RTV; Kaletra) in older patients, with Coronavirus symptoms, may increase the risk of bradycardia 29. Similar studies have suggested that there are no implications of renin-angiotensin-aldosterone system medications with COVID-19 patients of all ages. Moreover, if any such implications are present, they may be non-significant 30-32.
Development of a vaccine (s) against COVID-19
Vaccines to prevent COVID-19 infection are considered the most promising approach for curbing the pandemic. By the end of 2020, several vaccines had become available for use in different parts of the world, over 40 candidate vaccines were in human trials, and over 150 were in preclinical trials. Now 33 vaccines are approved by at least one country and 10 vaccines are approved for use by WHO 57.
General Principles
A Brief Overview of vaccine development
As with the development of pharmaceuticals, vaccine development progresses through preclinical evaluation and three distinct clinical stages.
Phase I trials
This study is designed to test vaccine safety and immunogenicity, with dose-ranging studies also included.
Phase II trials
These expand the safety profile and assess the immune response involving a large number of participants.
Phase III trials
These are intended to test the effectiveness of an intervention for preventing a predefined endpoint, usually a laboratory-confirmed disease.
Traditionally, these means happen consecutively, and each generally requires quite a long while for completion. COVID-19 vaccine development has sped up to an uncommon speed, with each progression happening north of a few months. Additionally, in the COVID-19 vaccine initiative, phase I and II and phase II and III examinations have regularly been joined with consistent progress from one phase into the next. Nevertheless, safety criteria remain stringent; data safety and monitoring committees (DSMCs) composed of independent vaccine experts and study sponsors assess adverse events that are reported in each phase of a clinical study and approve advancement to the next phase. Nevertheless, safety criteria remain stringent; data safety and monitoring committees (DSMCs) composed of independent vaccine experts and study sponsors assess adverse events that are reported in each phase of a clinical study and approve advancement to the next phase. In the United States, the Food and Drug Administration (FDA) plays a very important role to approve progression to each next step in human trials, from initiation of phase I trials through progression to phase III trials, based on data generated in the prior step 56.
Overview of vaccine development
A COVID‑19 immunization is a biotechnology item expected to give invulnerability against Covid illness 2019 (COVID‑19). There were 321 immunizations up-and-comers are in various phases of advancement or have been created and in the clinical preliminary stage, a 2.5-crease increment since April. Be that as it may, no up-and-comer has finished clinical preliminaries to demonstrate its wellbeing and viability yet 51. Some 42 antibodies up-and-comers were in the clinical examination: specifically, 33 in Stage I–II preliminaries and 9 in Stage II–III preliminaries 50-53.
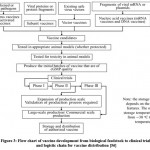 |
Figure 3: Flow chart of vaccine development from biological feedstock to clinical trials and logistic chain for vaccine distribution 56 |
Table 2: Development of various vaccines for the treatment of COVID-19 [37, 42, 49-52]
| Name of the vaccine | Manufacturer | Properties | Clinical Trial stages |
| mRNA-1273 | Moderna and NIAID | mRNA vaccine | Phase-III |
| BNT162 | BioNTech and Pfizer | mRNA vaccine | Phase-II/ III |
| INO-4800 | Inovio Pharmaceuticals | DNA vaccine | Phase-II |
| AZD1222 | University of Oxford and AstraZeneca | Adenovirus vaccine | Phase-III |
| Ad5-nCoV | CanSino Biologics | Adenovirus vaccine | Phase-III |
| PiCoVacc | Sinovac | Inactivated virus, plus adjuvant | Phase-III |
| NVX-CoV2373 | Novavax | Protein subunit | Phase-II |
| mRNA-1273
|
Moderna
|
LNP-encapsulated mRNA vaccine encoding S protein | Phase-II |
| LV-SMENP-DC
|
Shenzhen Geno-Immune Medical Institute
|
DCs modified with lentiviral vector expressing synthetic minigene based on domains of selected viral proteins; administered with antigen-specific CTLs | Phase-II |
| Pathogen-specific aAPC
|
Shenzhen Geno-Immune Medical Institute
|
aAPCs modified with lentiviral vector expressing synthetic minigene based on domains of selected viral proteins | Phase-I |
Table 3: Development of various vaccines for the treatment of COVID-19 UpToDate [57]
| Name of the vaccine
|
Manufacturer | Properties | Approved | Trials |
| 1. Nuvaxovid | Novavax | Protein Subunit | Approved in 32 countries
|
11 trials in 7 countries
|
| 2.COVOVAX
|
Serum Institute of India(Novavax formulation) | Protein Subunit | Approved in 3 countries
|
2 trials in 1 country
|
| 3.Spikevax | Moderna | RNA | Approved in 85 countries
|
45 trials in 19 countries
|
| 4.Comirnaty | Pfizer/BioNTech | RNA | Approved in 137 countries
|
58 trials in 24 countries
|
|
5.Ad26.COV2. S |
Janssen
(Johnson& Johnson) |
Non-Replicated Viral Vector | Approved in 106 countries
|
18 trials in 18 countries
|
| 6.Vaxzevria | Oxford/AstraZeneca | Non-Replicated Viral Vector | Approved in 137 countries
|
56 trials in 30 countries
|
| 7.Covishield | Serum Institute of India
(Oxford/AstraZeneca formulation) |
Non-Replicated Viral Vector | Approved in 47 countries
|
2 trials in 1 country
|
| 8.Covaxin | Bharat Biotech | Inactivated | Approved in 13 countries
|
7 trials in 1 country
|
| 9. Sinopharm
|
(Beijing)
Covilo |
Inactivated | Approved in 88 countries
|
22 trials in 8 countries
|
| 10.CoronaVac | Sinovac | Inactivated | Approved in 53 countries | 29 trials in 9 countries
|
| 11. Sputnik V | Gamaleya | Non-Replicated Viral Vector | Approved in 74 Countries | 22 trials in many countries |
| 12. Sputnik Light | Gamaleya | Non-Replicated Viral Vector | Approved in 26
Countries |
4 trials in 2 countries |
| 13. EpiVacCorona | FBRI | Protein Subunit | Approvals in 4
Countries |
4 trials in 1 country |
| 14. KoviVac | Chumakov Center | Inactivated | Approvals in
3 Countries |
3 trials in 1 country |
| 15. Aurora-CoV | FBRI | Protein Subunit | Approvals in
1 Country |
2 trials in 1 country |
The advancement of Coronavirus vaccines is critical for the world to get back to pre-pandemic normalcy, and a total around the world effort has been put into protection against SARS-CoV-2.
Numerous vaccines have been arranged and broad preclinical examination has been conducted. It starts with animal models, studies, and those effective procedures to clinical preliminaries in humans. Under typical conditions, these vaccines can be approved for marketing after the stage III clinical preliminaries have completely confirmed that the vaccines are secure and effective. In any case, due to the criticalness of this pandemic. COVID-19 vaccines have been given restrictive, emergency, or momentary use authorization with high reconnaissance on their viability and safety profile post-authorization. While such advancement is promising, it is important to fortify the promotion of counteraction information and regulation measures to by and large control the spread of the pandemic with measures, for example, social separating, continuous hand washing, and cover wearing in open regions. The whole people ought to alter to these modern preventive measures to fix everything out, fundamentally until secured and powerful vaccines are made open to the overall population, especially those most vulnerable.
New Antiviral drugs
Presently, The FDA has approved two antiviral pills for the treatment of Coronavirus 19.
Paxlovid: (from Pfizer) is an oral antiviral treatment, that interferes with the ability of the coronavirus to replicate (Harvard Article). According to interim studies, Paxlovid significantly reduced the risk of COVID-related hospitalization and death compared to a placebo (Harvard Article). On December 22, 2021, the FDA authorized Paxlovid: (from Pfizer) itis an oral antiviral treatment, that interferes with the ability of the coronavirus to replicate (Harvard Article). According to interim studies, Paxlovid significantly reduced the risk of COVID-related hospitalization and death compared to a placebo (Harvard Article). On December 22, 2021, the FDA authorized 58.
Impact Of COVID-19
Health Care System and Education
The Coronavirus pandemic has affected the whole world. It has touched so many areas of individual lives, affecting the health of individuals and the scope of social activities. The capacity of health care systems is also challenged in the course of the current pandemic. It has also affected the education and working sectors 36
According to UNESCO (2020), almost 90% of the world’s understudy populace had encountered disturbance of their scholastic advancement because of the safety measures and strategies carried out by the public authority to forestall the spread of Coronavirus. This records for over 1.5 billion students in 165 nations. The standards of schooling framework which incorporate vis-à-vis homeroom experience has been supplanted by online training frameworks 37.
This has been depicted as the “biggest synchronous shock to all training frameworks in the course of our lives “by the Worldwide Head of Schooling [38]. The global wellbeing emergency because of Coronavirus has especially influenced the youth schooling frameworks. It has not just prompted uncommon and sensational changes in the existence of youngsters, yet in addition, their folks, educators, coaches, and different tutors engaged with molding the instructive society. Furthermore, the dread of falling youth training framework has called for approaches like for Coronavirus monetary bundles to ensure the equivalence 39-40.
Hence Coronavirus has not just suspended the ordinary youth exercises, including school participation, family communications, and outside exercises with companions, yet in addition, disturbed the social-enthusiastic advantages that kids get from taking part in these experiences. Small children are increasingly affected by this experience because they are naturally vulnerable to the belief that adults are capable of controlling the surrounding incidents. They are dependent on adults for their basic needs. Hence, a situation where the adults in the family cannot devise a coping mechanism to deal with the immediate and the adaptive demand of the situation leaves a strong impact on the basic confidence and analysis potential of a toddler.
In this specific circumstance, Xafis (2020) has noticed that the people who have most normally confronted foul play made by the maldistribution of influence, cash, and assets are the people most contrarily influenced. This is valid, especially for youngsters who are living in destitution and experience instabilities identified with food and asylum. Also, kids dwelling in distant regions or sidelined by the standard society (e.g., native individuals and transient laborers) are similarly influenced. What’s more, these pandemics compound the issues experienced by kids who are persistently sick or have handicaps, as well as are experiencing disregard or misuse 41.
As worried as these quick and perceptible results of Coronavirus might appear, truly the future holds a few difficulties in adapting to the drawn-out impacts of the pandemic. According to a researcher’s point of view, one might say that we are present “members in the greater impromptu analysis that schooling has ever had in the course of our lives” 42. On the positive side, a review has demonstrated further developing cleanliness rehearses among people during the Coronavirus pandemic. It has changed the disposition of individuals towards viral contaminations and they are currently more ready in going to preventive lengths 43.
Global Economy
COVID-19 pandemics related to sickness and mortality are lower than the circuitous misfortunes brought about by the emergency. Numerous nations are encountering a downturn, despite the fact that Coronavirus has not seriously affected them as far as wellbeing. In the presence of Coronavirus, the worldwide economy changes to an abrupt quit, causing shocks to the organic market. Beginning in January 2020, a large number of nations endured episodes of the new Covid, with each confronting epidemiological shocks that prompted monetary and monetary shocks as an outcome. The effect of Covid on the worldwide economy will stretch out past 2020. As per estimates from the Global Money related Asset and World Bank, Gross domestic product per capita toward the end of 2021 is as yet expected to be lower than December 2019 in many nations (Figure 4).
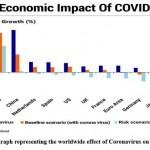 |
Figure 4: Barograph representing the worldwide effect of Coronavirus on economy [38] |
The impact of the Corona pandemic on global financial markets / financial globalization shows very clearly due to some factors, such as low oil prices, feelings of fear, and panic which affected investors because of the pandemic. Lack of control significantly decreased the global economy and also what caused it to slow down the failure of the production machine, the best example of this scenario is China. 38
Artificial Intelligence Effects
The implementation of artificial intelligence has played a very important role during this pandemic, by helping scientists with information related to testing methods, machine learning, and data mining tools. These tools have impacted the COVID-19 advance research in a way that has led to an intellectually demanding society36. COVID-19pandemic has most intriguing challenged the theories and practices of infectious disease control and prediction.
Security
Interpol revealed an increment in counterfeit clinical items to battle against the Coronavirus pandemic, to get quick money. The lawbreakers are exploiting higher market interest for individual assurance and cleanliness items 44.
Management In Human Life
The elective evaluating models for Coronavirus treatment were delivered by the Institute for Clinical and Economic Review (ICER). As indicated by ICER, the primary course of ordinary Coronavirus treatment with Remdesivir was $US10 for an a10-day treatment course. The subsequent model represented a limit of $US50,000. This ICER-Coronavirus model demonstrated the mortality advantage of a10-day treatment course from the ACCT. The inexact cost of this treatment was recommended to be $4500. It is consequently recommended by ICER that the clinical proof, vulnerability, and cost-viability examination ought to be seen with alertness. They further demonstrated that the arrangement creator’s ought to consider lower limits by gauging factors like vulnerability and reason ableness to advance prompt and expansive utilization of such models 45.
According to different examinations, there exist six strategy choices for estimating the clever Coronavirus antibodies and medicines. They offer various methodologies relying upon the job of government and the private market. American researchers have inferred that Coronavirus treatment is generously higher than other normal irresistible diseases. It has been seen that financially tested gatherings are relying upon the more established type of medications for the treatment of COVID-19.
However, several acute side effects of these drugs have been reported. Hence it is crucial to increase access to safe and effective opioid agonist treatment (OAT) and prevent the consumption of harmful drugs during this pandemic. In this effect, Ireland opted for one of the best methods for overcoming the challenges of the COVID-19 pandemic. They delivered telephonic and video consultation to save the time and energy of healthcare professionals as well as the patients 46
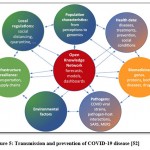 |
Figure 5: Transmission and prevention of COVID-19 disease [52] |
Implications On Research
COVID-19 is a serious health problem. Most patients of COVID-19 show positive results by RT-PCR assays. However, many patients show multiple negative tests of the same assay. This may be because these patients are carriers of the virus, and the collected samples do not contain the required titer of these viruses that is necessary for detection. It is prescribed to do the full clinical test and knows the patient’s openness history, clinical indications, lab tests, and common imaging discoveries like chest radiography and CT examination. These elements assume a fundamental part in making primer conclusions and guide early disengagement and treatment. The RT-PCR is suggested for the treatment of the patients and avoidance of spreading of disease.
It can be summarizedCOVID-19 has an impact on current health system challenges, education, security, management, global business, culture, and other aspects of human life. The fundamental goals of this paper are to give mindfulness and recognize the examination regions identified with Coronavirus 19 by identifying the best methods to minimize the negative impact on these aspects of controlling the pandemic COVID-19. Clinical studies should continue for the antiviral drug investigation and its limitations. And also accelerate the study of vaccinations.
Result and Discussion
COVID-19 pandemic is a public health crisis of global concern. Our study emphasizes the global health, education system, economy, security, management, and other aspects of human life which are impacted by COVID-19.
It is required to concentrate on minimizing the negative effects of this pandemic by protecting yourself from the virus by social distancing, masking, and isolation of infected individuals as well as taking care of our immune system.
There are many points and destinations which assessed, for the model’s history of pandemics, SARS-CoV2, Serious Intense Respiratory Disorder (SARS), Center Eastern Respiratory Infection (MERS), Developmental beginning, Transmission of the infection, Pathophysiology, Clinical show, Testing, Therapy and Suggestions for research. Additionally referenced with regards to the harmful impacts of the ordinarily utilized medications for the treatment of Coronavirus and the intriguing models for limiting these impacts and further studies.
It is likewise imperative to take note of the treatment of Coronavirus 19. Greatly depends on the immunity system of the patients for asymptomatic, mild, moderate, severe, and critical cases. The advanced cases, however, need personal attention and medical care. Due to socio-economic ramifications on society and also affecting all aspects of life, the future exploration will be multi-disciplinary and trans-national.
This is another influx of exploration into the natural and the clinical sciences, to expand the accessibility of antiviral medications and assess the degree of poisonousness of these drugs. This is for the prosperity of the civilized.
Future Perspective
The EU leaders have agreed on its reconstruction after the COVID-19 plane on21 July 2020. In this regard, they have announced a profoundly expected arrangement, the ‘Cutting edge EU’, to together get €750 billion to react to the Covid pandemic.
New a few methodologies are being examined to work on the action and decrease the unwanted results of antiviral medications executed for the treatment of Coronavirus patients since. Researchers are searching for remedial regimens with the demonstrated adequacy and endorsement of immunizations. They are likewise looking for new medications, mixes of medications, and more current conveyance techniques.
One such technique includes a novel approach to targeting drugs more specific at the cellular level to maximize the antibody response through nanoparticles to produce effective treatment measures with minimum side effects. Moreover, there are numerous ranges of inquiry about required with respect to especially undertake extensive clinical research area which will be significantly impacted on this pandemic, which leads to a normalized life of the human being. Appealing to WHO and government institutions to create a health system to protect human beings from viral infection by transmission and put the suitable treatment for all human beings in entering the world without exception.
Conclusion
Presently, COVID-19 could be a ‘once in a lifetime’ widespread, that has affected hundreds of nations all throughout the planet causing noteworthy misfortune of property and life. There is no compelling treatment for Coronavirus. Infected people are approached to remain in segregation while getting treatment for their indications. Since every one of the three zoonotic coronaviruses is comparative, it is essential the world keeps on pursuing a fight against an imperceptible adversary and the fight must be won completely with the help of science, to also appreciate the pathogenesis and medicinal focal points of Covid, a greater number of animal investigations and clinical starters are required.to gain from SARS and MERS to see how these might be identified with this new flare-up. Which lead to manufacturing more vaccines, and speeding up the distribution of it for all countries. Probiotics play a very important role in both the prevention and treatment of COVID-19. Also, treatment strategy by antiviral drugs chloroquine /hydroxychloroquine & respiratory therapy. Additionally, by the utilization of logically demonstrated treatments, which is in manufacturing processes.
Acknowledgment
I wish to acknowledge Princess Tharwat University College, affiliated with Balqa Applied University, Muhawish Yusuf, Amman, Jordan, for providing help, support, and assistance throughout my study. I am grateful to (Dean), and (Vice-Dean) of the Princess Tharwat University College, affiliated to Balqa Applied University, Muhawish Yusuf, Amman, Jordan for making available research, and development facilities to undertake the present work. And Technical support with regard to the computation of the data is gratefully acknowledged.
Conflict of Interest
The authors declare that there are no conflicts of interest relevant to this article.
Funding Source
There is no fund was received.
References
- Kupferschmidt, K., and Cohen, J. (2020). Can China’s COVID-19 strategy work elsewhere? Science, 367,1061–2. doi: 10.1126/science.367.6482.1061.
CrossRef - Deng, S. Q., and Peng, H. J. (2020). Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. Journal of Clinical Medicine, 9(2), 575. doi: 10.3390/jcm9020575.
CrossRef - Spector-Bagdady, K. (2020). Hospitals should act now to notify patients about research use of their data and biospecimens. Nature Medicine, 26(3), 306-308. doi: 10.1038/s41591-020-0795-6.
CrossRef - Lewis, IK., Zhou, M., and Wang, EJY. (2020). The China Experience – Understanding the Evolution of the COVID-19 Crisis: Perspectives & Events: Mayer Brown,” Perspectives & Event Mayer Brown, Retrieved November 01, 2020, from https://www.mayerbrown.com/en/perspectives-events/publications/2020/03/the-china-experience-understanding-the-evolution-of-the-covid-19-crisis.
- Kraemer, M. U., Yang, C. H., Gutierrez, B., Wu, C. H., Klein, B., Pigott, D. M., … Brownstein, J. S. (2020). The effect of human mobility and control measures on the COVID-19 epidemic in China. Science, 368(6490), 493-497. doi: 10.1126/science. abb4218.
CrossRef - Gorbalenya, A. E., Baker, S. C., Baric, R. S., de Groot, R. J., Drosten, C., Gulyaeva, A. A., … and Penzar, D. (2020). The species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology, 5, 536–544. doi: 10.1038/s41564-020-0695-z.
CrossRef - Henry R. (2020). Etymologia: Coronavirus. Emerging Infectious Diseases, 26(5), 1027. doi: 10.3201/eid2605.190940.
CrossRef - Shen, M., Zhou, Y., Ye, J., Al-Maskri, A. A. A., Kang, Y., Zeng, S., and Cai, S. (2020). Recent advances and perspectives of nucleic acid detection for coronavirus. Journal of Pharmaceutical Analysis. doi: 10.1016/j.jpha.2020.02.010.
CrossRef - Noh, J. Y., Yoon, S. W., Kim, D. J., Lee, M. S., Kim, J. H., Na, W., … and Kim, H. K. (2017). Simultaneous detection of severe acute respiratory syndrome, Middle East respiratory syndrome, and related bat coronaviruses by real-time reverse transcription PCR. Archives of Virology, 162(6), 1617-1623. doi: 10.1007/s00705-017-3281-9.
CrossRef - Huang, P., Wang, H., Cao, Z., Jin, H., Chi, H., Zhao, J., … and Jiao, C. (2018). A rapid and specific assay for the detection of MERS-CoV. Frontiers in Microbiology, 9, 1101. doi: 10.3389/fmicb.2018.01101.
CrossRef - De Wit, E., Van Doremalen, N., Falzarano, D., and Munster, V. J. (2016). SARS and MERS: recent insights into emerging coronaviruses. Nature Reviews Microbiology, 14(8), 523. doi: 10.1038/nrmicro.2016.81.
CrossRef - Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., … and Cheng, Z. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497-506. doi: 10.1016/S0140-6736(20)30183-5.
CrossRef - Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., … and Zhao, Y. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Journal of the American Medical Association, 323(11), 1061-1069. doi: 10.1001/jama.2020.1585.
CrossRef - Dong, Y., Mo, X., Hu, Y., Qi, X., Jiang, F., Jiang, Z., and Tong, S. (2020). Epidemiology of COVID-19 among children in China. Pediatrics, 145(6). doi: 10.1542/peds.2020-0702.
CrossRef - Wu, Z., and McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Journal of the American Medical Association, 323(13), 1239-1242. doi: 10.1001/jama.2020.2648.
CrossRef - (2020). Coronavirus (COVID-19) Update: FDA Reiterates Importance of Close Patient Supervision for ’Off-Label’ Use of Antimalarial Drugs to Mitigate Known Risks, Including Heart Rhythm Problems. Retrieved November 01, 2020, from https://www.fda.gov/news-events/press-announcements/coronaviruscovid-19-update-FDA-reiterates-importance-close-patient-supervision-label-use 803472005.
- Gérard, A., Romani, S., Fresse, A., Viard, D., Parassol, N., Granvuillemin, A., … and Drici, M. D. (2020). “Off-label” use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: a survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers. Therapies. 371-379. doi: 10.1016/j.therap.2020.05.002.
CrossRef - Chandler, R. E., McCarthy, D., Delumeau, J. C., and Harrison-Woolrych, M. (2020). The Role of Pharmacovigilance and ISoP During the Global COVID-19 Pandemic. Drug Safety, 1. doi: 10.1007/s40264-020-00941-4.
CrossRef - Davies, M., Osborne, V., Lane, S., Roy, D., Dhanda, S., Evans, A., and Shakir, S. (2020). Remdesivir in Treatment of COVID-19: A Systematic Benefit–Risk Assessment. Drug Safety, doi: 10.1007/s40264-020-00952-1.
CrossRef - European Medicines Agency. (2020). EMA Advises Continued Use of Medicines for Hypertension, Heart or Kidney Disease During COVID-19 Pandemic. Retrieved November 01, 2020, https://www.ema.europa.eu/en/news/ema-advises-continued-use-medicineshypertension-heart-kidney-disease-duringcovid- 19-pandemic 803467953.
CrossRef - US Food and Drug Administration. (2020). The FDA advises patients on the use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19. Retrieved November 01, 2020, https://www.fda.gov/drugs/drug-safety-and- availability/FDA-advises-patients- use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19.
- European Medicines Agency (EMA). (2020). EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19. Retrieved November 01, 2020, from https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19.
- European Medicines Agency (EMA). (2020). COVID-19: reminder of the risks of chloroquine and hydroxychloroquine. Retrieved November 01, 2020, from https://www.ema.europa.eu/en/ news/ covid-19-reminder-risks-chloroquine-hydroxychloroquine.
- Danish Medicines Agency. (2020). Clinical trials of hydroxychloroquine are stopped temporarily. Retrieved November 01, 2020, from URL: https:// laegemiddelstyrelsen. Deck/en/news/2020/clinical-trials-of-hydroxychloroquine-are-stopped-temporarily/ 803479840.
- Guidon, A. C., and Amato, A. A. (2020). COVID-19 and neuromuscular disorders. Neurology, 94(22), 959-969. doi: 10.1212/WNL.0000000000009566.
CrossRef - European Medicines Agency (EMA). (2020). EMA advises continued use of medicines for hypertension, heart or kidney disease during a COVID-19 pandemic. Retrieved November 01, 2020, from https://www.ema.europa.eu/en/news/ema-advises-continued-use-medicines-hypertension-heart-kidney-disease-duringcovid-19-pandemic 803467953.
CrossRef - US Food and Drug Administration. (2020). The FDA advises patients on the use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19. Retrieved November 01, 2020, from https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-nonsteroidal-anti-inflammatory-drugs-nsaids-covid-19.
CrossRef - European Medicines Agency (EMA). (2020). EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19. Retrieved November 01, 2020, from https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19. 803466172.
CrossRef - Beyls, C., Martin, N., Hermida, A., Abou-Arab, O., and Mahjoub, Y. (2020). Lopinavir-ritonavir treatment for COVID-19 infection in intensive care unit: risk of bradycardia. Circulation: Arrhythmia and Electrophysiology, 13(8), e008798. doi: 10.1161/CIRCEP.120.008798.
CrossRef - Mancia, G., Rea, F., Ludergnani, M., Apolone, G., and Corrao, G. (2020). Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. New England Journal of Medicine, 382, 2431-2440. doi: 10.1056/NEJMoa2006923.
CrossRef - Reynolds, H. R., Adhikari, S., Pulgarin, C., Troxel, A. B., Iturrate, E., Johnson, S. B., … and Katz, S. D. (2020). Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. New England Journal of Medicine, 382, 2441-2448. doi: 10.1056/NEJMoa2008975.
CrossRef - Jarcho, J. A., Ingelfinger, J. R., Hamel, M. B., D’Agostino Sr, R. B., and Harrington, D. P. (2020). Inhibitors of the renin–angiotensin–aldosterone system and Covid-19. New England Journal of Medicine,382, 2462-2464. doi: 10.1056/NEJMe2012924.
CrossRef - Borba, M. G. S., Val, F. F. A., Sampaio, V. S., Alexandre, M. A. A., Melo, G. C., Brito, M., … and Hajjar, L. A. (2020). Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA network open, 3(4), e208857-e208857. doi: 10.1001/jamanetworkopen.2020.8857.
CrossRef - Fihn, D., Perencevich, E., and Bradley, S. M. (2020). Caution needed on the use of chloroquine and hydroxychloroquine for coronavirus disease 2019. JAMA network open, 3(4), e209035-e209035. doi: 10.1001/jamanetworkopen.2020.9035.
CrossRef - Shafaat, IN. (2020). Serious cardiac AEs with hydroxychloroquine during COVID-19 pandemic. Reactions Weekly, 1806(1), 9. doi: 10.1007/s40278-020-79027-9.
CrossRef - Editor’s Note: Applied Intelligence and COVID-19 Research. (2020).Applied Intelligence, 1. Retrieved November 01, 2020, from https://doi.org/10.1007/s10489-020-01721-4. doi: 10.1007/s10489-020-01721-4.
CrossRef - United Nations Educational, Scientific and Cultural Organization (UNESCO) Web Site (2020). Retrieved November 01, 2020, from https://en.unesco.org/news/covid-19-educational-disruption-and-response.
- Rogers, F. H., and Sabarwal, S. (2020). The COVID-19 Pandemic: Shocks to Education and Policy Responses (Spanish). Washington, D.C.: World Bank Group. http://documents.worldbank.org/curated/en/804001590734163932/The-COVID-19-Pandemic-Shocks-to-Education-and-Policy-Responses.
- National Association for the Education of Young Children (2020). NAEYC COVID 19 statement. Retrieved November 01, 2020, from https://www.naeyc.org/sites/default/files/globally shared/downloads/PDFs/resources/topics/PS_technology_WEB.pdf.
- Zero to Three Website (2020). Retrieved November 01, 2020, from https://www.zerotothree.org/resources.
- Xafis, V. (2020). ‘What is Inconvenient for You is Life-saving for Me’: How Health Inequities are playing out during the COVID-19 Pandemic. Asian Bioethics Review, 1. doi: 10.1007/s41649-020-00119-1.
CrossRef - Thomas, M. S., and Rogers, C. (2020). Education, the science of learning, and the COVID-19 crisis. Prospects, 1. doi: 10.1007/s11125-020-09468-z.
CrossRef - Vandormael, A., Adam, M., Greuel, M., and Bärnighausen, T. (2020). A short, animated video to improve good COVID-19 hygiene practices: a structured summary of a study protocol for a randomized controlled trial. Trials, 21(1), 469. https://doi.org/10.1186/s13063-020-04449-1. doi: 10.1186/s13063-020-04449-1.
CrossRef - (2020). Global operation sees a rise in fake medical products related to COVID-19. Retrieved November 01, 2020, from https://www.interpol.int/en/News-and-Events/News/2020/Global-operation-sees-a-rise-in-fake-medical-products-related-to-COVID-19#:~:text=Criminals%20are%20cashing%20in%20on%20COVID%2D19&text=Law%20enforcement% 20agencies%20taking%20part,during%20the%20week%20of%20action.
CrossRef - Hill, A., Wang, J., Levi, J., Heath, K., and Fortunak, J. (2020). Minimum costs to manufacture new treatments for COVID-19. Journal of Virus Eradication, 6(2), 61. doi: 10.1016/S2055-6640(20)30018-2.
CrossRef - Crowley, D., and Delargy, I. (2020). A national model of remote care for assessing and providing opioid agonist treatment during the COVID-19 pandemic: a report. Harm Reduction Journal, 17(1), 1-5. doi: 10.1186/s12954-020-00394-z.
CrossRef - Wilder-Smith, A., Chiew, C. J., and Lee, V. J. (2020). Can we contain the COVID-19 outbreak with the same measures as for SARS. The Lancet Infectious Diseases. doi: 10.1016/S1473-3099(20)30129-8.
CrossRef - Zou, H., Shu, Y., and Feng, T. (2020). How Shenzhen, China avoided widespread community transmission: a potential model for successful prevention and control of COVID-19. Infectious diseases of poverty, 9(1), 1-4. doi: 10.1186/s40249-020-00714-2.
CrossRef - Grasselli, G., Zangrillo, A., Zanella, A., Antonelli, M., Cabrini, L., Castelli, A., … and Iotti, G. (2020). Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. Journal of the American Medical Association, 323(16), 1574-1581. doi: 10.1001/jama.2020.5394.
CrossRef - Le, T. T., Cramer, J. P., Chen, R., and Mayhew, S. (2020). Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov, 19(10), 667-8. doi: 10.1038/d41573-020-00151-8.
CrossRef - Vaccine Centre, London School of Hygiene and Tropical Medicine. (2020). Retrieved November 01, 2020, from https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/.
- COVID-19 vaccine tracker, Milken Institute Website. (2020). Retrieved November 01, 2020, from https://covid-19tracker.milkeninstitute.org/
- World Health Organization (WHO). (2020). DRAFT landscape of COVID-19 candidate vaccines. World.
- Zinovkin, R. A., and Grebenchikov, O. A. (2020). Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochemistry (Moscow), 85(7), 833-837. doi: 10.1134/S0006297920070111.
CrossRef - Salman, J. A. S.; Mahmood, N. N.; Abdulsattar, B. O.; and Abid, H. A. (2020). The effectiveness of probiotics against viral infections: A rapid review with focus on SARS-CoV-2 infection. Open Access Macedonian Journal of Medical Sciences; 8(T1):496-508, 2020. https://doi.org/10.3889/oamjms.2020.5483.
CrossRef - Yuxin, Y., Yoongxin, P., Zhuoyi, L., Ruiqi, W., Xinyun, W., Chong, Y., Haitao Z., Sivakumar, M., Edward, L., Tao, W., and Cheng, H. P. (2021). The COVID-19 Vaccines: Recent Development, Challenges and Prospects. Vaccines (Basel). 2021 Apr; 9(4): 349. doi: 10.3390/vaccines9040349.
CrossRef - World Health Organization (WHO). (2022). Covid-19 Track Vaccines. Last Updated 2 February 2022, from https://covid19.trackvaccines.org/agency/who/.
- Harvard Article (2022). Treatments for COVID-19 January 31, 2022, from https://www.health.harvard.edu/diseases-and-conditions/treatments-for-covid-19

This work is licensed under a Creative Commons Attribution 4.0 International License.





