How to Cite | Publication History | PlumX Article Matrix
Electrospun Collagen Based Nanofibrous Mats for Wound Healing: An Integrative Review
Mugdha A. Kulkarni , Anoushka R. Gangal
, Anoushka R. Gangal , Sameeha S. Khare
, Sameeha S. Khare , Harshal G. Mundada
, Harshal G. Mundada , Ashwini R. Gawade
, Ashwini R. Gawade and Rohini R. Pujari*
and Rohini R. Pujari*
School of Pharmacy, Dr. Vishwnath Karad MIT World Peace University, Kothrud, Pune- 411038, India, Maharashtra.
Corresponding Author E-mail: rohinirpujari@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3006
ABSTRACT: Wound healing has always been an important issue that needs to be addressed, especially where second-and third-degree burns are considered. These types of burns extend to the dermis in second-degree burns and the tissues in third-degree burns and this process is extremely slow. Hypertrophic scarring and infection which are caused due to decreased immunity are some of the obstacles that need to be tackled. Recent studies show that fish collagen is useful in preventing infections due to its natural antimicrobial properties. Increased cellular respiration, exudate removal and retention of moisture are a few events that promote wound healing and these are aided by electrospun nanofibrous mats. Combining the properties of collagen into the nanofibrous mats in order to facilitate wound healing can potentially serve as an alternative to the current wound healing therapies. This article covers the various types of collagen that can be used with a focus on the synthetic polymeric blending into the collagen structures and its electrospinning process.
KEYWORDS: Burns; Collagen; Electrospinning; Nanofibrous Mats; Scaffolds; Wound Healing
Download this article as:| Copy the following to cite this article: Kulkarni M. A, Gangal A. R, Khare S. S, Mundada H. G, Pujari R. R. Electrospun Collagen Based Nanofibrous Mats for Wound Healing: An Integrative Review. Biosci Biotech Res Asia 2022;19(2). |
| Copy the following to cite this URL: Kulkarni M. A, Gangal A. R, Khare S. S, Mundada H. G, Pujari R. R. Electrospun Collagen Based Nanofibrous Mats for Wound Healing: An Integrative Review. Biosci Biotech Res Asia 2022;19(2).Available from: https://bit.ly/3mBQYKs |
Introduction
One of the largest organs in our body is the skin and is made up of the epidermis, dermis and hypodermis. All of these three layers have different functions. Skin is the body’s primary defense that protects the internal organs from external damage.1
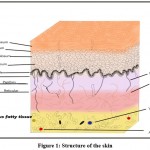 |
Figure 1: Structure of the skin. |
The four kinds of cells present in the epidermis are:
Keratinocytes
Melanocytes
Langerhans cells
Merkel cells
Stratum basale is made up of cuboidal or columnar cells that actively undergo mitosis and produce keratinocytes. Melanocytes are also found in this layer. Stratum spinosum contains dendritic cells. Diamond-shaped cells containing keratohyalin are present in stratum granulosum. Eliedin, which is obtained on the transformation of keratohyalin is found in the cells of stratum lucidum. The stratum corneum is the topmost layer and consists of dead keratinocytes.2
The main function of keratinocytes is to produce keratin as well as the secretion of lipids, which act as a water barrier. They also regulate calcium absorption for the production of vitamin D. Melanocytes produce melanin which is responsible for skin pigmentation and protects the skin from UV B rays. Langerhans cells or dendritic cells are a part of the mononuclear phagocytic system. Merkel cells which act as mechanoreceptors are present in the stratum basale.2.3
The dermis contains two layers, papillary and reticular tissue. Loose connective tissue is present in the papillary layer, while the reticular layer is made up of dense connective tissue. This layer is composed of sweat glands, hair follicles, sensory neurons and blood vessels. The hypodermis is mainly composed of adipose globules.3
Wound Healing
It is a complex and systematic process that is divided into four parts.
Hemostasis
In the hemostatic phase, a blood clot is formed by aggregation of platelets, which are activated due to contact with collagen.
Inflammatory phase
The inflammatory phase includes the production of a bed which facilitates tissue growth. On activation of the immune system, the first step in response to the injury is degranulation of platelets and mast cells at the site of injury. Proinflammatory mediators such as IL-1, IL-6, IL-8 (interleukins), TNFα (Tumor necrosis factor alpha), PAF (Platelet activating factor), Interferon-γ, PG’s (Prostaglandins), LT’s (Leukotrienes), activate immune cells such as macrophages. Neutrophils followed by macrophages enter the site of injury to clear out any bacteria and debris and thus cause inflammation.
Proliferative phase
The proliferative phase can be further divided into three stages a) filling of the wound b) constriction of margins c) epithelialization. The wound is filled by connective tissue and angiogenesis is the formation of new blood vessels occurs. This is followed by contraction of the wound due to actin-positive myofibroblasts.
Remodeling
In the final phase of wound healing, the wound begins to contract due to contractile fibroblasts. The other prominent step is the collagen remodeling that occurs through the rearrangement of collagen fibers. It constitutes the most dominant and abundant macro-protein present in the extracellular matrix which surrounds all the connective tissues in our body. Along with collagen and other macromolecular components like glycoproteins, proteoglycans, elastin fibers and growth factors, this three-dimensional fibrinous matrix functions to provide structural integrity and also plays a part in major cellular functions like adhesion, migration and differentiation.4,5
Factors affecting steps in wound healing
Hemostasis: Some drugs such as Aspirin and Ibuprofen are responsible for anti-platelet action. Chemotherapeutic agents are known to cause abnormal fibroblast proliferation as well.
Inflammatory phase: While inflammation is important to get rid of any contaminants, such a prolonged state can cause an increase in the cytokine and interleukin levels and thus stop efficient wound healing. Inflammation can be increased due to bacterial infections. For example, in chronic ulcers, Bacteria such as Pseudomonas aeruginosa can avoid attacks by polymorphonuclear neutrophils. Prolonged hypoxia can also contribute to delayed wound healing in cases of diabetes mellitus by increasing the inflammatory phase.
Proliferative phase: Drugs such as glucocorticoids that reduce inflammation, suppress proliferation of cells, reduce wound contraction as well as reduce collagen synthesis.
Other factors that have an overall systemic effect are obesity, stress, alcohol consumption, nutrient deficiency
Thus, from the above examples, the major limitations of wound healing can be summarized as less cell proliferation, an increase in cytokines due to inflammation. If we consider natural wound healing, challenging factors such as poor oxygenation, bacterial infections, age and co-morbidities and medications come into play.6,7
Treatment strategies for wound healing
Acute wound
The current treatment scenario for acute wound healing is relatively easier as compared to chronic wounds. The steps in treatment are as follows:
Cleaning the wound.
Pain management
Dressing for open wounds.
The dressing for open wounds varies according to what the injury is. For example, skin flaps should be covered by a non-adhesive dressing. Hydrocolloid dressings are used to cover skin graft injuries and a simple gauze for wounds that leak blood or wound fluids.8,9
Chronic wound
Chronic injury treatments are more complicated as they reach deeper into the skin and sometimes even into the subcutaneous fat tissue as in the case of severe burns. The typical type of care required is taking swabs of the wound to detect infections, cleaning, dressing and may require debridement of the wound.
The treatment options include:
Split thickness autograft
Here, a graft from the donor’s site is placed over the wound, but this results in a lot of pain and there is a risk of infection. The potential of the cells to proliferate varies with age which is why it is not the best option.
Hyaluronic acid dressings
These are dressings that play a role in angiogenesis and inflammatory response but pose a challenge in patients with comorbidities.10,11
Collagen and wound healing
Collagen has an indispensable role in our bodily processes. Along with being necessary for joints and skin elasticity, all stages of wound healing are also highly receptive to the extracellular matrix (ECM), especially collagen. In the initial wound healing stage, platelets that mediate the hemostasis in blood vessels, get activated only after interacting with collagen by activation of its two collagen receptors, integrin a2b1 and GPVI (Glycoprotein VI). This further enables fibrin clot deposition at the site. The wound collagen degradation liberates more fragments which cause the fibroblasts to proliferate and also help release the growth factors which help in new blood vessel formation and re-epithelialization. The fibroblasts which get recruited in the inflammatory stage also contribute to collagen deposition.
The significance of collagen is that it helps in:
Breakdown of the damaged tissue at the site of the wound (autolytic debridement)
Facilitation of the formation of new blood vessels (angiogenesis)
Formation of new epithelium (re-epithelialization)12,13
Structure of collagen
It is composed of three alpha polypeptide chains, arranged to form a right-handed triple helix. The collagen fibers length is around 280 nm and weighs around 300kDa. Amino acid, glycine is present as every third residue in the structure with the other two being proline and 4-H proline residues, in most of the cases. The three alpha peptide chains coil around in such a way that all the glycine residues lie along a central axis, which makes glycine very important for collagen stabilization. In addition, glycine also provides a -NH group which makes hydrogen bonding and cross-linking possible in the structure expansion. Based on the presence of identical or non-identical alpha chains, the trimer formed can be a homotrimer or a heterotrimer. This difference along with changes in the amino acid sequence forms the basis of the existence of different structural types of collagens in our body. Collagen types II, III, VII, VIII and X are composed of homo-trimers while types I, IV, V, VI, IX and XI are made of hetero-trimers.5,14
Further, they can also be categorized based on their function or structural features into different families such as:
Fibril-forming collagens (I, II, III, V, XI)
Fibril-associated collagens (IX, XII, XIV, XVI)
Collagen forming hexagonal networks (VIII and X)
Basement membrane-associated type IV family
Type VI family, forming beaded collagens.6,15
Amongst all, type I fibril-forming collagen, a heterotrimer composed of two equivalent α1 and one α2 chains, makes 90% of all the collagen in the body and is also functionally the most important subtype in tissue regeneration and wound healing. Its superiority is backed by its characteristics like excellent chemical and thermal stability, mechanical strength and presence of intra-strand hydrogen bonds at secondary and tertiary structural levels. The abundance and ease of extraction of specifically collagen I from porcine and bovine sources is another coincidence that has been exploited for the fabrication of different collagen dressings for accelerated wound healing in humans. To date, it is the most widely used subtype used as a surrogate ECM matrix in tissue engineering. Collagen III and V are present at similar locations as type I but in fewer numbers. Collagen II meanwhile, is mainly found in the cartilaginous tissues near bones. They are made use of in the making of ECM-mimicking matrix but it is limited to an extent.
Hence, broadly, the fibril-forming collagen family has been widely sought after for its application in chronic burns and wounds where rapid healing with the aid of exogenous collagen natural scaffolds can be extremely beneficial.16,17
Collagen extraction
Collagen naturally has a water-insoluble nature due to the crosslinking in its structure. The soluble collagen is only preferably found in the skin of young animals. Hence, different methods like salt precipitation, acidic treatment or enzymatic isolation are implemented to extract the collagen. It is followed by careful steps to eliminate any virus or pathogen that may be present in the raw collagen so that the transmission of disease can be avoided.16
Salts like NaCl have been used to precipitate the collagen, but the efficiency was found to be low due to crosslinking. Inorganic (HCl) and organic (acetic acid, citric acid) acids break the crosslinking bonds and help collagen solubilization. They also allow the preservation of the triple helical structure while extraction. This is achieved by keeping the terminal telopeptides of the collagen intact. Dilute acetic acid is especially preferable for type I collagen.18
Meanwhile, the addition of acidic solutions of enzymes like papain, tryptase or pepsin also breaks the bonds of collagen, lowering the tediousness of the process. Unlike the former method, this treatment causes hydrolysis of the terminal telopeptides. This obtained collagen, called atelocollagen, proves to be more processable. It additionally does not impart any hypersensitivity or adverse reactions, which cannot be denied completely in the case of pure intact collagen due to its animal origin.19
Extraction needs to be done carefully as all crucial properties of the collagen are subject to differ on the basis of the efficiency with which the process is carried out. For a successful extraction, proper cleaning of the animal hides or bones, its alkaline pretreatment, and complete collagen solubilization is necessary along with further purification, demineralization and defatting of the obtained collagen.8,18 Another factor that plays a decisive role in the quality of collagen is its source. The use of human collagen is limited due to the lack of availability of huge amounts of raw collagen. Recombinant processing of collagen has been worked on but alternatives were necessary because it proved to be a very costly process commercially. This brought the extraction of animal-based collagen to the forefront.9,18,19
Sources of collagen
Bovine, porcine and equine are the most used sources while chicken-derived, human-derived and recombinant collagen are amongst the newer and alternative sources for collagen isolation and extraction. Lately, marine species like fish, sharks, sea urchins and even jellyfish have been looked upon as promising sources of collagen due to no reported event of transmitted disease, lack of religious constraints and good quality of collagen. Waste from fish processing industries like bones and scales are revealed to have a good concentration of collagen and are also being studied as a potential source.20,21
Detailed information about the animal and marine-derived collagen5,20,21 is included in tables 1 and 2.
Table 1: Animal sources of collagen.
| Primary animal source | Parts used | Type of collagen used | Specifications |
| Bovine | Achilles tendon and bone | Collagen 1 | The amount of collagen isolated depends on the age of the bovine tissue; young tissue yields more amount. Bovine collagen shows good biocompatibility and illicit negligible immune response in normal populations. |
| Nasal an articular cartilage | Collagen 2 | ||
| Skin | Collagen 3 | ||
| Placental Villi | Collagen 4 | ||
| Porcine | Bones, skin and small intestinal mucosa | Collagen types 1 and 3 | Porcine collagen is known to be almost similar to the human collagen and no allergic response has been noted. |
| Equine (horse) | Skin, articular cartilage, flexor tendon, adult equine pericardium | Collagen 1 and 2 | Used for tendon reinforcement, skin and wound healing and hernia |
| Chicken | Chicken Neck | Collagen types 1, 2, 3 and 4 | Chicken derived collagen has been looked upon as an alternative source because of religious constraints, allergies and transmissible diseases related to bovine and porcine sources. |
| Skin | Collagen 1 and 3 |
Table 2: Marine sources of collagen.
| Marine Invertebrates | Fishes | Marine by-products |
| Jellyfish | Atlantic Salmon | Skin of Japanese Sea bass |
| Sea cucumber | Cod | Bladder of yellow fish Tuna |
| Squids | Olive flounder | Scales, skin, bones, and bladder of Carp |
| Starfish | Catfishes | Cartilages of Octopus, Sea urchin |
| Sponges | Nile Tilapia | Skin of Bullhead Shark |
| Crustaceans | Haddock | Skin of Mackerel |
| Mussels | Blacktip shark | Scales of Nile Tilapia |
Collagen for scaffolds and tissue engineering
Scaffolds which can be called biopolymeric substitutes for the endogenous biological components are extensively used to offer structural support, hasten tissue repair and cell proliferation in cases of severe wound healing.22 Collagen-made scaffolds, it is found, provide a promising microenvironment similar to that of the ECM and can directly help in tissue regeneration in case of chronic wounds and burns. They can act in a similar way to the endogenous collagen thus aiding healing greatly. In addition, they are extremely flexible and can be used in the form of sheets, foams, fleeces and nanomats in modern tissue engineering.23
Designing of scaffolds
Pure collagen
Pure collagen from the listed sources has long been studied as a potential scaffold in tissue healing but the main problem lies in its very low mechanical strength to sustain further manufacturing processes. They also show rather poor thermal and solvent stability. This has shifted the focus to inducing crosslinking between the fibers so that the former shortcomings can be overcome.24
Physical methods like elevated temperature vacuum treatment and UV crosslinking in pure collagen nanofibers are well known. In UV irradiation, a photo-initiator decomposes in presence of UV light to release ions that end up inducing a double bond between the collagen fibers.9 In chemical methods, citric acid has been experimented upon as a crosslinking agent in marine collagen and the results obtained were promising.25 Few other works have reported using quaternary ammonium organosilane (QOS) as a cross-linking agent which can also confer antimicrobial properties in addition to increasing the electrospinnability of the fibers. QOS is mixed with collagen in solution form and then treated on a desiccator with the ammonium carbonate powder which yields siloxane, the main agent involved in crosslinking.26
Other cross-linking chemical agents that have been researched and used are glutaraldehyde, dialdehyde starch, an iridoid glucoside named Genipin and Chitosan. Even, enzymatic treatment using lysyl oxidase and microsomal transglutaminase has been used to induce crosslinking.
In regards to electrospinning, HFIP (1,1,1,3,3,3 hexafluoro-2-propanol) has been found as the solvent of choice for pure collagen but few studies claim that they destroy the collagen structure hence alternative solvents are still under experimentation. 2,2,2 trifluoroethanol has also been used. A new study even reports successful electrospinning of pure collagen self-supported membranes without the aid of fluorinated solvents.27
The morphological characteristics and mechanical strength of the collagen nanofibres may be improved by incorporating synthetic polymers like hydroxyapatite, PCL, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and PGA (Polyglycolic acid). Natural polymers are used less frequently to make composite collagen nanofibers. Nonetheless, in prior experiments, collagen was combined with elastin and chitosan to create composite nanofibers.28
Multilayering electrospinning, in which multiple polymeric solutions were spun successively, is another method for producing composite collagen fiber mesh. Multilayering electrospinning allows multilayered scaffolds creation. The structural properties, biological activities, and physical attributes of each layer are unique. The multilayered scaffold may be used to tissue engineer the layered tissues like blood veins and skin. thus through multilayered scaffolds vital organs and tissues can be protected.29
Also, another method for making composite collagen fibers is coaxial electrospinning, which creates fibers with a core-shell configuration. The inner (core) or outer (sheath) layer of collagen can be electrospun. Due to its high biological activity, collagen is frequently electrospun as the outermost surface and a synthetic polymer as the internal part to strengthen mechanical strength. the synthetic polymer which is the innermost part of the core-shell configuration brings a lot of biological activity and good mechanical strength.30
PCL and Collagen
Due to its outstanding mechanical strength and biocompatibility, PCL is a biodegradable synthetic polymer that is electrospun with collagen on a routine basis. Powell and Boyce used electrospun collagen/PCL nanofibers as the framework for modified skin and discovered that mixing a little amount of PCL with collagen nanofibers increased mechanical properties without sacrificing biocompatibility. Collagen/PCL fiber scaffolds developed with human epidermal keratinocytes also dermal fibroblasts helped to create a well-stratified epidermis and dermis with continuous basal cell layers and basement membrane.31
In more recent work, researchers used electrospun collagen/PCL nanofibers as a scaffold for skin tissue regeneration and observed that they stimulated dermal fibroblast proliferation and collagen synthesis. Human adipose stromal cells have also been used to test electrospun collagen/PCL nanofibers for wound healing purposes.32 These composite nanofibers with insulin-containing chitosan nanoparticles were generated as well. The hydrophilicity, water absorption, and blood compatibility were all enhanced when insulin-chitosan particles were added.
In another study, to stimulate skin dermis regeneration, electrospun collagen/PCL nanofibers were covalently linked with EGF(Epidermal Growth Factor).33 Using a dual-needle electrospinning system in which a single needle extrudes hyaluronic acid loaded with bFGF(basic Fibroblast Growth Factor) and VEGF(Vascular Endothelial Growth Factor)-loaded gelatin nanoparticles, while the other needle extrudes collagen filled with EGF and PDGF-laden gelatin nanoparticles, it forms a composite fiber mesh which allows for staggering growth factor release. This design allowed for the rapid release of bFGF and EGF in the early phases to promote re-epithelialization and angiogenesis, accompanied by the prolonged release of VEGF and PDGF in the later stages to promote vascular maturation. Wound closure, collagen deposition, and vascularization were all accelerated in diabetic rats implanted with the scaffold.34
PGA and collagen
It’s a different synthetic biomaterial that’s been electrospun with collagen. On wound healing, electrospun collagen/PGA nanofibers were compared to commercial collagen matrix, and the collagen/PGA nanofibers were shown to enhance not only cell migration but also neovascularization. They theorized that it was owing to the nanostructure effect, which allows for increased cell infiltration, as well as the presence of PGA, which is known to promote angiogenesis. Clearly, in this work, the combination of collagen and PGA combined the high biocompatibility and proangiogenic properties of each material to form a scaffold with exceptional characteristics for skin tissue engineering applications.35
Electrospinning using collagen and natural biomaterials is less prevalent. GTA-cross-linked electrospun collagen/elastin nanofibers facilitated human dermal fibroblast adhesion, migration, and proliferation, according to researchers who mixed collagen and elastin for electrospinning. The scaffold’s performance was equivalent to Integra (cross-linked bovine tendon collagen and glycosaminoglycan) after 6 weeks of in vivo implantation, promoting not only fibroblast migration but also collagen deposition, allowing vascular infiltration, and stimulating a minor immunological response.36
Electrospun collagen/PLGA nanofibers with Glucophage (an anti-diabetic medication) allows for long-term medication administration, and it has been shown to speed up wound healing in diabetic rats when used in vivo. MMP-9 suppression by Glucophage caused a larger buildup of collagen in wounds, according to wound analysis. so this helped in diabetic patients having wound cases.37
PCL/Vitamin A nanofibers and collagen/silver nanoparticles shell nanofibers were made by coaxial electrospinning. Silver nanoparticles were used as an antibacterial agent and vitamin A palmitate was encapsulated as a healing-promoting medication. The antibacterial and wound-healing capabilities of the coaxial nanofibers were acquired by integrating both compounds. The coaxial nanofibers prevented the development of Staphylococcus aureus, while vitamin A palmitate was released gradually over 72 hours, with an initial burst release for the first 5 hours.38,39 Furthermore, the coaxial nanofibers showed high biocompatibility, which aided cell adhesion.
Because of the presence of numerous hydroxyl groups, hydrocolloids are polymers with hydrophilic characteristics. Because hydrocolloid dressings are occlusive, they maintain a hypoxic environment, which promotes necrotic tissue liquefaction and facilitates autolytic debridement. Granulation tissue formation on exposed bone wounds (without periosteum) is often a reconstructive surgical difficulty due to the wound bed’s low vascularization. However, after applying a hydrocolloid dressing to the lesion, a good granulation layer was detected. The present market for hydrocolloid dressings in medical applications is used to treat diabetic foot ulcers Example of hydrocolloid gel is as follows – agar, alginate, carrageenan, pectin, gelatin, hydrogel, etc.40,41
Electrospun nanofibers a novel approach for wound healing
As an alternative to the current wound healing techniques, a novel approach of using a technique known as ‘Electro spinning’ can be used to create a nanofibrous mat containing collagen to speed up the wound healing process in an efficient manner. It is a broadly used technology for electrostatic fiber formation which utilizes electrical forces to produce polymer fibers with diameters ranging from 2 nm to several micrometers using polymer solutions of both natural and synthetic polymers has seen a tremendous increase in research and commercial attention over the past decade. This process offers unique capabilities for producing novel natural nanofibers and fabrics with controllable pore structures. A variety of natural and synthetic polymers such as polylactic acid, polyurethanes, silk, collagen, hyaluronic acid, cellulose, chitosan/collagen, have been used for tissue engineering.42,43
Electrospinning techniques of nanomats
The use of polymer solutions of both natural and synthetic polymers has made electrospinning a popular method for electrostatic fiber formation since the 1990s. It produces fibers with diameters ranging from 2 nm to several micrometers. Academics and the commercial world are taking note. A unique capability this process offers is the ability to create fabrics and nanofibers with controllable pores. It is possible to tailor the pore structures of natural nanofibers and fabrics resulting from this process. Furthermore, spun nanofibers can be controlled in properties and functionality to achieve the desired outcome.44 Furthermore, the composition of nanofibers can be controlled, as well as the ratio of surface to volume. Since electrospun nanofibers exhibit these advantages, they are heavily studied for their applications in a wide range of fields, including filtration, optical and chemical sensors, electrode materials, and biological scaffolds. It has been over 60 years since nonwoven fiber fabrics were developed in this manner. The exploitation of nanoscale fiber produced via this technology has become increasingly critical in recent years. In the case of tissue engineering applications involving polylactic acid, polyurethanes, silk, collagen, hyaluronic acid, cellulose, and collagen/chitosan scaffolds, these considerations are especially relevant.45
Electro-spinning process
By electrostatically forcing polymer solutions or melts to form fibers, electrospinning produces fine fibers. Compared with conventional spinning methods, fiber produced by this technique has a smaller diameter (between nanometers and micrometers) and a larger surface area. Moreover, it is necessary to generate tens of kVs of DC voltage in order to achieve electro-spinning. Different technologies, including electrostatic precipitators and pesticide sprayers, function similarly to the electrospinning process based mainly on the mutual electrostatic forces that overcome weaker surface tension forces in the liquid polymer. During electrospinning, atmospheric conditions are at room temperature.46 The typical set up of electro-spinning apparatus is shown in Figure 2.
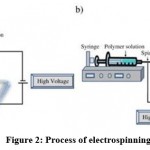 |
Figure 2: Process of electrospinning. |
Electrospinning apparatus
An electric charge of a specific polarity is injected into a polymer solution or melting material using a high voltage source. A polarity-opposed collector is used to accelerate the charge. A power supply, a spinneret (e.g., a pipette tip) and a grounded collecting plate (typically a metal screen, a plate or a rotating mandrel) constitute the primary elements. In most polymer solutions, polymers are dissolved in a solvent before electrospinning. When they dissolve entirely, they turn into a polymer solution. Electrospinning is carried out by adding the polymer fluid into the capillary tube. As some polymers may release unpleasant or even harmful odors, it is important to conduct processes in rooms with ventilation systems. By bringing an electric field to the end of a capillary tube, a polymer solution at the end of the tube induces an electric charge on the liquid surface. The application of a critical field overcomes surface tension forces when electrical forces prevail. Within the transition between the tip of the capillary and the collector, there is a rapid whipping of the jet that causes a charged jet of the solution to emerge from the tip of the taylor cone, leaving behind a polymer. At the point of the spinneret, the jet is stable, but after that, it becomes infeasible. Electrospinning is an easier method for forming fibers because of this.44-46
Effects of various parameters on electrospinning
Numerous parameters relating to the electrospinning process are dependent entirely on it, which can be grouped into three categories: solution parameters, process parameters, and ambient parameters. A solution has several parameter characteristics such as viscosity, conductivity, molecular weight, and surface tension. By contrast, the process includes parameters such as tip-to-collector distance, feeding rate, and applied electric field. Using electrospinning, fibers with various morphologies can be made by analyzing parameters appropriately. By doing so, we can achieve nanofibers of virtually any morphology and diameter. Aside from the humidity and temperature of the surrounding environment, ambient parameters can also influence the morphology and diameter of electrospun nanofibers. Effects of various parameters on electrospinning shown in Table 3.47
Table 3: Effects of various parameters on electrospinning.
| Parameters | Effect on fiber morphology |
| Solution parameters | |
| Viscosity | The number of beads generated is low, the fiber diameter is growing, and beads disappear |
| Polymer concentration | Concentration increases fiber diameter |
| Molecular weight of polymer | With increasing molecular weight, there is a reduction in beads and droplets |
| Conductivity | Fiber diameter decreases as conductivity increases |
| Surface tension | High surface tension leads to jet instability. No definitive relationship with fiber morphology |
| Processing parameters | |
| Applied voltage | An increase in voltage leads to a decrease in fiber diameter. |
| Distance between tip and collector | There were too few and too many beads generated, the distance required for uniform fibers being too small and too large. |
| Feed rate/flow rate | The diameter of the fiber decreases as the flow rate decreases, generating beads with an excessive flow rate. |
| Ambient parameters | |
| Humidity | Circular pores are created on the fibers when the humidity is high. |
| Temperature | The diameter of fiber decreases as the temperature increases. |
Solvents used for electrospinning
A suitable solvent must be present in the electrospinning process before the polymer is dissolved. As a result, the solvent used in preparing polymer solutions has an impact on its spinnability. Solvents that preserve polymer solutions must exhibit certain qualities, such as being capable of boiling, having a good boiling point pressure, being volatile, and being able to evaporate below a certain temperature. For electrospinning to succeed, the solvent system must be suitable. Intermolecular interactions between polymers and solvents (binary systems) are either positive or negative. The type of solvent affects these interactions. Electrospinning, when carried out with jet thinning, results in rapid solvent evaporation and phase separation. Determining the evaporation rate and drying time of a solvent depends on its vapor pressure. During phase separation, which is the process by which nanostructures are formed, it is equally important to consider the volatility of the solvent.48 The size and morphology of electro-spun nanofibers depend on many properties of solutions, including viscosity and surface tension. The surface tension of different solutions may vary. Electrospinning relies on solvents for two reasons: first, they form an electrified jet in which polymer molecules dissolve in water, and second, they allow the molecules to flow toward the collector. Other solvents include chloroform, dimethylformamide, hexafluoropropanol, tetrahydrofurane, trifluoroethanol, acetone, water, Methanol, Acetic acid, Formic acid, Dichloromethane, Ethanol and trifluoroacetic acid. It is very helpful to understand how properties of solutions such as conductivity affect electrospun polymers by studying the solvents used in electrospinning. The electrospinning of poly(acrylonitrile) and polyurethane urea copolymers was successfully accomplished by using dimethylformamide (DMF), which is a highly dielectric aprotic solvent with a high dipole moment. Researchers have also tested chitosan electrospinning using acetic acid for conducting solutions with poly(p-dioxanone co-L-lactic acid)-block poly(ethylene glycol) copolymer electrospinning. Thinner fibers can be produced by changing the solvent concentration. Solvent concentration in the solution causes surface tension to decrease gradually.49
Characterizations of electrospun nanofibers
As the chances of getting a single fiber are extremely rare when electrospinning, the characterization of fibers produced by the process remains one of the hardest tasks. An evaluation of the entire process, including a selection of the polymer and mechanical testing, is needed in order to empirically understand electrospinning. Electrospinning polymers can typically be classified into three distinct classes: structural and mechanical polymers, mechanical polymers, and chemical polymers. Nanofibers are currently attracting the attention of researchers for their remarkable micro and nanostructural characteristics, high surface areas, and small pores, as well as their ability to produce three-dimensional structural patterns, which could be used to create advanced materials with a diverse range of applications. A number of experiments conducted by different research groups have been conducted to understand the characteristic features of nanofibers as a function of process parameters, material properties, as well as the different electrospinning processes.50
Geometrical characterizations
Nanofibers have a specific internal structure that determines their physical and mechanical properties. Therefore, in order to determine the physical and mechanical properties of the sample, we need to examine the structure and morphology of the nanofiber. Fiber diameter, diameter distribution, fiber orientation, and fiber morphology (such as cross-section shape and surface roughness) are some of the geometric properties of nanofibers. SEM, FESEM, TEM, atomic force microscopy (AFM), and transmission electron microscopy (TEM) are some of the methods employed for the characterization of geometric properties. Fiber diameters and morphology can be detected by microscopy, but the resolution is reduced at high magnifications.51
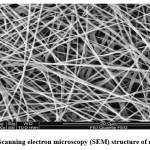 |
Figure 3: Scanning electron microscopy (SEM) structure of nanofibers. |
Chemical characterizations
Nanofibers can be characterized by FTIR and NMR techniques. These methods can be used not only to study the chemical structure of two polymers blended for nanofiber fabrication but also to study their interactions between molecules. In the case of a collagen and polyethylene oxide (PEO) blend used for electrospinning of nanofibers, the NMR spectrum revealed a new phase structure caused by hydrogen bonds forming between oxygen atoms in PEO and proton atoms in amino and hydroxyl groups in collagen.52
Mechanical characterizations
Scaffolds must withstand forces exerted by growing tissue and physiological activity like pulsed blood flow, in order to be used for biomedical applications. Measurements of nanofibrous matrix mechanical properties are therefore indispensable. We conducted tensile tests on ultrafine nonwoven fiber mats electrospun from ultrafine fiber in order to characterize their mechanical properties. While mechanically characterizing single nanofibers, samples must be handled with sufficient care so as to prevent severe damage. Nanoindentation, bending tests, reflection frequency measurements, and microscale tension measurements have all proven useful for the characterization of nanofibers and nanowires.51,53
Application of electrospun nanofibers in wound healing
Nanofibers synthesized by electrospinning technology have exhibited outstanding role in endorsing wound healing. The microstructure of electrospun nanofibers is highly tailored for the extracellular matrix structure of human body, which is extremely favorable for cell growth, proliferation and adhesion. Similarly, because of their high permeability and absorption rate they can easily absorb the exudates produced on the surface of wound, thus maintaining a moist healing environment. Moreover, the large surface area facilitates loading and transportation of bioactive substances such as growth factors and drugs. Therefore, electrospun nanofiber materials are considered to be the ideal choice for wound dressings. Applications of electrospun nanofibers in other fields shown in Figure 4.54
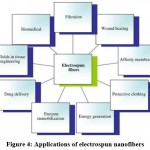 |
Figure 4: Applications of electrospun nanofibers. |
Conclusion
Fibers produced by electrospinning are simple, versatile, and cost-effective because they have high surface-to-volume ratios. This process has several properties which make it a promising candidate for various applications, including tissue engineering. Furthermore, there is a significant interaction between solution and processing parameters such as viscosity, molecular weight, polymer concentration, tip-to-collector distance, conductivity, etc. These parameters can be adjusted to get desired properties. Additionally, melting electrospinning is also possible, in which polymer melts are used instead of solvents, which eliminates the need for solvents. Nanofibers can be characterized on the physical, chemical, and mechanical levels using a variety of techniques, such as scanning electron microscopy, transmission electron microscopy, atomic force microscopy, nuclear magnetic resonance, etc. In addition to tissue engineering scaffolds and wound healing, spun fibers can be used in drug delivery, enzyme immobilization, protective clothing, cosmetics, affinity membranes, and filtration applications, among many others. It is important to note, however, that electrospinning, despite being beneficial and successful, has some critical limitations, including small pore sizes and insufficient cellular infiltration. Through multilayering, blending with a polymer with a different degradation profile, or inserting heprasil, attempts are being made to improve cell migration and design. A general conclusion for tissue engineering and regenerative medicine is that electrospinning holds great promise.
Conflict of Interest
The author declare that no conflict of interest.
Funding Sources
There are no funding Source.
References
- Yousef H, Alhajj M, Sharma S. Anatomy. Skin (Integument), Epidermis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022 Jan-Guo S, DiPietro LA. Factors Affecting Wound Healing. J Dent Res. 2010;89(3):219-29.
CrossRef - Joseph M. Abdo, Nikolai A. Sopko, Stephen M. Milner. The applied anatomy of human skin: A model for regeneration. Wound Medicine. 2020;28:100179.
CrossRef - Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Exp Dermatol. 2016;25:92-98.
CrossRef - Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing – A literature review. An Bras Dermatol. 2016;91(5):614-620.
CrossRef - Wallace HA, Basehore BM, Zito PM. Wound Healing Phases. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022 Jan.
- Luis Cañedo-Dorantes, Mara Cañedo-Ayala. Skin Acute Wound Healing: A Comprehensive Review. International Journal of Inflammation. 2019;2019;1-15.
CrossRef - El Ayadi A, Jay JW, Prasai A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int J Mol Sci. 2020;21:1105.
CrossRef - Ubbink DT, Brölmann FE, Go PMNYH, Vermeulen H. Evidence-Based Care of Acute Wounds: A Perspective. Adv Wound Care. 2015;4(5):286-94.
CrossRef - Cañedo-Dorantes L, Cañedo-Ayala M. Skin Acute Wound Healing: A Comprehensive Review. Int J Inflam. 2019;2019:3706315.
CrossRef - Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C. 2015;48:651–62.
CrossRef - Han G, Ceilley R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017;34(3):599-610.
CrossRef - Mathew-Steiner SS, Roy S, Sen CK. Collagen in Wound Healing. Bioengineering (Basel). 2021;8(5):63.
CrossRef - Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821-833.
CrossRef - Sato Kenji, Asai Tomoko T., Jimi Shiro. Collagen-Derived Di-Peptide, Prolylhydroxyproline (Pro-Hyp): A new low molecular weight growth-initiating factor for specific fibroblasts associated with wound healing. Cell Dev Biol. 2020;28:1-10
CrossRef - Holly N. Wilkinson and Matthew J. Hardman. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223.
CrossRef - Hayuningtyas, R.A., Han, M., Choi, S. et al. The collagen structure of C1q induces wound healing by engaging discoidin domain receptor 2. Mol Med. 2021;27:125.
CrossRef - Pawel Olczyk, Łukasz Mencner, Katarzyna Komosinska-Vassev. The Role of the Extracellular Matrix Components in Cutaneous Wound Healing. BioMed Research International. 2014;2014:1-8.
CrossRef - Karami A, Tebyanian H, Sayyad Soufdoost R, Motavallian E, Barkhordari A, Nourani MR. Extraction and Characterization of Collagen with Cost-Effective Method from Human Placenta for Biomedical Applications. World J Plast Surg. 2019;8(3):352-358.
- Mofieed Ahmed, Amit Kumar Verma, Rajan Patel. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustainable Chemistry and Pharmacy. 2020;18:100315.
CrossRef - Davison-Kotler E, Marshall WS, García-Gareta E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering. 2019;6(3):56.
CrossRef - Cruz, M.A., Araujo, T.A., Avanzi, I.R. et al. Collagen from Marine Sources and Skin Wound Healing in Animal Experimental Studies: A Systematic Review. Mar Biotechnol. 2021;23:1-11.
CrossRef - Law JX, Liau LL, Saim A, Yang Y, Idrus R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng Regen Med. 2017;14(6):699–718.
CrossRef - Meyer M. Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng OnLine. 2019;18(1):24.
CrossRef - Rnjak-Kovacina J, Wise SG, Li Z, Maitz PKM, Young CJ, Wang Y, et al. Electrospun synthetic human elastin:collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012;8(10):3714–22.
CrossRef - Pallaske F, Pallaske A, Herklotz K, Boese-Landgraf J. The significance of collagen dressings in wound management: a review. J Wound Care. 2018;27(10):692–702.
CrossRef - Hernández-Rangel A, Martin-Martinez ES. Collagen based electrospun materials for skin wounds treatment. J Biomed Mater Res A. 2021;109(9):1751–64.
CrossRef - Kirti, Khora SS. Marine Collagen as a Source of Biomaterial. In: Encyclopedia of Marine Biotechnology. John Wiley & Sons, Ltd; 2020. p. 1175–93.
CrossRef - Senadheera TRL, Dave D, Shahidi F. Sea Cucumber Derived Type I Collagen: A Comprehensive Review. Mar Drugs. 2020;18(9):471.
CrossRef - Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue- specific considerations. Eur Spine J. 2008;17(4):467–79.
CrossRef - Law JX, Liau LL, Saim A, Yang Y, Idrus R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng Regen Med. 2017;14(6):699–718.
CrossRef - Dhand C, Balakrishnan Y, Ong ST, Dwivedi N, Venugopal JR, Harini S, et al. Antimicrobial quaternary ammonium organosilane cross-linked nanofibrous collagen scaffolds for tissue engineering. Int J Nanomedicine. 2018;13:4473–92.
CrossRef - Dems D, Rodrigues da Silva J, Hélary C, Wien F, Marchand M, Debons N, et al. Native Collagen: Electrospinning of Pure, Cross-Linker-Free, Self-Supported Membrane. ACS Appl Bio Mater. 2020;3(5):2948–57.
CrossRef - Chen ZG, Wang PW, Wei B, Mo XM, Cui FZ. Electrospun collagen-chitosan nanofiber: a biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010;6(2):372–82.
CrossRef - Rnjak-Kovacina J, Wise SG, Li Z, Maitz PKM, Young CJ, Wang Y, et al. Electrospun synthetic human elastin:collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012;8(10):3714–22.
CrossRef - Duan N, Geng X, Ye L, Zhang A, Feng Z, Guo L, et al. A vascular tissue engineering scaffold with core-shell structured nano-fibers formed by coaxial electrospinning and its biocompatibility evaluation. Biomed Mater Bristol Engl. 2016;11(3):035007.
CrossRef - Sekiya N, Ichioka S, Terada D, Tsuchiya S, Kobayashi H. Efficacy of a poly glycolic acid (PGA)/collagen composite nanofibre scaffold on cell migration and neovascularisation in vivo skin defect model. J Plast Surg Hand Surg. 2013;47(6):498–502.
CrossRef - Lee C-H, Chang S-H, Chen W-J, Hung K-C, Lin Y-H, Liu S-J, et al. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. J Colloid Interface Sci. 20151;439:88–97.
CrossRef - Powell HM, Boyce ST. Engineered human skin fabricated using electrospun collagen-PCL blends: morphogenesis and mechanical properties. Tissue Eng Part A. 2009;15(8):2177–87.
CrossRef - Gümüşderelioğlu M, Dalkıranoğlu S, Aydın RST, Cakmak S. A novel dermal substitute based on biofunctionalized electrospun PCL nanofibrous matrix. J Biomed Mater Res A. 2011;98(3):461–72.
CrossRef - Fu X, Wang H. Spatial arrangement of polycaprolactone/collagen nanofiber scaffolds regulates the wound healing related behaviors of human adipose stromal cells. Tissue Eng Part A. 2012;18(5–6):631–42.
CrossRef - Wei Q, Xu F, Xu X, Geng X, Ye L, Zhang A, et al. The multifunctional wound dressing with core–shell structured fibers prepared by coaxial electrospinning. Front Mater Sci. 2016;10(2):113–21.
CrossRef - Chen S, Liu B, Carlson MA, Gombart AF, Reilly DA, Xie J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine (Lond). 2017;12(11):1335-1352.
CrossRef - Lai H-J, Kuan C-H, Wu H-C, Tsai J-C, Chen T-M, Hsieh D-J, et al. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014;10(10):4156–66.
CrossRef - Xue J, Wu T, Dai Y, Xia Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem Rev. 2019;119(8):5298-5415.
CrossRef - Islam MS, Ang BC, Andriyana A. et al. A review on fabrication of nanofibers via electrospinning and their applications. SN Appl Sci. 2019;1:1248.
CrossRef - Xiaomin Shi, Weiping Zhou, Delong Ma, Qian Ma, Denzel Bridges, Ying Ma, Anming Hu. Electrospinning of Nanofibers and Their Applications for Energy Devices. Journal of Nanomaterials. 2015;2015:1-20.
CrossRef - Beachley V, Wen X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater Sci Eng C Mater Biol Appl. 2009;29(3):663-668.
CrossRef - Luo CJ, Nangrejo M, Edirisinghe M. A novel method of selecting solvents for polymer electrospinning. Polymer. 2010;51(7):1654–62.
CrossRef - Srujan Mishra, S. Phillip Ahrenkiel. Synthesis and Characterization of Electrospun Nanocomposite TiO2 Nanofibers with Ag Nanoparticles for Photocatalysis Applications. Journal of Nanomaterials. 2012;2012:1-6.
CrossRef - Mir M, Ali MN, Barakullah A, Gulzar A, Arshad M, Fatima S, et al. Synthetic polymeric biomaterials for wound healing: a review. Prog Biomater. 2018;7:1–21.
CrossRef - Bhardwaj N, Kundu SC. Electrospinning: A fascinating fiber fabrication technique. Biotechnology Advances. 2010;28(3):325–47.
CrossRef - Scaffaro R, Maio A, Lopresti F, Botta L. Nanocarbons in Electrospun Polymeric Nanomats for Tissue Engineering: A Review. Polymers. 2017;9(2):76.
CrossRef - Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS. Electrospinning of nanofibers. Journal of Applied Polymer Science. 2005;96(2):557–69.
CrossRef - Lu P, Ding B. Applications of electrospun fibers. Recent Pat Nanotechnol. 2008;2(3):169-82.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





