How to Cite | Publication History | PlumX Article Matrix
Formulation Development and Evaluation of Floating Tablets of Zolmitriptan
Sapana Ahirrao1* , Deepak Bhambere1
, Deepak Bhambere1 , Kunal Todkar1
, Kunal Todkar1 , Moreshwar Patil1
, Moreshwar Patil1 , Pallavi Khairnar1
, Pallavi Khairnar1 , Pavan Udavant1
, Pavan Udavant1 , Piyushgir Gosavi1
, Piyushgir Gosavi1 , Anagha Baviskar2
, Anagha Baviskar2
1MET’s Institute of Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, India-422003.
2Assistant Professor at PRES's College of pharmacy (for women), chincholi, Nashik. PRES's College of pharmacy (for women), chincholi, Nashik.
Corresponding Author E-mail: sapana.58ahirrao@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2994
ABSTRACT:
Zolmitriptan is the antimigraine agent widely used for the treatment of the migraine. It shows the half-life of 2.5 – 3 h which indicate the frequent dosing to achieve proper pharmacological action of the drug. Gastro Retentive Drug Delivery System (GRDDS) is a common approach to decrease the dosing frequency and increase patient compliance, and delivery of drug through such an approach of floating tablet formulation will meet the requirement. This system showed substantial effect on the drug release through floating and swelling properties. The tablets were formulated by using direct compression technique along with hydrophilic polymers and gas generating system. In the preliminary trials, we observed that the tablets were within pharmacopeial limit. % Drug release of the formulations (F1 – F9) was studied up to 6 h and it was found from 52 to 91 %. The cumulative percentage drug release was inversely proportional to polymer concentrations of HPMC K100 M and PVP K 30. The batches follow Higuchi model of drug release which involves the diffusion mechanism. In-vitro dissolution studies showed good percent drug release, which is in accordance with robinson-errikson equation. Good buoyancy for additional 6 h, trailed by the diffusion.
KEYWORDS: Floating Tablet; Gastro Retention; HPMC K 100M; Migraine; Zolmitriptan
Download this article as:| Copy the following to cite this article: Ahirrao S, Bhambere D, Todkar K, Patil M, Khairnar P, Udavant P, Gosavi P, Baviskar A. Formulation Development and Evaluation of Floating Tablets of Zolmitriptan. Biosci Biotech Res Asia 2022;19(2). |
| Copy the following to cite this URL: Ahirrao S, Bhambere D, Todkar K, Patil M, Khairnar P, Udavant P, Gosavi P, Baviskar A. Formulation Development and Evaluation of Floating Tablets of Zolmitriptan. Biosci Biotech Res Asia 2022;19(2). Available from: https://bit.ly/3NDIIFn |
Introduction
Migraine is a disease characterized by a headache, usually occurs on one side, lasting up to 4-48 h with nausea, vomiting, light sensitivity, vertigo, loose movement and other symptoms [1]. The two major types are migraine with aura in which the headache is preceded by visual or other emotional symptoms, and migraine without aura. Pulsatile stretching of certain large cranial vessels is a rapid cause of pain. Vascular theory catches the first vasoconstriction or blood clotting by carotid artero-venous anastomoses producing cerebral ischemia and initiating invasion 2.
Oral administration is the most convenient mode of drug delivery and is associated with superior patient compliance as compared to other modes of drug intake. Oral drug delivery systems account for around half of all drug delivery systems on the market, and they offer more benefits owing to patient acceptability and simplicity of administration. Due to a short gastric retention time (GRT), or the time it takes for the contents of the stomach to enter the small intestine, oral medication absorption is frequently reduced 3-5. Gastro retentive systems can stay in the gastric area for several hours, considerably extending the duration medications spend in the stomach. Prolonged stomach retention enhances bioavailability, minimizes drug waste, and improves solubility for medicines that are less soluble at high pH. Gastro retentive systems (GRDDS) are intended to constrain and localize the drug delivery device in the stomach or upper sections of the small intestine until all of the medication is delivered, based on delayed gastric emptying and controlled principles. Flotation or buoyancy (floating systems), mucoadhesion (bioadhesive systems), sedimentation (high-density systems), swelling and expanding (swelling and expanding systems), and geometry are some of the methods (approaches) for establishing gastric retention (modified shaped systems). Floating systems are the most often utilized approach for gastric retention among the methods listed above. Because floating systems are less thick than gastric fluid, they stay buoyant in the stomach for longer periods of time. The medicine is gently delivered at the desired pace while the system is floating over the stomach contents [6]. As a result, the gastro retention duration increases and the variability in plasma drug concentration decreases. Two unique technologies have been used in the creation of FDDS, based on the mechanism of buoyancy: A. Effervescent System, and B. Non-Effervescent System. Effervescent systems utilize gas-generating agents, carbonates (such as sodium bicarbonate), and other organic acids (such as citric acid and tartaric acid) in the formulation to create carbon dioxide (CO2) gas, lowering the system density and allowing it to float atop the stomach fluid. Non-effervescent FDDS is based on the process of polymer swelling or bioadhesion to the mucosal layer of the GI tract. Gel forming or highly swellable cellulose type hydrocolloids, polysaccharides, and matrix forming material such as polycarbonate, polyacrylate, polymethacrylate, polystyrene, and bioadhesive polymer such as chitosan and carbopol are the most often utilised excipients in non-effervescent FDDS. Floating tablets are a sort of sustained release drug delivery device that floats on stomach fluids for a longer time by producing CO 2 gas or swelling and releasing the medicine for a longer duration. Various polymers, such as various grades of HPMC, Eudragit, chitosan, carbopol, guar gum, and xanthum gum 2, can be used to prolong drug release. Drugs that are easily absorbed from the stomach and have a short half-life are eliminated quickly from the blood circulation, require frequent dosing. To avoid this problem, the oral Gastroretentive formulations have been developed in an attempt to release the drug slowly into the Gastric region 4.
Neurogenic theory is considered to be a stress-induced. The stress of cortical electrical activity followed by vascular conditions. Another dramatic event appears to indicate neurogenic inflammation of the affected blood vessels which is enhanced by retrograde transfer to the arteries associated with the release of drugs such as 5- HT3, neurokinin, substance P, calcitonin-related peptide [3]. Changes in blood / urine levels of 5 HT and their metabolites during migraine attacks, its precipitation by 5HT manufacturers and the effectiveness of active drugs in the serotonergic system to prevent migraine attacks shows an importance of 5-HT in this disease [4]. Drugs that are readily available in the stomach and have a short half-life are quickly eliminated from the bloodstream, requiring constant dosage. To overcome this problem, oral Gastroretentive formulations were prepared in an effort to release the drug gradually into the Gastric region 5.
Zolmitriptan is 5-HT1 receptors agonist. There is a lot of hepatic metabolisms, especially to indole acetic acid, as well as N-oxide and N-desmethyl analogues. Metabolism is primarily regulated by CYP1A2, and monoamine oxidase is responsible for the continuation of N-desmethyl metabolite metabolism 6. The first pass metabolism reduces the oral bioavailability of zolmitriptan to 40% and has an elimination half-life of 2.5-3 h. It shows the absorption zone from the upper intestine. For these reasons, the Gastric Floating Drug Delivery System is able to extend the retention time of the medicine, thereby improving the oral availability of the drug 7. Therefore, the objectives of the study were as follows, zolmitriptan is easily absorbed in the stomach and has a short shelf life, is rapidly escape from the bloodstream thus requiring a higher dose. To avoid this problem, the formation of oral gastroretentive should be improved in an effort to release the drug slowly into the abdominal area and increase bioavailability and reduce the frequency of dosage. To test the prepared formulations for Response Surface Analysis’9-12.
Materials and Methods
Materials
Zolmitriptan was obtained as gift sample from Dr Reddy’s laboratories Ltd, Hyderabad. HPMC K100M, and PVP K30 polymers were from Glenmark Pharma, Nasik, India. Talc and magnesium Stearate were purchased from S.D. fine chemicals Pvt. Ltd’ Mumbai, India. Microcrystalline cellulose was procured from Signet Chemicals. All other ingredients used were of analytical grade and purchased from SD fine chemicals Pvt Ltd, Mumbai, India.
Methods
Preparation of floating tablets: The study is carried out for the detection of the suitable polymer blends and their ratios that will satisfy the requirements. Various polymers are studied in different like HPMC K100M, K4M, ethyl cellulose, sodium bicarbonate, citric acid, lactose, magnesium stearate, aerosol, povidone, the polymer blends were taken in different concentrations to evaluate the effect on the drug release, floating time and floating lag time. The powder mixtures along with drug and grinded uniformly, passed from sieve no 40 and compressed using 9 mm punch. Based from the preliminary batches results HPMC K100M selected as matrix forming agent, PVP K30 as a binding agent, the effect of ethyl cellulose on overall release is not considerable so it is neglected.
Dosage calculation
The Robinson Erikson equation was used to calculate dose [8, 9].
Dose – 2.5 mg
Half Life – 3 hr
Time to reach peak concentration (TOP) – 1 hr
Time up to which dosage form need to be controlled – 6 hr
Elimination Rate constant (Ke)
Ke = 0.693/3 = 0.231
Loading dose = Xo/Ke × t
= 2.5 / 0.231 × 6
= 1.80 mg
Desired rate of drug release (Ks) = Xo × ke
= 2.5 × 0.231
= 0.5775 mg/hr
Maintenance Dose = Ks × t
= 0.5775*6
= 3.465 mg
Corrected initial dose = loading dose – (Ks × TOP)
= 1.80 – (0.5775 × 1)
= 1.22 mg
Total dose = Maintenance Dose + Corrected initial dose
= 3.465 + 1.22
= 4.6875 mg
= 4.70 mg
Factorial design: Based on the preliminary study a 3 level factorial design is applied where HPMC K100M and PVP K 30 were selected as factors and their 3 levels were selected. The other factors were remaining invariant during all study. Factors, X1 was HPMC K100 and X2 was PVP K30 and responses, Y1 was cum % drug Release at 6 hrs and Y2 was Floating lag time.
Formulations as per 32 factorial designs
The floating tablets of Zolmitriptan were formulated as mentioned in the table 1. By direct compression technique using 9 mm biconvex punch.
Table 1: Formulations as per 32 factorial design
| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| HPMC K100 M | 70 | 70 | 70 | 75 | 75 | 75 | 65 | 65 | 65 |
| PVP K 30 | 7 | 10 | 13 | 7 | 10 | 13 | 7 | 10 | 13 |
| Sodium bicarbonate | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Citric acid | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Magnesium stearate | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Aerosil | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Lactose | q. s. | q. s. | q. s. | q. s. | q. s. | q. s. | q. s. | q. s. | q. s. |
| Zolmitriptan | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 |
| Total | 190 | 190 | 190 | 190 | 190 | 190 | 190 | 190 | 190 |
Evaluation of floating tablets
Physical parameters
Thickness: The vernier caliper was used to measure the thickness of the tablets. Tablets from all batches were tested and average values were calculated and expressed in mm. 10-13.
Hardness: The hardness of tablet is the resistance to breaking of tablet during shipping, storage, transportation and handling. The hardness of tablets was determined by using the Monsanto hardness tester10.
Friability: Roche Friabilator was used for testing the friability. Percent friability (% F) was calculated as follows 10.
Weight variation test: The test was performed as per the conditions and specification given in pharmacopoeia 11.
Floating Behavior: The in-vitro buoyancy was determined by floating lag time and floating time. The tablets were placed in dissolution vessel containing 200 mL of 0.1N HCl. The time required for the tablet to rise to the surface and a float was determined as floating lag time. The duration for which the tablet remains float on surface of solution is known as floating time 12, 13.
Swelling Behavior of Tablets: A tablet was weighed (W1) and placed in to a glass beaker containing 200 mL of 0.1N HCl maintained at a temperature of 37 ± 0.5. After every hour the tablet was removed and excess water is absorbed by a filter paper and it is reweighed (W2). The swelling index was calculated by the formula 14-16
SI = (W2-W1)/W1 × 100
Assay: Ten tablets were weighed and average weight was calculated, they were crushed to fine powder. The powder equivalent to 10 mg of Zolmitriptan was transferred to the 100 mL volumetric flask and dissolved by shaking. The made the volume to get the final concentration of 100μg/mL concentration. The working solution of the drug was prepared from standard stock solution in ethanol. The absorbance of this solution was measured and amount of Zolmitriptan was calculated from calibration curve. 15
In-Vitro Dissolution Studies: The in-vitro dissolution study was performed on Electrlab TDT 06-P according to parameters given below.
Dissolution test apparatus – USP (Type II)
Speed – 100 RPM
Volume of dissolution medium – 900 mL
Dissolution Medium used – 0.1 N HCl (pH 1.2)
Temperature – 37 ± 0.5 0C
Aliquot (10 mL) of the solution was withdrawn at regular interval of 1 Hrs. and same volume of fresh dissolution medium was replaced to maintained volume constant 13, 16-19.
Results and Discussion
The bulk density for all prepared formulations was in the range of 0.382 to 0.416 (g/mL) and the tapped density was in the range of the 0.459 to 0.493 (g/mL). The angle of repose of the powder formulations indicated good flow property and it was in the range of 25.55° to 36.24° which is required for the proper flow of the powder blend in the die cavity. The Carr’s index was in range of 14.56 to 17.39 which showed good flow property and compressibility of the powder blend. The Hausner’s ratio was in the range of 1.16 to 1.20 which is within the reported limits. All the results indicate that all the formulations possess good flow property and compressibility characteristics.
Table 2: Physical Parameters of Powder Blend.
| Formulations | Bulk Density
(g/mL) |
Tapped Density
(g/mL) |
Angle of Repose
(0) |
Carr’s Index
(%) |
Hausner’s Ratio
|
| F1 | 0.405±0.00346 | 0.474±0.0142 | 29.30±0.25 | 14.56±1.89 | 1.16±0.025 |
| F2 | 0.398±0.0075 | 0.480±0.0095 | 31.18±0.67 | 16.079±0.85 | 1.2±0.01 |
| F3 | 0.394±0.00346 | 0.477±0.0052 | 31.55±0.34 | 17.39±1.41 | 1.20±0.025 |
| F4 | 0.402±0.00651 | 0.483±0.010 | 32.37±0.62 | 16.63±3.11 | 1.19±0.04 |
| F5 | 0.390±0.006 | 0.471±0.009 | 33.27±1.79 | 17.18±1.43 | 1.20±0.025 |
| F6 | 0.382±0.00346 | 0.459±0.00462 | 36.24±0.96 | 16.83±0.080 | 1.19±0.019 |
| F7 | 0.413±0.0040 | 0.500±0.01 | 25.55±0.028 | 17.26±1.05 | 1.20±0.015 |
| F8 | 0.416±0.07 | 0.493±0.015 | 26.26±0.495 | 15.61±1.24 | 1.18±0.017 |
| F9 | 0.409±0.0118 | 0.483±0.005 | 29.51±0.378 | 15.22±3.02 | 1.19±0.06 |
* Mean± S.D., n=3
Floating tablets of Zolmitriptan were formulated using different grades of HPMC, PVP K 30, Ethyl cellulose. The batches of HPMC K 4M and K15M (F2 and F3) were good in floating lag time but these tablets get dispersed after 4 – 5 hours so were rejected. The formulation containing high HPMC K 100M were showed very less release in (formulation A7 and A8) so these are rejected. The effect of ethyl cellulose on overall release is not considerable so it is neglected. The formulation A9 having 65 mg of HPMC showed optimum release.
Based on the preliminary study a 3 level factorial design is applied where HPMC K100M and PVP K 30 were selected as factors and their 3 levels were selected. The other factors were remaining invariant during all study.
As per Table 3, the hardness of the tablets was in the range of 4.53 to 5.33 kg/cm2. The thickness was in the range of 3.886 to 3.966 mm. The % friability was in the range of 0.3 to 0.87 % which is within the limits. All formulations show weight variation within the range of the pharmacopoeial limits.
Table 3: Physical parameters of formulation F1 to F9
| Formulations | Hardness
(kg/cm2± SD) |
Thickness
(mm ± SD) |
% Friability
(± SD) |
Wt. Variation
(± SD) |
| F1 | 4.833±0.288 | 3.953±0.011 | 0.78±0.13 | 188.2±1.15 |
| F2 | 4.83±0.288 | 3.626±0.5254 | 0.87±0.43 | 188.75±1.44 |
| F3 | 5.00±0.5 | 3.940±0.02 | 0.41±0.11 | 189.35±1.66 |
| F4 | 4.83±0.288 | 3.92±0.04 | 0.59±0.45 | 189±1.52 |
| F5 | 5.33±0.288 | 3.966±0.011 | 0.74±0.84 | 188.6±1.42 |
| F6 | 4.66±0.288 | 3.886±0.030 | 0.3±0.13 | 189.05±1.35 |
| F7 | 5.16±0.288 | 3.923±0.050 | 0.78±0.68 | 188.75±1.332 |
| F8 | 4.83±0.288 | 3.933±0.00013 | 0.62±0.23 | 188.95±1.27 |
| F9 | 4.53±0.288 | 3.933±0.011 | 0.61±0.26 | 189.35±1.03 |
As the tablets immersed in to the medium, the interaction of the dissolution medium with sodium bicarbonate results into the formation of the carbon dioxide gas generation, the gas is get entrapped in to the swollen gel, this cause the expansion of the gel matrix and cause reduction in the density of the system. The effervescent system was so chosen to compromise the matrix integrity with the shortest possible lag time. It was found that the tablets were floated within the 20-47 seconds.
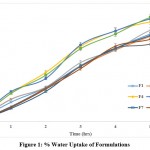 |
Figure 1: % Water Uptake of Formulations. |
In case of the hydrophilic matrices when they immersed in to water it gets swell and eventually dissolve in to water. Initially water hydrate the polymer and it causes the swelling of the polymer, water molecules are absorbed by the hydrophilic groups of the polymer and swelling of the polymers occurs initially. As the more water gets absorbed by the polymer the chains become more hydrated and the gel become more diluted, above a critical concentration the polymer chains disentangle and detached from the gellified matrix. Thus, the process undergoes simultaneous swelling, dissolution and diffusion in to the bulk medium resulting in to erosion of the polymer.
Tablets from each batch showed drug content in the range 95.90% to 98.70% which is within pharmacopoeia specifications.
Table 4: Floating Lag Time, Assay and responses of Formulation F1-F9
| Formulation Code | Factor 1
A:HPMC |
Factor 2
B:PVP K
|
Response1:
%CDR |
Response2: FLT
|
Floating Lag Time (sec) | Assay (%) |
| F1 | 70
70 |
7 | 72.79
|
28 | 47±2.0 | 95.90±3.21 |
| F2 | 70 | 10 | 68.43
|
32 | 20±3.05 | 97.12±1.82 |
| F3 | 70 | 13 | 62.473
|
30 | 38±3.0 | 97.35±1.53 |
| F4 | 75 | 7 | 62.66
|
38 | 24±3.46 | 96.93±2.57 |
| F5 | 75 | 10 | 58.59
|
42 | 42±2.0 | 97.17±1.51 |
| F6 | 75 | 13 | 52.72
|
47 | 32±2.0 | 98.49±1.41 |
| F7 | 65 | 7 | 91.94
|
20 | 25±3.20 | 96.73±2.21 |
| F8 | 65 | 10 | 89.87
|
24 | 28±2.0 | 97.76±0.73 |
| F9 | 65 | 13 | 81.6
|
25 | 30±2.30 | 98.70±0.69 |
* Mean± S.D., n=3
From cumulative % drug release results it is concluded that as the polymer concentration increases the viscosity of the gel layer increases as well as the diffusional path length of the drug increases this cause the less drug release at the higher level of the HPMC and vice versa. The formulation F7, F8 and F9 shows good drug release.
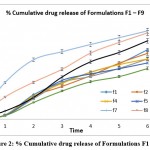 |
Figure 2: % Cumulative drug release of Formulations F1 – F9. |
The Release kinetics of the formulation was shown in the table 5, the best fit model for the drug release was found to be Higuchi. The mechanism involved for the drug release involved diffusion.
Table 5: The Dissolution Models for Formulations (F1-F9)
| Formulation code | R2 | ||||
| Zero order | First order | Higuchi | Hixon Crowell | Korsmeyer
Peppas |
|
| F1 | 0.9798 | 0.9681 | 0.994 | 0.9817 | 0.8801 |
| F2 | 0.9896 | 0.974 | 0.995 | 0.985 | 0.8815 |
| F3 | 0.9643 | 0.9839 | 0.991 | 0.9843 | 0.8745 |
| F4 | 0.9583 | 0.9873 | 0.993 | 0.9873 | 0.8703 |
| F5 | 0.9209 | 0.970 | 0.972 | 0.9661 | 0.8689 |
| F6 | 0.9771 | 0.9878 | 0.997 | 0.988 | 0.8698 |
| F7 | 0.9265 | 0.987 | 0.972 | 0.9653 | 0.9844 |
| F8 | 0.9932 | 0.9661 | 0.993 | 0.9925 | 0.9998 |
| F9 | 0.9865 | 0.9638 | 0.991 | 0.9821 | 0.9485 |
Experimental design and data analysis
Percentage Drug Release (%CDR)
Analysis of variance
A value of p < 0.05 was considered as significant and following equation suggest the result
% CDR = +1297.56741-32.12833* HPMC+1.72130 * PVP+4.16667E-003 * HPMC * PVP+0.20787* HPMC2-0.18481 * PVP2 ………………………………………..(1)
Diagnostics case statistics of experimental matrix
Predicted vs. actual plot of % CDR
The values shows that the predicted data, for percentage drug release, matches with the experimental results due to their low differences and showed the linear correlation between actual and predicted value.
Table 6: Composition of the Optimized Formulation.
| Ingredients (mg) | Optimized formulation |
| HPMC K 100 M | 65.51 |
| PVP K30 | 8.44 |
| Sodium bi carbonate | 30 |
| Citric acid | 10 |
| Magnesium stearate | 7 |
| Aerosil | 4 |
| Lactose | 60.35 |
| Zolmitriptan | 4.7 |
| Total | 190 |
Effect of HPMC K100
The % Drug release increases with decreasing the concentration of HPMC K100M. It was decided that the concentration of HPMC K100M might have individual effect on the % drug release.
Effect of PVP K 30
The % Drug release increases with decreasing the concentration of PVP K30. It was decided that the concentration of PVP K30 might have individual effect on the % drug release.
Effect of combined factors
Interaction of AB
It can be observed that the increase in % drug release mainly depends upon decreasing the concentration of HPMC K100M and PVP K 30. There is no interaction between factors A and B indicates that each variable affects individually in increasing % drug release. It was observed that the combined effect of HPMC K100M and PVP K30 which indicates that as decrease in HPMC K100M and PVP K30 might be responsible to increase % drug release.
Counter plot
The 3D surface Plot: 3D surface plot was obtained from Design Expert 8 software. On Analysis branch click the R1: Conversion node and go to Model Graphs to bring up the contour plot. Let’s quickly try some things here that you may find useful when making a presentation. In the vacant region of the AB contour plot right-click and select Add contour. Then drag the contour around (it will become highlighted). You may get two contours from one click like those with the same response value. On the Graphs Toolbar go to 3D Surface view. Modify the color range via a click on the color scale gradient in the graph legend, which brings up the Edit Legend dialog box. Change the Low and the High. Now click the design point sticking up in the middle. See how this is identified in the legend at the left by run number and conditions. Now try a handy feature for pulling up the right plot for any given run. On the Factors Tool select number 1 off the Run # dropdown-list. The 3D view now shifts to the correct ‘slice’ on factor C (catalyst). However, the colors are not ideal now. So right-click over the gradient and in the Edit Legend dialog box press the Defaults button. Your graph should now match the one shown below. The plot was drawn against response and variable. Fig 3 shows 3D surface plot of % drug release vs HPMC K100M and PVPK30. The figure shows the counter plot which concludes that factor A (HPMC K100M) have most significant effect on increase in % DR as compared to factor B (PVPK30).
 |
Figure 3: 3D View of %CDR With Respect to HPMC K100 and PVP K30. |
Approximation of desired response
As the concentration of HPMC K100 and PVP K30 is increases, percentage drug release also decreases. If still increased the concentration of HPMC K100 and PVP K30 then there is decrease in the percentage cumulative drug release.
At the point of Perturbation indicates that the levels of the entire 2 variable consider together for optimized response should be at their low-level value.
Table 7: In-vitro dissolution data and % water uptake of the optimized formulation.
| Time
(hrs) |
% Cumulative drug release | % Water uptake |
| 1 | 38.66±0.01 | 22.89±3.56 |
| 2 | 55.06±3.14 | 63.42±2.17 |
| 3 | 62.70±1.31 | 97.51±4.85 |
| 4 | 75.52±1.42 | 131.74±1.33 |
| 5 | 84.78±1.87 | 151.22±2.64 |
| 6 | 92.61±0.48 | 181.89±0.79 |
* Mean± S.D., n=3
Floating Lag Time
Analysis of variance
FLT =-112.44444+1.93333 * HPMC+0.88889 * PVP
From the equation 2 it was concluded that HPMC K100 (factor A), PVP K30 (factor B) having a induvidual as well as combined effect on the increasing in floating lag time (FLT)
Diagnostics case statistics of experimental matrix
Predicted vs. actual plot
The values prove that the predicted data, which were obtained from the empirical model for percentage drug release, are similar with the experimental results due to their low differences.
Linear correlation ship was observed between actual and predicted value.
Effect of experimental variables on the response
The effect of variables on the response was evaluated by Design expert software 8.0 and was plotted. In each plot, two factors remains constant and the other factor was in given range between its high and low levels, therefore its influence can be seen as a line that represents the demanded response
Effect of HPMC K100
The FLT increases with decreasing the concentration of HPMC K100M. It was concluded that the concentration of HPMC K100M might have individual effect on the FLT.
Effect of PVP K 30
The FLT increases with increasing the concentration of PVP K30. It was concluded that the concentration of PVP K30 might have individual effect on the FLT
Effect of combined factors:
Interaction of AB
It can be observed that the decrease in floating lag time mainly depends upon decreasing the concentration of HPMC K100M and PVP K 30. There is no interaction between factors A and B indicates that each variable affects individually in decreasing floating lag time. The combined effect of HPMC K100M and PVP K30 which indicates that as decrease in HPMC K100M and PVP K30 might be responsible to decrease floating lag time.
Counter plot
The 3D surface Plot: 3D surface plot was obtained from Design Expert 8 software. The plot was drawn against response and variable. Fig 4 shows 3D surface plot floating lag time vs HPMC K100M and PVPK30. The figure shows the counter plot which conclude that factor A (HPMC K100M) have most significant effect on increase in FLT as compared to factor B (PVPK30).
 |
Figure 4: 3D View of FLT With Respect to HPMC K100 and PVP K30. |
Shows the counter plot which conclude that factor A (HPMC K100M) have most significant effect on increase in FLT as compared to factor B (PVPK30).
Data analysis showed that from high levels to low level each factoer cause increase in the %CDR in the formulation. This software also suggests some formulations out of the range that was given at first, in regard to the results of analysis. Also the desirability of each item could be observed. All of the formulation can be chosen for percentage drug release at maximum level. Out of 18 solutions, solutions 1, 2, and 3 were considered. The optimized solution obtained from the model was formulated and the results are performed in the triplicates for determination of % CDR, FLT, hardness, friability, thickness and content uniformity. The solution no 1 was found to comply all specifications hence considered optimized.
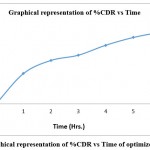 |
Figure 5: Graphical representation of %CDR vs Time of optimized formulation. |
Conclusion
Zolmitriptan is the antimigraine agent widely used for the treatment of the migraine. GRDDS is a common approach to decrease the dosing frequency and increase patient compliance, and delivery of drug through such an approach of floating tablet formulation will meet the requirement. This system showed significant impact on the drug release through floating and swelling properties. The floating tablets were prepared by using direct compression technique using hydrophilic polymers and gas generating system. The present study was carried out to develop the floating drug delivery of Zolmitriptan using HPMC K100M and PVP K30 polymers. In-vitro dissolution studies showed good percent drug release, which is in accordance with robinson-errikson equation. Good buoyancy for more than 6 hrs, followed by the diffusion transport. Thus, results of the current study clearly indicate, a promising potential of the zolmitriptan floating tablet as an alternative to the conventional dosage form. However, further clinical studies are needed to assess the utility of this system.
Acknowledgement
Authors are thankful to Dr. Reddy’s laboratories, Hyderabad, India, for providing sample of Zolmitriptan and R. C. Patel college of Pharmacy, Shirpur for their help in DSC, and Diya Lab; Mumbai for performing PXRD analysis. The authors are also thankful to Dr. S. J. Kshirsagar, Principal, and management of METs Institute of Pharmacy, Nashik, Maharashtra, India, for providing laboratory facilities.
Conflict of Interest
The authors declared that there is no conflict of interest.
Funding Source
No specific grant was received from any funding agency.
References
- Andreou, A.P., Edvinsson, L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain, 2019. 117 (20): p 1-17. https://doi.org/10.1186/s10194-019-1066-0
CrossRef - Kunz A, Iadecola C. Cerebral vascular dysregulation in the ischemic brain. Handb Clin Neurol, 2009. 92: p 283-305. doi:10.1016/S0072-9752(08)01914-3
CrossRef - Goadsby P. J, Holland P. R, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017. 97 (92): p 553-622. doi:10.1152/physrev.00034.2015.
CrossRef - Tripathi, k.d. Essentials of Medical Pharmacology, 5th Ed. Jaypee brother’s medical publishers Pvt. Ltd. 2003. pp 153-155.
- Mandal U. K, Chatterjee B, Senjoti FG. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J Pharm Sci. 2016. 11(5): p 575-584,
CrossRef - National Center for Biotechnology Information. PubChem Compound Summary for CID 60857, Zolmitriptan. Retrieved January 19, 2019, from https://pubchem.ncbi.nlm.nih.gov/ compound/Zolmitriptan.
- Maffat, A.C. Osselton, M.D. and Widdop, B. Clark’s analysis of drugs and poisons. 3rd Ed. Published by the Pharmaceutical Press. 2004. pp. 1714-1715.
- Irene, N. and Sasikanth, K. Preparation and in vitro evaluation of rosiglitazone maleate bilayered bio adhesive floating tablets. J Chem and Pharm Res. 2011. 3 (4): p.140-149.
- Robinson, J. N. and Eriksen, S. P. Therotical formulation of sustained release dosage form. Presented to the Basic Pharmaceutics section, academy of sciences, dallas meeting. 1966. 55 (11): p. 1254-1262.
CrossRef - Lachman, L. Lieberman, H.A. and Kanig J.L. The Theory and practice of Industrial pharmacy. 3rd edition. Varghese Publishing house. 1991. pp. 67, 183-184, 293-299.
- Indian Pharmacopoeia, Government of India, Ministry of Health and Family Welfare, Published by Indian Pharmacopoeal commission, Gaziabad, 2007, pp 505, 662-665. (04), pp 717-712.
- Tadros, M.I. “Controlled release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vivo evaluation in healthy human volunteers”. European J Pharmaceutics and Biopharm. 2010. 74: 332-339.
CrossRef - Charyulu, R.N., Patil A. B. Lakshi D. C. H., Prabhu P., Shastry C. H. Development of gastroretentive floating matrix tablets of diltizem hydrochloride. Nitte J health sci. 2011. 1 (1-3): p. 38-45.
CrossRef - Rao GK, Mandapalli PK, Manthri R, Reddy VP. Development and evaluation of gastroretentive delivery systems for cefuroxime axetil. Saudi Pharm J. 2013. 21 (1): p. 1-7.
CrossRef - Prajapati, S.T. Patel L. D., Patel D. M. Gastric floating matrix tablets: Design and optimization using combination of polymers. Acta Pharm. 2008. 58: p 221-229.
CrossRef - Dash, S., Murthy P. N., Nath L., Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta polonaiae pharmaceutica. 2010. 67 (3): p. 217 -223.
- Miehlke, S., Lucendo, A.J., Straumann, A., Jan Bredenoord, A. and Attwood, S. Orodispersible budesonide tablets for the treatment of eosinophilic esophagitis: a review of the latest evidence. Therapeutic Advances in Gastroenterology. 2020. 13, p.1756284820927282.
CrossRef - Kanathe, P., Jain, R., Jain, N. and Jain, S.K., Formulation and Evaluation of Orodispersible Tablet of Fluvastatin Sodium. Journal of Drug Delivery and Therapeutics, 2021. 11(1): p 42-47.
CrossRef - Pratiksha S. Deore, Yashpal M. More, Avish D. Maru. Formulation and Evaluation of Orodispersble Tablet. Asian Journal of Research in Pharmaceutical Sciences. 2021; 11(4): p. 267-2. doi: 10.52711/2231-5659.2021.00042
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






