How to Cite | Publication History | PlumX Article Matrix
Gene-Specific Drug Delivery System: An Art of War
Neetu R. Saudagar , Sahebrao S. Boraste
, Sahebrao S. Boraste , Dattatray M. Shinkar
, Dattatray M. Shinkar , Prashant L. Pingale*
, Prashant L. Pingale*  and Sunil V. Amrutkar
and Sunil V. Amrutkar
Gokhale Education Society’s Sir Dr. M. S. Gosavi College of Pharmaceutical Education and Research, Nashik-422005, India.
Corresponding Author E-mail: prashant.pingale@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3000
ABSTRACT:
Drug delivery key research aim is to support patients by designing clinically effective formulations. Drug delivery systems can enhance the treatment of a variety of diseases, including microbes’ infections, and cancers. Drug delivery systems preparation methods, on the other hand, remain difficult, particularly at the microscale. Some of the necessary criteria for speeding the transformation of drug delivery systems from a limited scale to an enormous scale include reducing batch-to-batch variance and increasing production volume. Gene-specific drug delivery system has a bright future as a preventive solution to severe diseases and has developed as an influential tool in recent years as a unique technology for disease management. Gene silencing, protein expression, or gene repair may be used to cure perhaps every illness with a gene-specific delivery system. The genetic material must be paired with a delivery additive to successfully transfer the nucleic acid payload to its target tissue. There are various non-viral and viral vectors involved along with the different mechanisms of gene entry into a cell which is discussed in this article. This review highlights that the gene-specific drug delivery system has vast scope in therapy and can prove advantageous over other therapies, because it includes several carriers and different methods of plasma membrane permeation. Very interestingly, it also includes various applications of the gene-specific drug delivery system in several diseases and recent trends in the Coronavirus vaccine.
KEYWORDS: Chitosan; Cancer; Dendrimers; COVID-19; Transfection; Viral and Non-viral vectors
Download this article as:| Copy the following to cite this article: Saudagar N. R, Boraste S. S, Shinkar D. M, Pingale P. L, Amrutkar S. V. Gene-Specific Drug Delivery System: An Art of War. Biosci Biotech Res Asia 2022;19(2). |
| Copy the following to cite this URL: Saudagar N. R, Boraste S. S, Shinkar D. M, Pingale P. L, Amrutkar S. V. Gene-Specific Drug Delivery System: An Art of War. Biosci Biotech Res Asia 2022;19(2). Available from: https://bit.ly/3xWUkhy |
Introduction
Gene therapy is a means of treating or avoiding illness by modifying the genes within the body’s cells. Genes that aren’t functioning correctly could be to blame for the disorder.
By deleting a faulty gene or introducing a new gene, gene therapy seeks to cure disease or enhance the body’s ability to battle illness. Among other disorders, gene therapy carries the potential for the treatment of tumors, cystic fibrosis, cardiovascular disease, obesity, and hemophilia.
Gene therapy works by:
Substituting transformed genes
Anchoring transformed genes
Thus, increasing the visibility of infected cells to the body’s defense system. Putting it up, the endogenous genome is manipulated.
One of the problems related to gene-specific treatment is that the gene cannot be specifically imbibed into the cell’s nucleus. It requires a transporter or a conveyer (a vector).
Viruses have the property to recognised particular cells and pass DNA/RNA into the cells’ total sets of genes, due to which they are mainly used as carriers. The infected gene is replaced with the gene of interest in the virus.
When administered encapsulated or conjugated to the surface of nanoparticles, gene silencing therapeutics, siRNAs, have been shown to have slightly longer half-lives. In certain cases, these therapeutics are used to treat ‘undruggable’ cancer proteins. Furthermore, it has been shown that the improved stability of genetic therapies provided by nanocarriers, which is also paired with controlled release, prolongs their effects 1.
One of the challenges faced in gene therapy is delivering the gene to the right place and switching it on. As a consequence, gene therapy often necessitates a case-by-case approach. This could be beneficial, but it could also be very costly 2.
In today’s drug treatment, vast patient populations are treated as individuals, regardless of the possibility of human, genetically dependent variations in drug reaction. Pharmacogenomics, on the other hand, could be able to better target successful treatment to smaller patient subpopulations that, though sharing the same disease phenotype, have different genetic profiles. It’s also uncertain if this plan would result in successful, more cost-effective care 3,4,5.
Plasmids with the fundamental constituents of gene expression
One of the keys to effective gene therapy is selecting the best vector. There are several different types of vectors, both viral and non-viral, and the most widely used ones are mentioned below.
Viral Vectors
For protection, uptake, and performance, there has been a renovation in the viral carriers for them to be utilized in gene-specific delivery. The vectors can be said to be of two types based on their cell entrance, one that enters the cell’s nucleus and the other that stays in the cytoplasm to show action. These vectors or carriers are obtained from deoxyribonucleic acid or ribonucleic acid 6.
Viral vectors are broadly divided on the basis of the derivative host, as represented in figure 1.
In a perfect scenario, the viral carriers that show implementations in gene-specific drug delivery show benefits of the relatively sophisticated viral infection mechanism whilst also escaping the pronouncement of an infected part that results in viral multiplication and fatal effect in a person who’s being exposed 7.
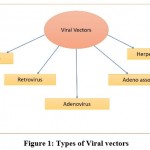 |
Figure 1: Types of Viral vectors. |
There are 5 major types of clinically effective viral vectors currently known:
Retroviral vectors
In gene therapy, retroviruses are one of the most commonly used viral vectors. During their life cycle, they integrate their complementary DNA into the host genome, resulting in faithful transgene transmission into the transduced cell progeny. 8
AAV
Among the most widely studied gene therapy engines is adeno-associated virus (AAV). It was first found in adenovirus formulations as a contaminant. Recombinant AAV (rAAV), which lacks viral DNA, is a protein-based nanoparticle intended to cross the cell membrane and carry the DNA cargo to the cell’s nucleus 9.
Bacteriophage
Despite the fact that bacteriophages are prokaryotic viruses, some experiments have shown that they can be modified to carry genes to mammalian cells.Because of their versatility and ease of manipulation and development, they have applications in biotechnology and medicine for testing, therapeutics, and manufacturing 10.
Table 1: General Properties of Viral Vectors1
| Vectors | Retroviral | Adenoviral | AAV |
| Family | Retroviridae | Adenoviridae | Parvoviridae |
| Particle size | 80–100nm | 80–100nm | 20nm |
| Genome | ssRNA | dsDNA | dsDNA |
| Max. Transgene Capacity | 12kb | 36kb | 5kb |
| Advantages | Safety is paramount. Integration of genes onto the genome. Immunogenicity is low. | Titers are large. Move transgene efficiently into both dividing and dormant cells. | The chromosome’s integration.
A vast array of hosts is available. Sustainability. Immunogenicity is low. Both actively dividing and quiescent cells are affected. |
| Disadvantages | Titer is low. Just works with cells that are actively dividing. Induction in insertional mutagenesis. | Immunogenic in nature. Transgene expression within a short period of time. | Packaging capability is restricted.
Toxicity of stem cells is a probability. |
The general properties of the viral carriers such as their merits, demerits, size, families are mentioned in the above table 1.
Non-Viral Vectors
Non-viral nucleic acid carriers are used to transport genes to various cells and groups of cells. Non-viral vectors show properties like being easier to pick out, safe to stockpile, found to be copious, and can possibly be frequently given, showing limited defense by the body against them 11.
The bulk of non-viral carriers show characteristics of positively charged DNA/RNA condensation. Centered on nature, these vectors are divided into two sets, as represented in Figure 2.
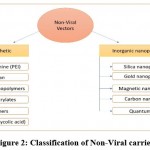 |
Figure 2: Classification of Non-Viral carriers. |
Natural or Synthetic
Naturally degradable positively charged ions are studied extensively due to their low toxicity in the body. In the majority of these carriers, positively charged proteins, lipid chains of monomers, or combinations of these are used 12.
Methods for using polymers as gene carriers:
DNA/RNA condensation
Direct form of conjugation
The structures of various polymers used as natural or synthetic, naturally degrading carriers are shown in figure 3.
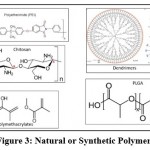 |
Figure 3: Natural or Synthetic Polymers. |
Polyethyleneimine
It is a positively charged (cationic) polymer that commonly shows its application in the transmission of the gene of interest. Because of their ability transport huge amounts of genes, showing adaptable output, these cationic carriers are perfect contestants for DNA/RNA delivery 13. PEI can be branched (BPEI) or liner (LPEI). In salt-containing conditions, branched PEIs are highly reactive and seem to conjugate with DNA. Compared to square PEIs, Linear PEIs show decreased toxicity and an elevated carrier performance capacity 14.
A CIJ, which is a unit confined impinging jet mixer was applied under fast mixing conditions to manufacture nanoparticles that were complex type of polyelectrolyte. Prior to processing, each stream is filled separately with the polyethyleneimine or the gene nanoparticles and put inside a tiny chamber 15. Since native PEI is non-degradable, it has a poor clearance rate in the systemic circulation. As a result, several studies have centered on the progress of naturally degradable PEI 16. The delivery assisted by PEI can perhaps result in a potential treatment option in curing disorders that require an amalgam of chemotherapy and DNA therapy 13.
Chitosan
Chitosan is one of the most commonly used cationic polymers due to its potential to release incorporated drugs for a longer duration of time. For the preparation of Chitosan/DNA nanospheres, a unique and easy osmosis-linked process was patented 17. The DNA integration was relatively large (up to 30%) using this approach, as it’s a laborious, and slow process of liberation. Several formulation parameters influence transfection performance, including salt type, molecular weight, acidity/basicity, N/P ratio, and so on. Taking into account all of the research, seems there are various molecular weight values and DDA parameters that shape complexes with optimum stability and transfection efficiency 18. To improve the complex’s cell-penetrating capacity and endosomal release, octaarginine-modified chitosan (R8- CS) was synthesized 19. Gene-specific DDS which includes a matrix mechanism containing chitosan may be an exciting area of study, with growing usefulness in rejuvenating dosage forms. Work involving stem cells is significant as the coated gene that monitors the seeded cells’ dedifferentiation can elevate the signals at the implantation area 20.
Cationic block co polymers
Due to the potential to solely aggregate in solid form and having limited solvents, block co-polymers are fascinating polymeric materials 21. Polyplexes are stable nanoparticles formed by the electrostatic reaction of the DNA (phosphate end) alongside cationic block co-polymers. PDMAEMA, shows the property of being biostable, pKa (7.4-7.5), water-dissolving while considering MW[21a,22]. It can be used as a non-viral DNA carrier and can be endocytosed into cells. Various factors like size of molecules, DNA attachment, buffer, form, affect the transfer of genes. DNA transfer, precipitation and attachment are all affected by the form and flexibility of the co-polymer. The capacity of this co-polymer to be able to coil on all sides of RNA/DNA resulting in a nice cast, is improved by the linear chain and the mass generating unit of polymers 22.
Polymethacrylates
In both in vivo and in vitro model systems, polymethacrylates, a vinyl-based cationic polymer, have been used for gene delivery. The polymer has evolved to increase its biodegradability, gene distribution effectiveness, and toxicity. It was conjugated with a hydrolysable cationic side chain to improve the polymer’s biodegradability 23. PMMA has poor contact with the cell membrane, so it may need to be modified further to improve penetration. To improve the transfection performance in a sample this polymer was mixed with a peptide which was cell pungent and soluble.
PLGA – Poly (lactic-co-glycolic acid)
Of all possible vectors, PLGA has been regarded as one of the most potent candidates. It’s a lactic and glycolic acid co-polymer that’s connected by an ester bond. The DNA entrapment time in a polymer is affected by constituents of the polymer, its weight, size and morphological structure. Because of its effective cellular absorption, fast endosomal escape, and continuous release of the therapeutic molecule, a PLGA nanoparticle is useful in gene silencing 24. The PEI-based PLGA formulation has been shown to have lower cytotoxicity and improved serum stability 25.
Dendrimers
Dendrimers may form complexes with genomic material such as RNA, plasmid DNA, and antisense oligonucleotides, among other things. They are artificial macromolecules with a compact molecular structure and a classification of different functional groups. Dendrimers have the ability to form polycations in a variety of physiological conditions, as well as the ability to bind genetic molecules with a negative charge and associate with nucleic acid anionic groups. Dendrimers with structurally tailored structures are used to increase delivery performance thus reducing cytotoxicity 26. Figure 4, represents the various types of dendrimers broadly segregated.
Dendrimer-based delivery mechanisms have shown a lot of promise as methods for improving genetic therapies. PAMAM dendrimers are undoubtedly among the best and most commonly used transfection agents, and many cell and molecular biologists use them as a basic method 27.
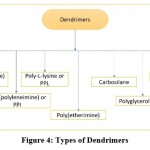 |
Figure 4: Types of Dendrimers |
Inorganic nanoscopic particles
Inorganic nanoscopic particles are miniature showing both unique chemistry and physical nature vary according to particle size. Gold (Au) and Silver (Ag) are used to make inorganic nanoparticles, magnetic nanoparticles consisting of Nickel, Cobalt, Iron, and iron oxide that are superparamagnetic, have massive magnetic motion in the magnetic arena, and fluorescent nanoparticles including quantum dots and SiO2, for example 38.
Silica nanoparticles
Mesoporous silica nanoparticles (MSNs) are made from amorphous SiO2 in a matrix form along with mesoporous permeability. MSNs are an appealing choice of dosage form due to characteristics like texture, durability, and uncomplicated changeability 29. MSNs may have their surfaces changed to produce cationic molecules which bind to anionic nucleic acid. The second choice which leads to MSNs being a reasonable method for gene transfer includes covering these with positively charged portion, while the inside includes an anionic nucleic acid that’s injected for travel. These are favorable candidates as co-delivery units.in animal studies, great therapeutic effectiveness can be demonstrated by deliberately picking the units that are to be bombarded into the site of interest and other structures. The successive move is to choose the best formula, optimize it for the mass manufacturing process, which leads to evaluation tests in patient applicable models and finally implementation in human trials 30.
Gold nanoparticles (GNPs)
Through the use of cationic co-carriers, GNPs containing synthetic microRNAs can invade cells 31. GNPs–microRNA conjugation is a novel method for microRNA delivery that could be used in a microRNA replacement scheme 32. In the ground operation of GNP, amino acids cause the genes to condense on the membrane. Powerful uptake and lower body defense have been demonstrated in DNA and gold nanoscopic particles, which are changed by an oligonucleotide 33. Using folic acid (FA)-based ligands, large transportability of the gene (80-90%) was obtained for GNPs which were surfaced by lipids in MCF-7 type of cell 34.
Magnetic nanoparticles(MNPs)
Magnetic nanoparticles are a kind of nanoparticle that can be controlled by applying magnetic fields to them. A magnetic material, such as iron, nickel, or cobalt, and a chemical component with usability, often with (bio)catalytic or bio-recognition properties, are typical components of such particles. The use of magnetic nanoparticles is seen in gene cloning, RNA/DNA purification 35. MNPs serve as monitors, evaluating the state of the disorder and dispensing medications to cure it. Their high charge adds to their stabilization by avoiding particle precipitation and settling due to gravitational forces 36.
Carbon nanotubes(CNTs)
Carbon nanotubes (CNTs) are carbon tubes with nanometer-sized diameters. Of all nanomaterials, carbon nanotubes have the largest surface area for chemical alteration. The use of nanotubes as a carrier for gene silencing has been shown to be effective in slowing the growth of cancer cells. The bits of advice for creating CNTs for implementation as a carrier, non-viral type in genetic drug delivery, the bits of advice are:
Engineering of the side chain groups during the preparation of naturally compatible nanotubes must be done while considering the solubility of the ultimate unit alongside.
More research would lead to greater understanding and regenerative forms of gene-specific drug delivery applications 37,38.
QD – Quantum dots
Nanoscopic lattice QDs can transport electrons and are made by humans. In recent years a number of techniques for DNA combination with QDs have been published, including electric, combined interaction, among others 38,39,40. mi RNA, tumor cell identification, single-stranded RNA delivery have all been shown to be successful with these higher-order assemblies 41.
Enhancing gene drug delivery by Physical Methods
Advanced physical gene distribution techniques, such as sonoporation, electroporation, gene gun, magnetoporation, and optoporation, have been extensively developed and are gaining popularity due to their shortness and lack of toxicity.
Electroporation
The physical process of inserting polar molecules such as DNA into eukaryotic cells via the cell membrane by exposing cells to electric pulses is known as electroporation or electropermeabilization (EP). Because of its high gene transmission quality and reduced side effects, electroporation is becoming more common as a nonviral gene delivery tool. It may transmit genes to a range of tissues, including muscle, skin, and even tumors specifically 42. Despite the fact that electroporation has been extensively studied and applied, it is still constrained by the following limitations: (i) electroporation’s transfection efficiency varies based on the tumor type, (ii) electroporation’s cell viability is still poor 43. The process of electroporation is represented by figure 5.
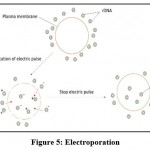 |
Figure 5: Electroporation. |
Sonoporation
The term “sonoporation” refers to the use of ultrasound to create tiny openings in cell membranes for the transport of nucleic acid materials. Sonoporation is analogous to electroporation, in which DNA is propelled along an electromagnetic field by an electrical force. Passive diffusion is used to mediate sonoporation. The duration and strength of ultrasound determine the efficiency of the transition. When used in conjunction with therapeutic genes, sonoporation has the potential to induce apoptosis. The use of combinations with chemicals and diagnostic ultrasound are optimistic ways to address the current drawbacks of sonoporation, which include poor efficiency of gene transfer and disruption to target cells 44.
This sonoporation process can be better understood by Figure 6.
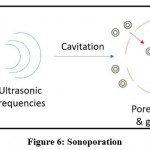 |
Figure 6: Sonoporation. |
Magnetoporation
The aim of magnetoporation is to bring genetic material into the cell while being influenced by a magnetic field A biomolecule/magnetic reagent composite is formed by mixing exogenous nucleic acids with magnetofection reagent. The composite is then transported into the cell under the influence of a magnetic field. The magnetic field stimulated cell membrane phagocytosis and pinocytosis 43.
Optoporation
Optoporation, a method for inserting specific genes into targeting cells that uses directed laser pulses to create a temporary fracture in the plasma membrane, has been extended to a range of cell types in vitro. It has been discovered that when DNA plasmid is exposed to radiation with higher-energy, isolated pulses, it piles up on the cell membrane at the incinerated site. It has been discovered that when a DNA plasmid is exposed to radiation with higher-energy, isolated pulses, it piles up on the cell membrane at the incinerated site 45.
Gene therapy in COVID-19 vaccine
Gene therapy in COVID-19 has implementation such as a multivalent vaccine which targets the coronavirus antisense Ribonucleic acid and a double-stranded ribonucleic acid which aim at the ORF1ab of SARS-CoV-2 and the N, E, S and M region of the genome were recently patented. Additionally, 2 single-stranded RNAs targeting the CoV-2 gene area which is conserved were created which together inhibited the SARS-CoV-2 gene significantly 46. Pfizer, Moderna Inc., and BioNTech recently developed vaccines mRNA-1273 and BNT162, which were recently approved for emergency use and vaccination by the US FDA. This study covers the coronavirus related anatomy, methodology of the viral spreadability, methods of identification of viral strains and human manifestations 47.
Gene therapy in COVID-19 vaccine:
Implementations
AAV8 vector infusion results in strong Factor IX gene expression and is hence used to treat Hemophilia B48.
Platelet gene therapy for hemophilia A is made possible by nongenotoxic antibody-drug conjugate conditioning 49.
In the treatment of cystic fibrosis, a gene-specific drug delivery system is used 50,51.
Parkinson’s Disease is also treated using gene specific drug delivery 52.
Huntington’s disease is treated using gene specific drugs 53.
Treating intrinsic metabolic errors, such as those of the liver 54,55.
In stomach cancer, MicroRNA-335-5p can act as a silencer of tumors and aggression 56.
Using myeloid cell-specific gene promoters, hematopoietic stem cell gene delivery for brain metastases has been created 57.
Combination cancer therapy employs tumour compressing genes 58.
m-RNA is delivered to specific tissues for more efficient outcomes 59.
Some gene-directed enzyme treatments have the potential to treat cancer 60.
To resolve numerous drug resistance and facilitate synergistic tumor inhibition, genes and chemotherapeutics are delivered simultaneously via co-polymeric micellar nanostructures 61.
Nanostructure carrier lipids are used as gene specific drug delivery systems in lung cancer 62.
By targeting BTG2, MiR-27a-3p acts as an oncogene in gastric cancer 63.
Cardiac remodeling in Type 2 diabetes suppressed due to gene therapy that targets cardiac p110α 64.
Gliomas are also treated using gene therapy 65.
Gene therapy to the blood–brain barrier and subsequent protein secretion for the treatment of Niemann Picks type C2 disease 66.
Using human HSC gene editing to dissect ELANE neutropenia pathogenicity 67.
Creating self-assembling mRNA vaccines 68.
Gene specific drug delivery as personalized medicine for neurological disorders 69.
Crispr CAS9-based gene therapy for lung cancer is being developed 70.
In sickle cell disorder, gene therapy will improve survival 71.
Spinal Muscular Atrophy treatment with Gene Therapy 72.
The cells of the hair are regenerated by using gene therapy 73.
Neovascular Eye disease is also treated using gene specific drug delivery systems 74.
Cancer immunotherapy is also carried out using gene therapy 75.
Using a Cocal-Pseudotyped Lentiviral Vector, In Vivo Gene Therapy for Canine SCID-X1 76.
Applications in dermatology have also been observed 77.
There are gene specific drug delivery systems developed for the treatment of intervertebral disc degeneration 78.
Gene therapy is also used in treating lysosomal storage diseases 79.
Inner ear gene delivery using viral vectors 80.
Glaucoma is also treated by gene therapy 81,82.
AIEgen probes with peptide or DNA modifications for biosensing are built in a modular fashion 83.
Tetrahedral self-assembled DNA nanostructures are formulated and used 84.
GNPs are used for viral detection 85.
DNA sensors are being used for the detection of genes 86.
Uveal melanoma hereditary prediction using gene therapy 10.
2D nanoparticles have been synthesized for gene therapy 87.
The mitotic cycle of prostate cancer cells is inhibited by gene specific drugs 88.
Neuromuscular drugs are treated using RNA-targeted drugs 89.
Development is seen in ocular single stranded RNA targets and drug delivery systems90.
Gene therapy by CRISPR seems to replace the antiviral drugs 91.
Gene delivery is also carried out using Electro spun material 92.
A gene biomarker is used to target cancer cells 93.
Cardiovascular diseases are treated using gene specific nanoparticles 94.
Elastin-like polypeptides are being developed for in vivo specific gene therapy 95.
Hepatocellular carcinoma was treated using RNA nanoparticles 96.
In the treatment of AL port Syndrome, adenovirus carriers are used to pass DNA or RNA in the cells of the glomerulus 97.
COVID-19 Vaccines are being developed using gene-specific drug delivery system 98.
Conclusion
The ability of gene therapy to have long-term health benefits, as shown by research advancements and clinical successes in recent years, justifies continued hope and increased attempts to make this therapy part of our regular armamentarium for treating severe human diseases. The progress made in discovering miRNA functions and their roles in cancer has sparked high hopes for a gene-specific drug delivery mechanism. Clinical gene therapy is based on delivering genetic information to tissues in vivo and is a popular procedure used in science. Gene-specific drug delivery systems shows multiplied effectiveness, monitoring, drug availability, and distribution over other drug delivery systems.
This review highlights that the gene-specific drug delivery system has vast scope in therapy and can prove advantageous over other therapies, because it includes several carriers and different methods of plasma membrane permeation. All of these factors can be modified as per convenience to achieve better and desired results. The article also includes recent advancements and the role of gene delivery in COVID-19 vaccines.
Acknowledgement
The authors wish to acknowledge the help provided by the technical and support staff of GES’s Sir Dr. M. S. Gosavi College of Pharmaceutical Education and Research, Nashik.
Conflict of interest
The authors declare no conflicts of interest.
Funding Sources
No specific grant was received from any funding agency.
References
- National Cancer Institute, Nanodelivery systems and Devices Branch,USA,2017. https://www.cancer.gov/nano/cancer-nanotechnology/treatment (Accessed on 5 march 2021).
- Mayo Clinic Staff, 29,2017. https://www.mayoclinic.org/tests-procedures/gene-therapy/about/pac-20384619 (Accessed on 5 march 2021).
- Mancinelli L, Cronin M, Sadée W. Pharmacogenomics: the promise of personalized medicine. Aaps Pharmsci. 2000 Mar;2(1):29-41.
- Ruan MZC, Guse K, Lee B., Genetics of Bone Biology and Skeletal Disease:Prospects of Gene Therapy.2nd Elsevier,USA, 2013.
- Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu. Rev. Biochem. 2005 Jul 7;74:711-38.
- Soofiyani SR, Baradaran B, Lotfipour F, Kazemi T, Mohammadnejad L. Gene therapy, early promises, subsequent problems, and recent breakthroughs. Adv. Pharm. Bull. 2013 Dec;3(2):249.
- Bilsland AE, Spiliopoulou P, Evans TJ. Virotherapy: cancer gene therapy at last?. F1000research. 2016;5.
- Miller, A. D. Development and applications of retroviral vectors. 2011.
- Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017 Aug;31(4):317-34.
- Dogrusöz M, Jager MJ. Genetic prognostication in uveal melanoma. Acta Ophthalmol.2018 Jun;96(4):331-47.
- Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J. Control. Release.2001 Feb 23;70(3):399-421.
- Kumar V, Wen D, Mahato RI. Non-viral delivery of nucleic acid complexes. InComprehensive Biomaterials II 2017 Jan 1. 506-526, Elsevier.
- Wang Q, Jiang H, Li Y, Chen W, Li H, Peng K, Zhang Z, Sun X. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials. 2017 Apr 1;122:10-22.
- Zakeri A, Kouhbanani MA, Beheshtkhoo N, Beigi V, Mousavi SM, Hashemi SA, Karimi Zade A, Amani AM, Savardashtaki A, Mirzaei E, Jahandideh S. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: a developing horizon. Nano reviews and experiments. 2018 Jan 1;9(1):1488497.
- Santos JL, Ren Y, Vandermark J, Archang MM, Williford JM, Liu HW, Lee J, Wang TH, Mao HQ. Continuous production of discrete plasmid DNA‐polycation nanoparticles using flash nanocomplexation. Small. 2016 Dec;12(45):6214-22.
- Huang FW, Feng J, Nie J, Cheng SX, Zhang XZ, Zhuo RX. Convenient Preparation of Biodegradable PEI‐Containing Polymers as Non‐Viral Vectors for Gene Transfection. Macromol. Biosci. 2009 Dec 8;9(12):1176-84.
- Masotti A, Bordi F, Ortaggi G, Marino F, Palocci C. A novel method to obtain chitosan/DNA nanospheres and a study of their release properties. Nanotechnology. 2008 Jan 14;19(5):055302.
- Surendra Nimish,2013, Chitosan nanoparticles, Gene therapy, Published by Woodhead Publishing Limited, first edition, pp.188.
- Zhao X, Li Z, Liu W, Lam W, Sun P, Kao RY, Luk KD, Lu WW. Octaarginine-modified chitosan as a nonviral gene delivery vector: properties and in vitro transfection efficiency. J. Nanopart. Res. 2011 Feb;13(2):693-702.
- Jayakumar R, Chennazhi KP, Muzzarelli RA, Tamura H, Nair SV, Selvamurugan N. Chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr. Polym.2010 Jan 5;79(1):1-8.
- Alhoranta AM, Lehtinen JK, Urtti AO, Butcher SJ, Aseyev VO, Tenhu HJ. Cationic amphiphilic star and linear block copolymers: synthesis, self-assembly, and in vitro gene transfection. Biomacromol. 2011 Sep 12;12(9):3213-22.
- Van de Wetering P, Zuidam NJ, Van Steenbergen MJ, Van Der Houwen OA, Underberg WJ, Hennink WE. A mechanistic study of the hydrolytic stability of poly (2-(dimethylamino) ethyl methacrylate). Macromol. 1998 Nov 17;31(23):8063-8.
- Arya, G., Kumari, R. M., Sharma, N., Gupta, N., Chandra, R., and Nimesh, S. (2018) Polymeric nanocarriers for site-specific gene therapy. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems,William Andrew Publishing, 689-714.
- Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polym. 2011 Sep;3(3):1377-97.
- Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int. J. Pharm.2009 Feb 9;367(1-2):195-203.
- Ojha B, Jain VK, Mehra NK, Jain K. Nanotechnology: Introduction and Basic Concepts. Dendrimers in Nanomedicine: Concept, Theory and Regulatory Perspectives. 2021 Mar 22:1.
- Ch D, Uchegbu IF, Schatzlein AG. Dendrimers in gene delivery [A Review]. Adv. Drug Deliv. Rev. 2005;57:2177-202.
- Zhang J, Chen L, Tse WH, Bi R, Chen L. Inorganic nanoparticles: engineering for biomedical applications. IEEE Nanotechnol. Mag. 2014 Oct 9;8(4):21-8.
- Manzano M, Colilla M, Vallet-Regí M. Drug delivery from ordered mesoporous matrices. Expert opin. drug deliv. 2009 Dec 1;6(12):1383-400.
- Paris JL, Vallet-Regí M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics. 2020 Jun;12(6):526.
- D’Agata R, Palladino P, Spoto G. Streptavidin-coated gold nanoparticles: critical role of oligonucleotides on stability and fractal aggregation. Beilstein J. Nanotechnol.2017 Jan 2;8(1):1-1.
- Wu S, Li D, Wang J, Zhao Y, Dong S, Wang X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sens. Actuators B Chem.2017 Jan 1;238:427-33.
- Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Regulating immune response using polyvalent nucleic acid− gold nanoparticle conjugates. Mol. Pharm. 2009 Dec 7;6(6):1934-40.
- Du B, Gu X, Han X, Ding G, Wang Y, Li D, Wang E, Wang J. Lipid‐coated gold nanoparticles functionalized by folic acid as gene vectors for targeted gene delivery in vitro and in vivo. ChemMedChem. 2017 Nov 8;12(21):1768-75.
- Katz, E. Magnetic nanoparticles. Magnetochemistry6(1),6.
- Paul W, Sharma CP. Inorganic nanoparticles for targeted drug delivery. Biointegration of Medical Implant Materials. 2020 Jan 1:333-73.
- Singh A, Hua Hsu M, Gupta N, Khanra P, Kumar P, Prakash Verma V, Kapoor M. Derivatized carbon nanotubes for gene therapy in mammalian and plant cells. ChemPlusChem. 2020 Mar;85(3):466-75.
- Banerjee A, Pons T, Lequeux N, Dubertret B. Quantum dots–DNA bioconjugates: synthesis to applications. Interface Focus. 2016 Dec 6;6(6):20160064.
- Zhang C, Ding C, Xiang D, Li L, Ji X, He Z, Xian Y. DNA functionalized fluorescent quantum dots for bioanalytical applications. Chin. J. 2016 Mar;34(3):317-25.
- Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem. Rev. 2013 Mar 13;113(3):1904-2074.
- Yang Y, Mao G, Ji X, He Z. DNA-templated quantum dots and their applications in biosensors, bioimaging, and therapy. J. Mater. Chem. B. 2020;8(1):9-17.
- Shirley SA, Heller R, Heller LC. Electroporation gene therapy. Cancer Gene Ther. 2014 Jan 1:93-106.
- Du X, Wang J, Zhou Q, Zhang L, Wang S, Zhang Z, Yao C. Advanced physical techniques for gene delivery based on membrane perforation. Drug Deliv. 2018 Jan 1;25(1):1516-25.
- Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Sueishi M. Sonoporation: Gene transfer using ultrasound. World J. Methodol.2013 Dec 26;3(4):39.
- Davis AA, Farrar MJ, Nishimura N, Jin MM, Schaffer CB. Optoporation and genetic manipulation of cells using femtosecond laser pulses. Biophys. J. 2013 Aug 20;105(4):862-71.
- Zhou W, Chen D. Emerging Patent Landscape for Gene Therapy as a Potential Cure for COVID-19. Math. Probl. Eng. 2021 Jan 8;2021.
- Chilamakuri R, Agarwal S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206.
- Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D, Riddell A. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N J. Med.2014 Nov 20;371(21):1994-2004.
- Gao C, Schroeder JA, Xue F, Jing W, Cai Y, Scheck A, Subramaniam S, Rao S, Weiler H, Czechowicz A, Shi Q. Nongenotoxic antibody-drug conjugate conditioning enables safe and effective platelet gene therapy of hemophilia A mice. Blood Adv. 2019 Sep 24;3(18):2700-11.
- Flotte TR, Laube BL. Gene therapy in cystic fibrosis. Chest. 2001 Sep 1;120(3):124S-31S.
- Maule G, Arosio D, Cereseto A. Gene therapy for cystic fibrosis: progress and challenges of genome editing. Int. J. Mol. Sci. 2020 Jan;21(11):3903.
- Feng LR, Maguire-Zeiss KA. Gene Therapy in Parkinson’s Disease. CNS drugs. 2010 Mar;24(3):177-92.
- Miniarikova J, Zanella I, Huseinovic A, van der Zon T, Hanemaaijer E, Martier R, Koornneef A, Southwell AL, Hayden MR, van Deventer SJ, Petry H. Design, characterization, and lead selection of therapeutic miRNAs targeting huntingtin for development of gene therapy for Huntington’s disease. Mol. Ther. Nucleic Acids 2016 Jan 1;5:e297.
- Ginocchio VM, Ferla R, Auricchio A, Brunetti-Pierri N. Current status on clinical development of adeno-associated virus-mediated liver-directed gene therapy for inborn errors of metabolism. Hum. Gene. ther. 2019 Oct 1;30(10):1204-10.
- Brunetti-Pierri N, Lee B. Gene therapy for inborn errors of liver metabolism, Mol. Genet. Metab. 2005 Sep 1;86(1-2):13-24.
- Sandoval-Bórquez A, Polakovicova I, Carrasco-Véliz N, Lobos-González L, Riquelme I, Carrasco-Avino G, Bizama C, Norero E, Owen GI, Roa JC, Corvalán AH. Correction to: MicroRNA-335-5p is a potential suppressor of metastasis and invasion in gastric cancer. Clin. Epigenetics. 2021 Dec;13(1):1-3.
- Andreou T, Rippaus N, Wronski K, Williams J, Taggart D, Cherqui S, Sunderland A, Kartika YD, Egnuni T, Brownlie RJ, Mathew RK. Hematopoietic Stem Cell Gene Therapy for Brain Metastases Using Myeloid Cell–Specific Gene Promoters. JNCI: J. Natl. Cancer Inst. 2020 Jun 1;112(6):617-27.
- Chen X, Zhu Q, Xu X, Shen S, Zhang Y, Mo R. Sequentially Site‐Specific Delivery of Apoptotic Protein and Tumor‐Suppressor Gene for Combination Cancer Therapy. Small. 2019 Oct;15(40):1902998.
- Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. nanotechnol. 2020 Apr;15(4):313-20.
- Ly CY, Kunnath AP. Application of Gene-Directed Enzyme Prodrug Therapy in Cancer Treatment. Int J Biomed Res Prac. 2021;1(1):1-8.
- Gao J, Chen L, Qi R, Zhou Z, Deng Z, Shi J, Qin T, Zhao S, Qian Y, Shen J. Simultaneous delivery of gene and chemotherapeutics via copolymeric micellar nanoparticles to overcome multiple drug resistance to promote synergistic tumor suppression. J. Biomater. Appl.2019 Jul;34(1):130-40.
- Han Y, Li Y, Zhang P, Sun J, Li X, Sun X, Kong F. Nanostructured lipid carriers as novel drug delivery system for lung cancer gene therapy. Pharm. Dev. Technol.2016 Apr 2;21(3):277-81.
- Zhou L, Liang X, Zhang L, Yang L, Nagao N, Wu H, Liu C, Lin S, Cai G, Liu J. MiR-27a-3p functions as an oncogene in gastric cancer by targeting BTG2. Oncotarget. 2016 Aug 9;7(32):51943.
- Prakoso D, De Blasio MJ, Tate M, Kiriazis H, Donner DG, Qian H, Nash D, Deo M, Weeks KL, Parry LJ, Gregorevic P. Gene therapy targeting cardiac phosphoinositide 3-kinase (p110α) attenuates cardiac remodeling in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2020 Apr 1;318(4):H840-52.
- Medina-Enríquez MM, Carlos-Escalante JA, Medrano-Hernández A, Wegman-Ostrosky T. Gene Therapy in Gliomas. InPrinciples of Neuro-Oncology 2021 (pp. 107-122). Springer, Cham.
- Hede E, Christiansen CB, Heegaard CW, Moos T, Burkhart A. Gene therapy to the blood–brain barrier with resulting protein secretion as a strategy for treatment of Niemann Picks type C2 disease. J. 2021 Feb;156(3):290-308.
- Rao S, Yao Y, de Brito JS, Yao Q, Shen AH, Watkinson RE, Kennedy AL, Coyne S, Ren C, Zeng J, Serbin AV. Dissecting ELANE neutropenia pathogenicity by human HSC gene editing. Cell Stem Cell. 2021 Jan 28.
- Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Advances in drug delivery rev. 2021 Jan 2.
- Jain KK. Personalized Therapy of Neurological Disorders. InTextbook of Personalized Medicine. Springer, 2021; 1(1): 107-122.
- Nair J, Nair A, Veerappan S, Sen D. Translatable gene therapy for lung cancer using Crispr CAS9—an exploratory review. Cancer gene therapy. 2020 Apr;27(3):116-24.
- Curtis SA, Shah NC. Gene therapy in sickle cell disease: possible utility and impact. Cleve Clin J Med. 2020 Jan 1;87(1):28-9.
- Waldrop MA, Karingada C, Storey MA, Powers B, Iammarino MA, Miller NF, Alfano LN, Noritz G, Rossman I, Ginsberg M, Mosher KA. Gene therapy for spinal muscular atrophy: safety and early outcomes. Pediatrics. 2020 Sep 1;146(3).
- Shibata SB, West MB, Du X, Iwasa Y, Raphael Y, Kopke RD. Gene therapy for hair cell regeneration: review and new data. Hear. Res.2020 May 5:107981.
- Lin FL, Wang PY, Chuang YF, Wang JH, Wong VH, Bui BV, Liu GS. Gene therapy intervention in neovascular eye disease: A recent update. Mol. Ther. 2020 Jun 30.2120-2138.
- Crowther MD, Svane IM, Met Ö. T-cell gene therapy in cancer immunotherapy: why it is no longer just CARs on the road. Cells. 2020 Jul;9(7):1588.
- Rajawat YS, Humbert O, Cook SM, Radtke S, Pande D, Enstrom M, Wohlfahrt ME, Kiem HP. In vivo gene therapy for canine SCID-X1 using Cocal-Pseudotyped lentiviral vector. Hum. Gene Ther. 2021 Jan 1;32(1-2):113-27.
- Sarkar T, Sarkar S, Gangopadhyay DN. Gene therapy and its application in dermatology. Indian J. 2020 Sep;65(5):341.
- Chen S, Luo M, Kou H, Shang G, Ji Y, Liu H. A review of gene therapy delivery systems for intervertebral disc degeneration. Curr. Pharm. biotechnol. 2020 Mar 1;21(3):194-205.
- Leal AF, Espejo-Mojica AJ, Sánchez OF, Ramírez CM, Reyes LH, Cruz JC, Alméciga-Díaz CJ. Lysosomal storage diseases: current therapies and future alternatives. J. Mol. Med. 2020 Jul;98(7):931-46.
- Maguire CA, Corey DP. Viral vectors for gene delivery to the inner ear. Hear. res. 2020 Feb 23:107927.107927.
- Komáromy AM, Koehl KL, Park SA. Looking into the future: Gene and cell therapies for glaucoma. Vet. Ophthalmol. 2021 Mar;24:16-33.
- Wu J, Bell OH, Copland DA, Young A, Pooley JR, Maswood R, Evans RS, Khaw PT, Ali RR, Dick AD, Chu CJ. Gene therapy for glaucoma by ciliary body aquaporin 1 disruption using CRISPR-Cas9. Mol. Ther. 2020 Mar 4;28(3):820-9.
- Xia F, Wu J, Wu X, Hu Q, Dai J, Lou X. Modular design of peptide-or DNA-modified AIEgen probes for biosensing applications. Acc. Chem. Res. 2019 Oct 28;52(11):3064-74.3064-3074.
- Li S, Tian T, Zhang T, Cai X, Lin Y. Advances in biological applications of self-assembled DNA tetrahedral nanostructures. Mater. Today. 2019 Apr 1;24:57-68.57-68.
- Draz MS, Shafiee H. Applications of gold nanoparticles in virus detection. Theranostics. 2018;8(7):1985.
- Pohanka M. Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Mater. 2018 Mar;11(3):448.
- Yin F, Gu B, Lin Y, Panwar N, Tjin SC, Qu J, Lau SP, Yong KT. Functionalized 2D nanomaterials for gene delivery applications. Coord. Chem. Rev.2017 Sep 15;347:77-97.
- Zhang Y, Wang Y, Meng L, Huang Q, Zhu Y, Cui W, Cheng Y, Liu R. Targeted micelles with chemotherapeutics and gene drugs to inhibit the G1/S and G2/M mitotic cycle of prostate cancer. J. Nanobiotechnol. 2021 Dec;19(1):1-5.
- Ferlini A, Goyenvalle A, Muntoni F. RNA-targeted drugs for neuromuscular diseases. Science. 2021 Jan 1;371(6524):29-31.
- Jiang J, Zhang X, Tang Y, Li S, Chen J. Progress on ocular siRNA gene‐silencing therapy and drug delivery systems. Fund Clin Pharmacol 2021 Feb;35(1):4-24.
- Hashmat R, Yousaf MZ, Rahman Z, Anjum KM, Yaqoob A, Imran M. Crispr-cas replacing antiviral drugs against hiv: An update. Crit. Rev. Eukaryot. Gene Expr. 2020;30(1).
- Rao GK, Kurakula M, Yadav KS. Application of Electrospun Materials in Gene Delivery. Electrospun Materials and Their Allied Applications. 2020 Apr 14:265-306.
- Zolotovskaia M, Sorokin M, Garazha A, Borisov N, Buzdin A. Molecular pathway analysis of mutation data for biomarkers discovery and scoring of target cancer drugs. InNucleic Acid Detection and Structural Investigations 2020 (pp. 207-234). Humana, New York, NY.
- Deng Y, Zhang X, Shen H, He Q, Wu Z, Liao W, Yuan M. Application of the nano-drug delivery system in treatment of cardiovascular diseases. Front. Bioeng. Biotechnol. .2020 Jan 31;7:489.
- Yi A, Sim D, Lee YJ, Sarangthem V, Park RW. Development of elastin-like polypeptide for targeted specific gene delivery in vivo. J. nanobiotechnol. 2020 Dec;18(1):1-4.
- Chen X, Chen T, Zhang L, Wang Z, Zhou Q, Huang T, Ge C, Xu H, Zhu M, Zhao F, Yao M. Cyclodextrin-mediated formation of porous RNA nanospheres and their application in synergistic targeted therapeutics of hepatocellular carcinoma. Biomater. 2020 Dec 1;261:120304.
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 2003 Jun 19;348(25):2543-56.
- Ho RJ. Warp-speed Covid-19 Vaccine development: beneficiaries of maturation in biopharmaceutical technologies and public-private partnerships. J. Pharm. Sci. 2021 Feb 1;110(2):615-8.

This work is licensed under a Creative Commons Attribution 4.0 International License.





