How to Cite | Publication History | PlumX Article Matrix
Prebiotic Profiling of Indigenous Selected dioscorea Spp. Using In-Vitro Techniques
Mayur Arjun Aswani1 , Suyash Arunrao Kathade2
, Suyash Arunrao Kathade2 , Akib Nisar2
, Akib Nisar2 , Pashmin Kaur Anand2
, Pashmin Kaur Anand2 , Bipinraj Nirichan Kunchiraman2
, Bipinraj Nirichan Kunchiraman2 and Suresh Dnyadeo Jagtap1*
and Suresh Dnyadeo Jagtap1*
1Department of Herbal Medicine, Interactive Research School for Health Affairs, Bharati Vidyapeeth (Deemed to be University), Pune, India.
2Department of Microbiology, Rajiv Gandhi Institute of I.T. and Biotechnology, Bharati Vidyapeeth (Deemed to be University), Pune, India.
Coresponding Author E-mail: chiritatml@rediffmail.com
DOI : http://dx.doi.org/10.13005/bbra/2993
ABSTRACT:
The current study used an in-vitro technique to evaluate the functional potential of Dioscorea alata L. and D. bulbifera L. extracts as prebiotics. Prebiotics are nondigestible carbohydrates that undergo a selective fermentation process in the gut to benefit the host, according to Gibson and Roberfroid in 1995. Many wild edible plants are high in carbohydrates and are utilised as both a staple food and medicine for a variety of stomach-related disorders. This study employed sweet tuber (ST), bitter tuber (BT), sweet bulbils (SB), and bitter bulbils (BB) from D. bulbifera, as well as tuber (AT) from D. alata and extracted prebiotics using standard method.The AT plant sample seemed to have the least reducing sugars, with a concentration of 2.83 mg/mL. The prebiotic activity of ST, BT, SB, BB, and AT samples was examined as the sole carbon source for microorganisms; among these, AT exhibited a considerable increase in the growth of recognised probiotics Lactobacillus plantarum, Saccharomyces cerevisiae, S. boulardii, and Pichia spp. in-vitro when compared to fructooligosaccharides (FOS). This preliminary investigation indicates that AT has the potential to be used as a promising prebiotic.
KEYWORDS: Dioscorea spp.,; Gut Microbiome; Prebiotic; Probiotics
Download this article as:| Copy the following to cite this article: Aswani M. A, Kathade S. A, Nisar A, Anand P. K, Kunchiraman B. N, Jagtap S. D. Prebiotic Profiling of Indigenous selected dioscorea Spp. Using In-Vitro Techniques. Biosci Biotech Res Asia 2022;19(2). |
| Copy the following to cite this URL: Aswani M. A, Kathade S. A, Nisar A, Anand P. K, Kunchiraman B. N, Jagtap S. D. Prebiotic Profiling of Indigenous selected dioscorea Spp. Using In-Vitro Techniques. Biosci Biotech Res Asia 2022;19(2). Available from: https://bit.ly/38Tsp8f |
Introduction
Humans have trillions of complex communities of microorganisms in their gastrointestinal tract, known as the gut microbiome. The preservation of the structure and function of the gut microbiome is critical for host homeostasis and immunity. This gut microbiome is impeded by a variety of factors such as nutrition, modern lifestyle, antibiotic usage, and so on, leading in dysbiosis. Dysbiosis is characterised as an excess of pathogens in the gut, which has the potential to induce inflammatory bowel disease (IBD), colon cancer, and other diseases. The use of functional foods such as prebiotics and probiotics, on the other hand, can assist to return a dysbiotic condition to a healthy one1–4. The use of functional foods such as prebiotics and probiotics, on the other hand, can assist to return a dysbiotic condition to a healthy one. Nowadays, there is an increasing trend of consumer awareness toward the demand for functional foods that are claimed to improve the consumer’s health. Apart from other food ingredients, prebiotics is among those which have attracted much attention recently4,5. Gibson and Roberfroid introduced the concept of prebiotics in 1995, defining them as “a non-digestible and selectively fermented ingredient that allows specific changes in composition and/or activity in the gastrointestinal microbiota that confers health benefits to hosts”5–7.Non-digestible substrates such as inulin, fructooligosaccharides (FOS), galactooligosaccharides (GOS), and lactulose have been identified as prebiotics since they serve as a medium for saccharolytic bacteria. Prebiotics selectively increase/decrease specific gut bacteria, that can result in health advantages such as Lactobacillus growth advantage over E. coli and Clostridium spp., which cannot utilize these substances, resulting in the Lactobacillus population domination.
Prebiotics can influence the makeup and function of gut bacteria by providing energy sources. Specific probiotic species can utilize a given prebiotic such as fermentation of inulin and FOS by Lactobacillus spp. Prebiotics can also change the environment of the gastrointestinal tract. The fermentation products of prebiotics are mainly acids, which lower the gut pH8–10. The pH shift can affect the population of acid-sensitive organisms like Proteobacteria and encourage Firmicutes. The role of these short-chain fatty acids is diverse that provokes antimicrobial, anti-inflammatory, immunomodulatory activities11–13.Furthermore, the molecular effect of prebiotics is mostly given through the stimulation of probiotics that generate short-chain fatty acids (SCFAs), which indirectly reduces pathogen development while changing the activity and composition of gut microbiota14–16. The incorporation of prebiotics into the diet is garnering global interest for human health purposes. Due to the obvious rising demand for prebiotics, there is a need to investigate new commercially feasible and sustainable sources of prebiotics. Prebiotics are mostly extracted from well-established Jerusalem plants such as artichoke, chicory root, onion, and leeks17–19.
However, the usage of wild edible plants in Maharashtra has not been intensively investigated. As a result, the purpose of this research is to investigate the prebiotic potential of Dioscorea alata and D. bulbifera. Dioscorea is a wild medicinal plant that has been used for food, immunomodulation, and the treatment of gastrointestinal diseases20,21. The genus has gained much importance, not just as a source of sustenance, but also to sustain tribal livelihoods. The genus has been associated with a plethora of phytochemicals such as diosgenin, dioscin, and others that have a promising potential in the pharmaceutical business.Since prebiotics are widely recognised from natural sources such as chicory root and Jerusalem artichokes, there is still room for improvement in finding better prebiotics that may have antimicrobial activities when coupled with probiotics.
Aside from being abundant in nutrients, Dioscorea tubers and bulbils are used to cure diarrhoea, constipation, stomach ulcers, and other ailments22. Their proximate investigation indicated the presence of significant amounts of polysaccharides and crude fibres, indicating the possibility of extracting a significant quantity of prebiotic from these tubers. Prebiotics like inulin and fructooligosaccharides have been used to treat gastrointestinal issues like dysentery, constipation, and ulcers. As a result, our research have shown another possible application for Dioscorea bulbifera and D. alata tubers and bulbils as a source of prebiotics. Hence, the objective of the study are: (i) to quantify the amount of water-soluble extract from D. bulbifera and D. alata tubers and bulbils; (ii) to characterize extract; and (iii) to elucidate the function of extract in supporting the growth of L. plantarum, S. boulardii, S. cerevisiae, and Pichia spp. using the in-vitro approach.
Material and methods
Sample Collection
Wild edible plant parts of Dioscorea bulbifera sweet and bitter tubers (ST, BT respectively), sweet and bitter bulbils (SB, BB respectively); and D. alata tubers (AT) were collected from the village, Chavani, Tal. Khalapur, Dist.- Raigad, Maharashtra State, India and were authenticated by Dr. Suresh Jagtap (Plant Taxonomist). Required prior approval of the biodiversity board was obtained (MSBB/Research/576/2021-22). These samples were washed with distilled water to remove dirt particles and were dried in a hot air oven at 60 °C till constant weight was achieved. Further, the dried samples were grinded to a fine powder and stored in airtight poly bags at room temperature for further use.
While probiotic cultures were isolated and identified as Lactobacillus plantarum (LB-VII) (NCBI Accession no. MK608674.1), Pichia kudriavzevii (S-I) (NCBI Accession no. LC528140.1), Saccharomyces cerevisiae (MD) (NCBI Accession no. LC528142.1), and Saccharomyces boulardii (SB) were procured from Pathology lab, Rajiv Gandhi Institute of I.T. and Biotechnology, Bharati Vidyapeeth (Deemed to be University), Pune 23–26.
In-vitro Gastrointestinal Environment Simulation for Enrichment of Prebiotic Content
Simulated gastrointestinal treatment on ST, BT, SB, BB and AT powders were performed as described by Yadav S et al., 2014 27. Accordingly, dried powders at a concentration of 10% w/v were added to gastric juice containing 0.6 mL of pepsin (pH 2.0) and the mixture was incubated for 2 hours at 120 revolutions per minute (rpm) to simulate the gastric environment; whereas for the intestinal environment, pH was adjusted to 7.5 along with the addition of bile pancreatin mixture (4 mL) which was incubated on a shaker for 2 hours at 37°C. The enzymatic reaction was nullified by incubating the resultant residue in cold distilled water for 1 hour. Finally, the residue was filtrated through a cheesecloth and the undigested residue was oven-dried at 55°C and used for further experiment.
Determination of Total Reducing Sugar
The reducing sugar content of undigested and digested residues was determined using the 3,5-dinitrosalicylic acid (DNS) assay28. In brief, 4 mg/mL concentrations of undigested and digested samples were treated with 1 mL of DNS reagent for 5 minutes at 100ºC. Following that, the tubes were diluted to a level of 10 mL with distilled water and spectrophotometric analysis was done at 520 nm. By plotting a standard graph with a known quantity of maltose, the concentrations of reducing sugars in these samples were estimated.
Assessment of Prebiotic Potential
The growth response of probiotic cultures was measured in presence of undigested, digested samples and standard prebiotic fructooligosaccharides (FOS). Prebiotics at a concentration of 4 mg/mL were added in Yeast extract Peptone Dextrose(YPD) medium26 (yeast extract- 10 g/L; peptone- 20 g/L) for yeasts while for Lactobacillus modified de Man Rogosa Sharpe (MRS) medium (Peptone- 10 g/L; yeast extract- 5 g/L; tween 80- 1 g/L; sodium acetate- 5 g/L; magnesium sulphate- 0.1 g/L; manganese sulphate- 0.05 g/L; dipotassium hydrogen phosphate- 2 g/L). These growth media were devoid of any other carbon source but prebiotics was autoclaved at 121°C for 15 min. Cultures S-I, MD, SB, and LB-VII were grown under respective broth conditions and were centrifuged for 5 min at 10,000 rpm; re-suspended in sterile saline before inoculation of 100 μl individual culture in prebiotic containing media and incubated at 37°C. Culture response to this prebiotic were assessed spectrophotometrically at 600 nm with time intervals of 0, 24 and 48 hours. Growth media devoid of cultures was used as negative control while for standard control FOS was used. Medium YPD and MRS along with glucose were used as a positive control29.
Statistical analysis
Each experiment was performed in triplicate and data were analysed using one-way analysis of variance (ANOVA) software and results expressed as Mean ± SD.Differences were considered statistically significant when p < 0.05 (p > 0.05 = ns, p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***).
Results and Discussion
Gastrointestinal Tolerance of Prebiotics
Prebiotics are short-chain carbohydrates that are resistant to the cleavage action of human digestive enzymes. As a result, for prebiotics to be optimum and efficient, they must enter the intestine30. These short-chain carbohydrates have 3 to 10 sugar moieties with a degree of polymerization (DP) ranging from 2 to 60. It has been proposed that resistance to digestion can be caused by either the arrangement of glycosidic linkages between monomeric sugar units or the substrate specificity of human digestive enzymes. However, to provide prebiotic effects, they must be resistant to the action of digestive enzymes as well as extreme pH conditions31. In this study, AT sample was found to possess high amounts of non-digestible matter with 7.71%; followed by SB (6.2%); ST (5.24%); BT (4.32%); and BB (4.08%) on a dry weight basis. Similarly, the proximate analysis of Dioscorea bulbifera and Dioscorea alata revealed fiber contents to be in a range of 4.1-11.0% respectively, on a dry weight basis32. Similar findings were found in this study when dried powders of ST, BT, SB, BB, and AT were subjected to harsh conditions of the gastrointestinal environment. Gastric juice composed of pepsin at lower pH degrades proteins into amino acids, while intestinal juice contains a mixture of digestive enzymes such as amylase, protease and lipase along with bile are responsible to catabolize carbohydrates, proteins and fats respectively into their monomeric form.
Estimation of reducing sugar
Prebiotics, which are non-digestible compounds, are made up of sugar units connected in such a way that they form non-reducing ends and so resist treatment with DNS reagent33. In this study, the sugar contents of undigested and digested samples were measured. The undigested BB sample had the most reducing sugars (20.09 mg/mL), while the digested BT sample contained the most (5.29 mg/mL) (Graph 1c and 1d respectively). There was, however, a considerable decline in digested samples, with the AT sample having the lowest quantity (2.83 mg/mL) (Graph 1e) Chard (9.5 mg/mL), Fennel leaves (11.7 mg/mL), and Mushroom buttons (11 mg/mL) all had comparable sugar profiles, according to Nowak R et al., 201734. Hence, the preliminary data reveals the presence of non-reducing and non-digestible compounds in digested samples.
Growth Stimulatory Effect
Carbohydrates that reach the intestine can indeed be fermented by the gastrointestinal microbiota. To meet the prebiotic criterion, it must be fermentable by gut microorganisms selectively, resulting in host benefit. Several studies have demonstrated that prebiotics like FOS and inulin stimulate the growth of probiotics like Lactobacillus and Bifidobacteria. Probiotics stimulate the production of short-chain fatty acids (SCFAs), which serve a variety of roles in the host. The method by which prebiotics influence microbial diversity in the colon is currently under investigation. Prebiotics have the advantage of promoting the growth of target microorganisms, which then compete with species that are specific to energy sources and exclude them by protecting or promoting the production of beneficial fermentation substances, such as SCFAs, which have immunomodulatory properties, influencing toll-like receptor signaling and the production of pro-inflammatory cytokines35.
In this study, the prebiotic potential of ST, BT, SB, BB, and AT samples were assessed with digestive treatment along with standard control FOS. Known probiotic cultures such as SB, SC, S-I, and LB-VII growth response to different digested samples were estimated spectrophotometrically. It was found that overall, AT sample was comparative with FOS. AT sample could stimulate SB, MD, and S-I with an optical density of 0.74, 0.84, 0.74 respectively after 24 hours of incubation. While FOS induced growth of 0.89, 0.99, 1.03 for SB, MD, and S-I respectively. The cultures further showed good growth after 48-hour incubation reaching an optical density of 1.442 at 600 nm (Graph 2a – 2d). A similar study reported by Sawangwan T et al., 2018 showed prebiotics extracted from mushroom supports the growth of probiotics such as Lactobacillus acidophilus and L. plantarum which is comparable to FOS28.
The control of the gut microbiome is critical since many illnesses are connected to microbial profiles; hence, prebiotics can be utilized as a therapeutic agent to prevent and reverse dysbiotic conditions. Prebiotics are widely established from plants such as Jerusalem artichoke, chicory, onions, and so on; nevertheless, the usage of wild edible plants of Maharashtra for prebiotic potential is yet unexplored. Exploring wild plants for prebiotics with higher prebiotic potential can also serve as a new commercial source of prebiotic extraction, as can introducing wild plants into agricultural fields.
 |
Graph 1: (a – e):Comparison of reducing the sugar by DNS method for undigested and digested residues of respective samples. |
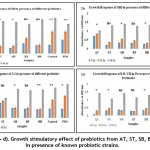 |
Graph 2: (a – d). Growth stimulatory effect of prebiotics from AT, ST, SB, BT, and BB in presence of known probiotic strains. |
Conclusion
Traditional medicine is used to cure ailments in the majority of the globe. However, the emergence of novel infectious illnesses, as well as the increased usage of traditionally used pharmaceutics, has prompted a quest for new biotherapeutics. The gastrointestinal system is home to diverse microbial communities known as the gut microbiome, which perform critical tasks. The number of probiotics in the gut is decreasing as a result of an urbanized lifestyle and the usage of medications. Prebiotics, on the other hand, are well defined as a growth-stimulating ingredient for probiotics. Hence, prebiotics as a source of biotherapeutics can accomplish the modernized need. As defined prebiotics pass through the digestive tract unchanged and modulate gut microbiota. Prebiotics were extracted from Dioscorea bulbifera bulbils (sweet and bitter), tubers (sweet and bitter), and D. alata tubers in this investigation, with AT exhibiting the least presence of reducing sugar after treatment with digestive enzymes. The growth of known probiotic cultures Lactobacillus plantarum, Saccharomyces cerevisiae, S. boulardii, and Pichia spp. was stimulated by all extracted prebiotics ST, BT, SB, BB, and AT; however, AT demonstrated superior growth potential than known prebiotic FOS. This provided a strong indication that AT can be used further as a prebiotic with additional therapeutic properties. In conclusion, Dioscorea alata tubers represent a promising source of natural prebiotics. However, more experimentation on pre-clinical trials is needed to validate the efficacy of prebiotics.
Acknowledgement
The authors would like to thank authorities of Bharati Vidyapeeth (Deemed to be University), Pune, Maharashtra for supporting this study.
Conflict of Interest
All authors declare no conflict of interest.
Funding Sources
There is no funding Source.
References
- Das B, Ghosh S, Kedia S, Rampal R, Saxena S, Bag S, Mitra R, Dayal M, Mehta O, Surendranath A, Travis P, Tripathi P, Nair G, and Ahuja V. Analysis of the Gut Microbiome of Rural and Urban Healthy Indians Living in Sea Level and High Altitude Areas. Sci. Rep.,2018; 8: 1-15.
CrossRef - Kaoutari E, Armougom F, Gordon I, Raoult D,and Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol.,2013; 11: 497–504.
CrossRef - Bäumler J,and Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature.,2016; 535: 85–93.
CrossRef - Hsiao A, Ahmed A, Subramanian S, Griffin N, Drewry L, Petri W, Haque R, Ahmed T, and Gordon J. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature.,2014; 515: 423–426.
CrossRef - Gibson R,and Roberfroid B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr.,1995; 125: 1401–1412.
CrossRef - Gibson R, Scott P, Rastall A, Tuohy M, Hotchkiss A, Dubert-Ferrandon A, Gareau M, Murphy F, Saulnier D, Loh G, Macfarlane S, Delzenne N, Ringel Y, Kozianowski G, Dickmann R, Lenoir-Wijnkook I, Walker C, and Buddington R. Dietary prebiotics: current status and new definition preparations. Food Sci. Technol. Bull. Funct. Foods.,2010; 7: 1–19.
CrossRef - Sonnenburg D, and Sonnenburg L. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab.,2014; 20: 779–786.
CrossRef - Simpson L, and Campbell J. Review article: dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther.,2015; 42: 158–179.
CrossRef - Depeint F, Tzortzis G, Vulevic J, I’Anson K,and Gibson R. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr.,2008; 87: 785–791.
CrossRef - Costabile A, Kolida S, Klinder A, Gietl E, Bauerlein M, Frohberg C, Landschutze V, and Gibson R. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br. J. Nutr.,2010; 104: 1007–1017.
CrossRef - Chung H, Pamp S, Hill J, Surana N, Edelman S, Troy E, Reading N, Villablnca E, Wang S, Mora J, Umesaki Y, Mathis D, Benoist C, Relman D, and Kasper D. Gut immune maturation depends on colonization with a host-specific microbiota. Cell.,2012; 149: 1578–1593.
CrossRef - Brennan A,and Garrett S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol.,2016; 70: 395–411.
CrossRef - Honda K, and Littman R. The microbiota in adaptive immune homeostasis and disease. Nature.,2016; 535: 75–84.
CrossRef - Markowiak P,and Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients.,2017; 9: 1-30.
CrossRef - LeBlanc G, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, and Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact.,2017; 16: 1–10.
CrossRef - Khisti U, Kathade S, Aswani M, Anand P,and Kunchiraman B. Isolation and identification of Saccharomyces cerevisiae from caterpillar frass and their probiotic characterization. Biosci. Biotechnol. Res. Asia.,2019; 16: 179–186.
CrossRef - Varzakas T, Kandylis P, Dimitrellou D, Salamoura C, Zakynthinos G, and Proestos C. Innovative and fortified food: Probiotics, prebiotics, GMOs, and superfood. in 2018; 67–129.
CrossRef - Alsheraji S, Manap A, Shuhaimi A, Yusof M, and Hassan F. Prebiotics as functional foods: A review. J. Funct. Foods.,2013; 5: 1542–1553.
CrossRef - Yang J,Kwon Y, Kim J, Kang S, Kim S, and Park S.Jerusalem artichoke and chungkookjang additively improve insulin secretion and sensitivity in diabetic rats. Nutr. Metab. (Lond).,2012; 9: 1-12.
CrossRef - Chen T, Hu S, Zhang H, Guan Q, Yang Y, and Wang X. Anti-inflammatory effects of Dioscorea alata L. anthocyanins in a TNBS-induced colitis model. Food Funct.,2017; 8: 659–669.
CrossRef - Wireko-Manu F, Oduro I, Ellis W, Robert A, and Maziya-Dixon B. Potential health benefits of water yam (Dioscorea alata). Food Funct.,2013; 4: 1496-1501.
CrossRef - Rahangdale S. Potential wild edible plant resources from maharashtra: future prospects for their conservation and improvement. Life Sci. Leafl.,2014; 57: 73–85.
- Kathade S, Aswani M, Anand P, Jagtap S,and Bipinraj N. Isolation of Lactobacillus from donkey dung and its probiotic characterization. Korean J. Microbiol.,2020; 56: 160–169.
- Kathade S, Aswani M, Anand P,and Nirichan B. Probiotic characterization and cholesterol assimilation ability of Pichia kudriavzevii isolated from the gut of the edible freshwater snail “Pila globosa”. Egypt. J. Aquat. Biol. Fish.,2020; 24: 23–39.
CrossRef - Kathade S, Aswani M,Anand P, Kale A, Shrivastava P, Sharma S, Badat U, Mohite J, Debbarma J, Sangma A, Wajravad B, Jagtap S, and Niricharan B. Isolation, characterization, and diversity of probiotic microorganisms from different postpartum milk of various animals. Int. J. Health Sci.,2022;12(3): 223-234.
CrossRef - Aswani M,Kathade S, Anand P, Kunchiraman B, Dhumma P, and Jagtap S.Probiotic characterization of cholesterol-lowering Saccharomyces cerevisiae isolated from frass of Pyrrharctia isabella caterpillars. Appl. Food Biotechnol.,2021; 8: 189–198.
CrossRef - Yadav S, Gite S, Lanjekar V, Nilegaonkar S, and Agte V. In vitro screening of indigenous plant materials for prebiotic potential. Int. J. Curr. Microbiol. Appl. Sci.,2014; 3: 137-150.
- Sawangwan T, Wansanit W, Pattani L,and Noysang C. Study of prebiotic properties from edible mushroom extraction. Agric. Nat. Resour.,2018; 52: 519-524.
CrossRef - Reza M, Hossain M,and Park S. In vitro prebiotic effects and quantitative analysis of Bulnesia sarmienti extract. J. Food Drug Anal.,2016; 24: 822-830.
CrossRef - De Giani A, Bovio F, Forcella M, Lasagni M, Fusi P, and Di Gennaro P. Prebiotic effect of maitake extract on a probiotic consortium and its action after microbial fermentation on colorectal cell lines. Foods.,2021; 10: 1-14.
CrossRef - Tang S, Koh C, and Hii S. Evaluation and characterisation of candidate prebiotics extracted from coconut husk by ultrasound-assisted extraction technique. IOP Conf. Ser. Mater. Sci. Eng.,2021; 1195: 1-17.
CrossRef - Obidiegwu J, Lyons J, and Chilaka C. The Dioscorea genus (Yam)—An appraisal of nutritional and therapeutic potentials. Foods.,2020; 9: 1-45.
CrossRef - Wong J, Hii S, and Koh C. Isolation of prebiotics from artocarpus integer’s seed. Int. J. Food Sci.,2021; 2021: 1-11.
CrossRef - Nowak R, Jechalke N, Juda M, and Malm A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: the stimulation effect on Lactobacillus strains growth. Eur J Nutr.,2017; 57: 1511-1521.
CrossRef - Gaurino M, Altomare A, Emerenziani S, Rosa C, Ribolsi M, Balestrieri P, Iovino P, Rocchi G, and Cicala M. Mechanisms of action of prebiotics and their effects on gastro-intestinal disorders in adults. Nutrients.,2020; 12(4): 1-24.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






