How to Cite | Publication History | PlumX Article Matrix
Akib Nisar1 , Suyash Arunrao Kathade1
, Suyash Arunrao Kathade1 , Mayur Arjun Aswani2
, Mayur Arjun Aswani2 , Abhay Madhukar Harsulkar3
, Abhay Madhukar Harsulkar3 , Suresh Dnyandev Jagtap2
, Suresh Dnyandev Jagtap2 , Bipinraj Nirichan Kunchiraman1*
, Bipinraj Nirichan Kunchiraman1*
1Rajiv Gandhi Institute of I.T. and Biotechnology, Bharati Vidyapeeth (Deemed to be University), Pune, Maharashtra, India.
2Interactive Research School for Health Affairs, Bharati Vidyapeeth (Deemed to be University), Pune, Maharashtra, India.
3Pharmaceutical Biotechnology, Poona College of Pharmacy, Bharati Vidyapeeth (Deemed to be University), Pune, Maharashtra, India.
Corresponding Author E-mail: bipinrajnk@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2992
ABSTRACT:
The COVID-19 had been emerged as a pandemic and resulted in more than 273 million reported cases and 5.3 million deaths worldwide. Concerns have been raised regarding this virus due to its unprecedented ability to move from human to human and cause infections, acute morbidity, and fatal outcome. Gut and lung microbiome profile substantially depends on dietary habits and plays a major role in modulating immunity thereby providing resistance to viral infections. The Gut-lung axis shows a correlation in microbial profile and the gastrointestinal microbiota can modulate lung microbiota majorly through the impact of microbial metabolites. Firmicutes and Actinobacteria specifically Bifidobacterium and Lactobacillus are responsible to modulate immunity and are widely used as probiotic species. In this review, we have concluded that different dietary habits affect microbial diversity and it can be a determining factor to fight SARS-CoV2 infections.
KEYWORDS: Gut Microbiota and Diet; Immunomodulation; Lung Microbiota; Probiotics; SARS-CoV2
Download this article as:| Copy the following to cite this article: Nisar A, Kathade S. A, Aswani M. A, Harsulkar A. M, Jagtap S. D, Kunchiraman B. N. Understanding the Correlation of Diet, Immunity, and Probiotics: A Credible Implication in SARS-CoV2 Infections. Biosci Biotech Res Asia 2022;19(2). |
| Copy the following to cite this URL: Nisar A, Kathade S. A, Aswani M. A, Harsulkar A. M, Jagtap S. D, Kunchiraman B. N. Understanding the Correlation of Diet, Immunity, and Probiotics: A Credible Implication in SARS-CoV2 Infections. Biosci Biotech Res Asia 2022;19(2). Available from: https://bit.ly/3kImd5G |
Introduction
The COVID-19 pandemic caused multiple deaths and a major burden on the healthcare system of the countries. According to the epidemiological report of WHO on 19th of December, 2021, over 273 million reported cases and 5.3 million deaths have been reported globally across 213 countries, areas, and territories 1. Countries strictly followed containment steps and lockdown measures to cope with the spread of this infection. These steps, while important for preventing the spread of COVID-19 and reducing the number of deaths in the absence of successful therapies and vaccines, have resulted in substantial short-term economic losses. Containment measures have had, on average, a very large impact on economic activity equivalent to a loss of about 15 percent in industrial production over 30 days following their implementation 2. Countries are trying several approaches based on a case-to-case basis and health care facilities. In general, the common drugs used in the pandemic by different countries are lopinavir, ritonavir, chloroquine, and remdesivir. According to the WHO’s Draft landscape and tracker of COVID-19 candidate vaccines, 186 vaccine candidates are currently in the pre-clinical phase and 87 vaccines in the clinical phase. Some of the vaccines like Comirnaty by Pfizer, Moderna COVID‑19 Vaccine by Moderna, Covishield by Astrazeneca, Sputnik V by Gamaleya Research Institute, CoronaVac by Sinovac, Covaxin by Bharat Biotech and COVID-19 Vaccine Janssen by Johnson & Johnson are currently in use in different countries3. In these vaccines, the Astrazeneca’s vaccine Covishield has been reported several cases of unusual thrombotic events and thrombocytopenia after administration to some of the candidates 4. These kinds of studies are limited with data and patient’s studies and it will be too hurry to conclude the side effects and efficacy of these vaccines. Also, the strains of the virus are mutating rapidly resulting in different variants and it is going to be more challenging for any of the vaccines. The current variants included in the variant of interest by the United States are B.1.526, B.1.525, and P.2; while those included in the variant of concern by the United States are: B.1.1.7, P.1, B.1.351, B.1.427, and B.1.429. The discovery of double and triple mutant variants in India has resulted in a substantial increase in the number of cases 5. Recently, a new SARS-CoV-2 variant of concern, omicron has been reported, which is showing the highest rate of transmissibility amongst other variants 6. Vaccines and other kinds of medications are sought to fight the infections that exist in the community but it’s not a precautionary measure. We need an alternative approach such as natural immunity boosters that may help us to fight and reduce the severity of this kind of infection.
The existing immunity is the first and most effective defensive barrier, responsible to prevent and fight different infectious diseases. Various studies have found that similar to the gut, human lungs also have a protective shield of microbes, specifically in the upper and lower respiratory zone that protects us from viral, bacterial, and fungal infections 7. Any disturbance in the microbial environment makes us prone to infections 8. Like other respiratory viruses, SARS-CoV2 also must face the microbial environment of the lining of the respiratory tract. It is therefore pertinent that a good microbiota profile may also play a role in the vulnerability of SARS-CoV2 infection. The microbial diversity of lung microbiota depends on lifestyle, exposed environment while some studies observed that gut microbiota environment also influences the diversity of the lung microbiota in many ways 9,10. More precisely, the diversity of gut microbiota depends majorly on the food habits of the people 9. In a final word, the severity and exposure of respiratory infections caused by viruses like SARS-CoV2 may be influenced by the gut and lung microbiota diversity and it is closely related to our food source and diet patterns. Nowadays, probiotics are renowned health supplements that are pillars of our immune system and helps us to fight different diseases and infections11–13. These probiotics are live microorganisms (MOs), bacteria, or yeast, when ingested in adequate amounts confer a health benefit to the host 14,15. The diet that is associated with health benefiting probiotics improves immunity and protects from the different infectious diseases through immunomodulation 16.
The current review speaks about probiotics as a therapy in this COVID-19 pandemic to reduce the chances of infection by improving the immune system. we have also discussed the role of diet for gut microbiota induced immunity.
Diet-Induced Microbiota Profile and Immunity
The human gastrointestinal tract (GI tract) is the site of focus where many kinds of reactions occur. However, recent discoveries have made it possible to answer the questions of how and why the GI tract is the focus of these reactions. The Human GI tract lining consists of trillion cells of MOs such as bacteria, yeast, and archaea that form a complex microbial community called the gut microbiome. The gut microbiome plays a vital role in digestion, fermentation of complex dietary compounds which are indigestible to humans, protect from virulent pathogens, acting as producers of vitamins, neurotransmitters, maintaining human health by modulating host immunity, production of signalling molecules such as cytokines, maturation of immune system, etc 17–23. Belkacem et al. reported the administration of Lactobacillus paracasei and L. plantarum in the GI tract modulated immune system via regulating cytokine secretion and increasing immune cells in the lungs such as natural killer cells, macrophages and dendritic cells in influenza virus infection 24. However, the balance of gut microbiota profile is of utmost importance as it plays a crucial role in maintaining human health throughout the life of an individual, and also, they are vital in providing the first line of defence 25–27. The gut microbiome seems to be very sensitive and does often change into several extrinsic and intrinsic factors such as genetics, dietary habits, age, geographic location, and ethnicity 26,28,29. Amongst the above-mentioned factors, dietary habit seems to affect the gut microbiome with a huge impact that is substantially observed from the research studies 30–36.
In the Eastern diet, the key meals are lunch and dinner, typically made up of basics such as rice or pasta, chilli, and some vegetables and meat dishes 37. South Asia harbours 26% of the world’s population in the Eastern zone that houses tremendous genetic and cultural diversity residing in India as the largest country with a denser population 38–40. Indians more often consume plant-based diets as per the studies conducted on gut and lung microbial profile, and effectiveness in immunity against various viruses. The data showed that Firmicutes and Actinobacteria specifically Bifidobacterium and Lactobacillus, play an important role in the stimulation of immune response against viruses. The high prevalence of Firmicutes that contains bacteria are responsible for fermentation and produces short-chain fatty acids (SCFA), these fatty acids fuels colonic epithelium thereby maintaining the integrity of epithelial cells, influencing metabolism and aiding in epithelial restitution which may be responsible to induce antigen-specific immune response 41. A phylum-level study from Tandon et al. 2018 reported from a cohort of 80 Indians residing in the urban area that the gut microbiome of these individuals was rich in Bacteroidetes (71.5%) followed by Firmicutes (18.7%), Proteobacteria (3.8%), and Actinobacteria (0.6%), occupying majorly 5 genera viz., Prevotella, Faecalibacterium, Alloprevotella, Roseburia, and Bacteroides with more than 80% of abundance. The typical diet reported in these people of the urban area was simple and complex carbohydrates such as rice, wheat, sorghum, and fibre rich components majorly fruits, vegetables, sprouts, etc 42. Contradictory to urban diet, tribal diet and rural diet show a much more balanced microbiome with the dominance of Firmicutes, followed by Proteobacteria, Bacteroidetes and Actinobacteria studied in south India. Tribal communities with this type of microbiome possessed a mixed diet rich in cereal millets such as pearl and finger millets along with moderate consumption of meat but did not consume milk or milk products. While rural diet used to be rich in rice and lentils along with milk, curd, and meat. At genus level, bacteria such as Clostridium (32.7% in tribal; 4.7% in rural) and Bacteroidetes (2.6% in tribal; 0.4% in rural) were abundant in tribal population than rural counterparts. While Streptococcus (0.4% in tribal; 2.7% in rural) and Enterobacteriaceae (0.4% in tribal; 1.2% in rural) were shown to be more prevalent in rural groups than in a tribal group. The study also stated an abundance of Firmicutes to an extent of 85.9% in tribal while 63.5% in the rural group 43. The change in dietary pattern and lifestyle among tribal, rural, and urban has a direct correlation with gut microbiota. The tribal, as well as rural cohorts, were found to be rich in microbial diversity aspects owing to their high fibre intake whereas less diverse in urban groups owing to the modern dietary lifestyle. However, urban individuals microbial profile reveals an abundance of Bacteroidetes phyla and low dominance of Firmicutes when compared to rural and tribal populations 44–46. The Western zone of the world mainly covers the American and European populations where they follow a similar pattern diet. Most Western populations consume overly processed and omnivorous foods with low dietary content, high in animal protein, total and saturated fats, and simple sugars 31,47.
The European diet resembles the Paleolithic age ancestors that include intake of vegetables, fruit, nuts, eggs, fish, lean meat while on the other hand excluding grains and dairy products 48,49. A recent study regarding the modern Paleolithic diet (MPD) by Barone et al (2019) was performed on participants from urban areas of Italy where they obtained, 51.02% of energy from fats, 30.14% from proteins, and 18.84% from carbohydrates. Further, the dominance of asaccharolytic bacteria such as Sutterella and opportunistic pathogens such as Odoribacter, Bilophila was reported. The abundance of these pathogens can be traced back to their diet which is rich in animal proteins and high consumption of saturated fats. As well as there was the presence of Akkermansia which is considered as potential next-generation probiotics i.e. directly correlated to consumption of unsaturated fats. Finally, the study reported the dominance of Firmicutes followed by Bacteroidetes, Proteobacteria, Actinobacteria, and Verrucomicrobia at the phyla level 50. Yet, the high diet supports the more growth of the Firmicutes but it hampers the microbial diversity and thus compromises the gut induced immunity.
The standard American diet comprises of consumption of refined carbohydrates, fatty meats, and added fats that lack many nutrients in grains, fruits, and vegetables. Studies show that this type of dietary pattern contributes to various chronic diseases 51–53. Furthermore, Americans follow a lavish diet to obtain 57.9% energy from ultra-processed foods involving sugar as well. The content of added sugar in these foods is usually eight-fold higher than in normally processed foods 54. American population shows the loss of microbial diversity to a greater extent when compared to the ancestral population of Hadza tribes. In the American group, the high abundance of Akkermansia muciniphila and Bacteroides were found compared to the Hadza tribes community 55. A study by David et al (2014) reported an increased abundance of Alistipes putredinis, Bilophila Wadsworth, Bacteroides sp. Along with genus Prevotella, phyla Bacteroidetes and Verrucomicrobia with a simultaneous decrease in the levels of Firmicutes resulting in reduced production of SCFAs 56. Due to the high-fat diet, and processed foods there are higher microbial counts of mucus degrading microbes in the American population that may result in a higher risk of infections and diseases. Lower levels of Firmicutes and Bifidobacteria are also stated in these individuals with a heavy loss of microbial diversity and functionality. Based upon the various findings, it looks like that, reduction in health-promoting groups of Firmicutes and Actinobacteria count may reduce the immunity driven by the gut microbiota profile.
The Lung Microbiome
A vast variety of microbial communities inhabits the human body that is found to be more prevalent on mucous membranes and play a vital role in various metabolic processes 57. Historically, the lungs were thought to be sterile and free from any microbial contact, yet it is constantly exposed to microbiota through inhalation. From the past decade, studies helped to understand how lung and microbiota interact and exist together 58,59. In comparison with gastrointestinal microbiota, lung microbiota hosts relatively lower microbial communities that range from 4.5 to 8.25 log CFU/ml as the lung hosts low nutrients than the intestinal tract 60–62. Several studies have been conducted to explore the healthy lung microbiome that comprises of two main phyla Bacteroidetes and Firmicutes 63,64. However, other studies also postulated the dominance of phyla such as Proteobacteria, Actinobacteria, and Fusobacterium along with a relative abundance of Firmicutes and Bacteroidetes 60,62,65–67. A genus-level study by Erb-Downward et al. (2011) showed a dominance of Pseudomonas, Streptococcus, Prevotella, Fusobacterium, Haemophilus, and Porphyromonas in the lower respiratory tract of healthy individuals 60. Others reported a lower abundance of genera, Veillonella, Leptotirchia, while an ample amount of Lactobacillus and Rothia 68.
The healthy lung microbiome is sensitive to factors such as oxygen tension, blood flow, luminal pH, temperature, inflammation, allergen, and more precisely to the pathogenic MOs that may result in respiratory ailments and disorders 69,70. The majority of respiratory infections are airborne which are caused by MOs that can travel and escape from mucosal and ciliary activity of epithelial cells present in the upper respiratory tract and adhere to the epithelial lining of the lower respiratory tract and profoundly multiple in lung alveoli. The result of infection would provoke immune-stimulating responses stimulating the respiratory microbiome to play a part in the prevention of respiratory infections 71–74.
Chronic obstructive pulmonary disease (COPD) is a group of respiratory diseases that are characterized by chronic obstruction of lung airflow which interferes with normal breathing. Many scientists have analyzed the lung microbiome of COPD patients and observed a lower bacterial diversity when compared to healthy populations 62. At the genus level, the relative abundance of Pseudomonas was found which is one of the known opportunistic pathogens 65. A similar study was stated by Huang et al. (2014) in COPD patients that observed enrichment of Proteobacteria viz. Moraxellaceae, Patuerellaceae, Pseudomonadaceae, and Enterobacteriaceae and concomitant reduction in the levels of Actinobacteria, Clostridia, and Bacteroidia 75. ARI also show similar microbial signatures as that of COPD patient with an enriched microbiota of Moraxella, Streptococcus, and Haemophilus 76. Pneumonia is characterized by flooding of fluid in the alveoli of lungs that contains enough nutrients and creates oxygen barrier conditions, hence impairing its clearance by ciliary action of epithelial cells and thereby facilitating the growth of the microbial community with the dominance of pathogen, progressing the disease 73,77. Recent data suggest a reduction in the pulmonary microbial diversity and reduction in Rothia, Lactobacillus, and Streptococcus which increases the risk of pneumonia, predominantly in the nasal mucosal lining 68. Additionally, patients with HIV in later stages showed dysbiosis in respiratory microbiota with an increase in Prevotella and Veillonella group amidst the treatment and this microbial signature persists for years 78.
Thus, it seems that a healthy lung microbiome responsible for the normal function of lungs, generally habitats the dominance of phyla such as Proteobacteria and Fusobacterium along with a relative abundance of Firmicutes and Bacteroidetes with a higher abundance of Lactobacillus. Phyla such as Proteobacteria and Fusobacterium are generally responsible to initiate a pro-inflammatory immune response that leads to the severity of the disease while on the other hand, Lactobacillus genera modulate the immune response by activation of Treg cells. These MOs are evidenced to play an important role in different respiratory diseases by creating an immunological barrier.
The Gut-Lung Axis
The gastrointestinal microbiota can modulate lung microbiota majorly through the impact of microbial metabolites produced by the gut microbiome. Dysbiosis in the gut is found to be linked with various diseases and respiratory infections are one of them 79,80. One study has reported a decrease in the density of Bifidobacteria while a simultaneous increase in Clostridia in the intestine is associated with asthma 81. Another research showed that the influenza virus infection in the respiratory tract significantly increased the count of Enterobacteriaceae with a concomitant reduction in Lactobacilli as well as Lactococcus levels were seen in gut microbiota 82. Furthermore, depletion in microbial diversity by antibiotics in the gut increased the infection rate of influenza virus infection in the lungs when studied in a mouse model 82,83. these findings corroborate that the gastrointestinal tract and lung are intensively linked organs that influence each other’s homeostasis.
Role of Probiotics in Viral Infections
The human lungs have been adapted and improved the protection mechanisms from last hundreds of years to fight the invading infective viruses using the first line of defence system viz. mucus induction, continuous motion of cilia, nonspecific inhibitors for viral replications, secretion of Immunoglobulin A (IgA) in respiratory tract infections, etc. 84. On the onset of a viral infection, a cascade starts that activates the body’s natural immune mechanism. Initially, Toll-like receptors (TLRs) mediate the antiviral immune responses by recognizing virus infection, activate the signalling pathway leading to the secretion of chemokines and cytokines such as interferons (IFN) type I. Chemokines activate the natural killer cells (NK cells) that result in disruption of viral RNA and stop replication. Furthermore, the dendritic cells (DCs) lead to an activation of CD4+ and CD8+ cells and develop antigen-specific T and B lymphocytes mediated immunity that works together to get rid of the invading infective stage 85. Microflora other than the digestive system, particularly in the lungs is also established to fight the incurring viral infections by modifying and supporting the natural immune process called immunomodulation. MOs and their secreted metabolites interact with TLRs, IFN, DCs, and T regulatory lymphocytes along with other chemokines and cytokines which is responsible to induce host immunity 83. Human microflora plays a key role to support innate and adaptive immunity whereas probiotics are proven to stimulate host immunity via immunomodulation of the immune system. These probiotic microbes translate the innate immunity and induce the acquired immunity that results in stimulation of specific and non-specific immunity 86,87
There are reports that probiotics such as Bifidobacterium breve shows anti-influenza effect by increasing the production of IgA, and IgG 88. Hepatitis A and B were found to be reduced by Lactobacillus acidophilus and Bifidobacterium bifidum while Thermophilus sp. is known to work as an anti-herpetic agent 89,90. Similarly, Bifidobacterium lactis and Saccharomyces boulardii can be used in antiviral therapy against Rotavirus 91. A clinical study has reported that daily consumption of probiotics by HIV infected people showed improvement of CD4+ count 92. It is also suggested that the consumption of probiotics like LAB and Bifidobacteria are found to reduce the risk of upper respiratory tract infections 93. An animal study demonstrated that oral administration of probiotic strains like Lactobacillus pentosus, L. casei, L. plantarum, L. bulgaricus, L. rhamnosus, L. gasseri, L. brevis, and B.breve helped to suppress symptoms of virus infection 94.
Anticipation of the Immunomodulatory Role of Probiotics in SARS Cov2 Infection
Yet, no direct relation and study are available to justify the role of probiotics against SARS-CoV2 infections but many previous studies regarding probiotics and viral infections can be used to implement the possible mechanisms and their role 95,96. The pathogenesis of SARS-CoV and SARS-CoV2 relied on a common entry point by interacting with the ACE2 receptor present on epithelial cell surfaces in the lung and intestine 97–100. In the certain report of SARS-CoV2 infection, it has been postulated a dysbiotic condition caused by Salmonella enterica, a member of Enterobacteriaceae family was found abundant that increased the level of ACE2 receptors in the epithelial cells of the intestine resulting it to be more prone to get infected from these viruses 97. The SARS-CoV2 virus has to surpass the immunologic barrier of respiratory tract epithelial to invade the cells through the ACE2 receptors whereas the probiotic microbes with commensal bacteria may help the immune system to reduce or inhibit this infection through immunomodulation 101,102.
Although, probiotics do not show a direct effect it creates an immunologic barrier by stimulating an immune response that supports the first line of defence of the body 103. Generally, the probiotics interact with lung and intestinal epithelial as well as specialized cells (M cells) for immunoregulation through interaction with macrophages and dendritic cells which leads to activation of T and B lymphocytes. It may hamper the viral attachment by competitive inhibition via blocking the binding sites on the epithelial lining. The probiotics induce the upregulation of mucin-1 (MUC1) and mucin-2 (MUC2) that can also prevent attachment of the virus to an epithelial cell and suppress the replication. Finally, it also produces antimicrobial peptides and dehydrogenase and nuclease enzymes which can break down the viral nucleic acid, and also the co-aggregation of probiotics with viral particles interferes with the attachment of the virus to the epithelial cell line 104. Probiotics also have a significant role in the induction of type 1 T helper (Th1) cell which is specific for antimicrobial/antiviral mediated immunity whereas IFN which is a glycoprotein and IgA are considered antiviral agents 16,105. One of the important molecules produced by probiotic MOs by breaking down the prebiotic compound is short-chain SCFA. It influences the immune system and induces pattern recognition receptors (PRR) by activating tumour necrosis factor- α (TNF-α) 106,107. More precisely, probiotics like Lactobacillus and Bifidobacterium modulates the immune system by regulating the cytokines, increasing the production of IgA and IgG antibodies 108. Specifically, the Lactobacillus species like L. acidophilus, L. casei, L. rhamnosus, L. helveticus are effective to enhance phagocytosis and improve the secretion of cytokines, immunoglobulin and plasma cells, as shown in a study, L. casei and L. acidophilus induced the interleukin (IL) such as IL-10 and CD4+ regulatory T (Treg) cells (Susan and Terry, 2009, Markowiak and Śliżewska, 2017). Moreover, the administration of L. plantarum and L. reuteri reduced inflammation while L. rhamnosus and B. lactis increased IFN-γ, IL-4, IL-10, and IL-6 in bronchoalveolar lavage 94. Besides, probiotics can induce the level of Bcl2 (B cell lymphoma 2), which is responsible for the activation of cellular and humoral immunity leading to the activation and production of the cytokines along with Th1/Th2 expression.
Probiotics have also been studied for their influence on immune-related gene expression and activation of cytokines, depending on the contact-based mechanism. A study suggested that probiotics like Lactobacillus mediates the expression of TLR2 which stimulates TNF-α while Bifidobacterium longum mediated expression of IL-10 and IL-12 via a contact-based mechanism that resulted in the modulation of T helper cell response in the gut and lung 109.
The oral administration of 109 CFU of probiotics are known to be more effective that may exert long term homeostasis and immunomodulatory effect on the host 110,111. Oral administration of Bifidobacterium bifidum and B. breve have also been shown to increase humoral immune response such as stimulation of IgA 111. Thus, probiotics also show the possibility to use as a live vaccine for oral immunization. Moeini et al. (2011) used L. acidophilus as a live vehicle for oral immunization against chicken anemia virus (CAV). The AcmA-binding domains present on the surface of Lactococcus lactis were used to display the viral protein 1 (VP1) CAV on L. acidophilus to immunize specific-pathogen-free chickens through the oral route. The immunization increased the levels of Th1 cytokines, such as IL-2, IL-12, and IFN-γ 112. Furthermore, some studies have shown that probiotics can enhance the outcome of influenza virus infection when administered through the nasal pathway. The nasal administration of Lactobacillus rhamnosus strains CRL1505 and CRL1506 were able to improve respiratory antiviral defences and beneficially modulated the immune response by triggering the TLR3 and PRR (RIG-I, a retinoic acid-inducible gene I) against the respiratory syncytial virus (RSV) 113.
It has been now clear that probiotics are microbiota that works as a potential barrier in the case of any viral attack through immunomodulation as described earlier (Figure 1). It may act indirectly through competitive inhibition or directly via the interaction of immune cells by producing chemokines, cytokines, and also be involved in other immunologic pathways. In light of this information, we can anticipate the possible role of these probiotics in the protection or reduction of the SARS-CoV2 infection. In this context, a model has been represented here showing the expected immunomodulatory role of probiotics along with prebiotics which may take place on the onset of SARS-CoV2 infection in a more or less similar way (Figure 2) (Table 1).
 |
Figure 1: Process of probiotic and immunomodulatory activity |
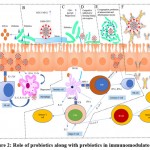 |
Figure 2: Role of probiotics along with prebiotics in immunomodulatory. |
Table 1: List of microorganisms showing various immunological functions.
| Microorganisms | Functions | References |
| Bifidobacterium breve | Increases the production of IgA and IgG for anti-influenza effect | 88 |
| Lactobacillus acidophilus, Bifidobacterium bifidum Thermophilus sp | Improved hepatitis A and B. | 89,90. |
| Bifidobacterium lactis, Saccharomyces boulardii | Improvement of CD4+ count | 91,92 |
| L. pentosus, L. casei, L. plantarum, L. bulgaricus, L. rhamnosus, L. gasseri, L. brevis, and B.breve | Help to suppress symptoms of virus infection | 94 |
| Lactobacillus and Bifidobacterium | Modulates the regulation of the immune system by cytokines, IgA and IgG production | 108 |
| L. rhamnosus and B. lactis | Increase IFN-γ, IL-4, IL-10, and IL-6 in bronchoalveolar lavage | 94 |
| L. plantarum and L. reuteri | Reduce inflammatory parameters | |
| Probiotic MOs | Produce SCFAs and induce PRR by activating TNF-α | 106,107 |
| L. lactis, L. acidophilus | Antigen-specific immune response, increase Th1 cytokines, such as IL-2, IL-12, and IFN-γ. | |
| L. Plantarum, L. reuteri | Improvement against pneumonia viral lethal infection | 110 |
| L. acidophilus, L. casei and L. bulgaricus | Production of IgG antibodies | 110 |
| Bifidobacterium bifidum, B. breve | Increase humoral immune response | |
| L. rhamnosus | Against rotavirus, leads to stimulate IL-4 | 114 |
| L. casei, L. acidophilus | Induce IL-10 and CD4+ and T regulatory cells | 109 |
| L. acidophilus, L. casei, L. rhamnosus, L. helveticus | Enhance phagocytosis, cytokines and immunoglobulin production | 86 |
| Lactobacillus and Bifidobacterium longum mediated toll-like receptors | Stimulate TNF-α, IL-10, IL-12 also modulate T helper cell response in the gut and lung | 16 |
Conclusions
Various studies have proven the role of dietary habits in determining the gut-microbiota profile and its likeliness to fight different viral infections. It has been shown that change in dietary pattern and lifestyle among tribal, rural, and urban has a direct correlation with gut microbiota. Specifically, the diet habits impart a direct role in the ratio of Bacteroidetes and Firmicutes in the gut that eventually participates in the immunomodulation activities against different diseases. These phyla encounter many probiotics genera which appear to be effective to maintain intestinal epithelial barrier integrity, modulating the immune response, and also directing the microbiota profile of the lung environment through the gut-lung axis. It is being correlated with the studies that diversity in microbial population in the gut provides may provide more immune response and lowers the risk of severe infections from SARS-CoV2. Thus, administration of probiotics such as Lactobacillus, Bifidobacterium, and Saccharomyces are subject to preference to fight the Covid-19 infection.
Acknowledgement
The authors are thankful to Rajiv Gandhi Institute of IT and Biotechnology, Bharati Vidyapeeth (Deemed to be University) for the support and encouragement.
Conflict of Interest
The authors declared no conflict of interests.
Funding Sources
There are no funding source.
References
- COVID-19 Weekly Epidemiological Update. World Heal. Organ. 2021.
- Deb P, Furceri D, Ostry JD, Tawk N. The economic effects of COVID-19 containment measures. 2020.
- Draft landscape and tracker of COVID-19 candidate vaccines. World Heal. Organ. 2021.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021. doi:10.1056/NEJMoa2104840.
- Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P. review of COVID-19 variants and covid-19 vaccine efficacy: what the clinician should know? J Clin Med Res 2021; 13: 317–325.
- Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 2021; 398: 2126–2128.
- de Steenhuijsen Piters WAA, Sanders EAM, Bogaert D. The role of the local microbial ecosystem in respiratory health and disease. Philos Trans R Soc B Biol Sci 2015; 370: 20140294.
- Hanada S, Pirzadeh M, Carver KY, Deng JC. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia . Front. Immunol. . 2018; 9: 2640.
- Anand S, Mande SS. Diet, microbiota and gut-lung connection. Front Microbiol 2018; 9: 2147.
- Dickson RP, Huffnagle GB. The lung microbiome: Nnew principles for respiratory bacteriology in health and disease. PLoS Pathog 2015; 11: e1004923.
- Aswani MA, Kathade SA, Anand PK, Kunchiraman BN, Dhumma PR, Jagtap SD. Probiotic characterization of cholesterol-lowering saccharomyces cerevisiae isolated from frass of pyrrharctia isabella caterpillars. Appl Food Biotechnol 2021; 8: 189–198.
- Kathade S, Aswani M, Anand PK, Nirichan B. Probiotic characterization and cholesterol assimilation ability of pichia kudriavzevii isolated from the gut of the edible freshwater snail “pila globosa”. Egypt J Aquat Biol Fish 2020; 24: 23–39.
- Kathade SA, Aswani MA, Anand PK. Isolation , characterization , and diversity of probiotic microorganisms from different postpartum milk of various animals. 2022. doi:10.52403/ijhsr.20220332.
- Khisti U, Kathade S, Aswani M, Anand P, Kunchiraman B. Isolation and identification of saccharomyces cerevisiae from caterpillar frass and their probiotic characterization. Biosci Biotechnol Res Asia 2019; 16: 179–186.
- Kathade SA, Aswani MA, Anand PK, Jagtap S, Bipinraj NK. Isolation of Lactobacillus from donkey dung and its probiotic characterization. Korean J Microbiol 2020; 56: 160–169.
- Markowiak P, Ślizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017; 9. doi:10.3390/nu9091021.
- Das B, Ghosh TS, Kedia S, Rampal R, Saxena S, Bag S et al. Analysis of the gut microbiome of rural and urban healthy indians living in sea level and high altitude areas. Sci Rep 2018; 8: 10104.
- El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013; 11: 497–504.
- Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016; 535: 85–93.
- Hsiao A, Ahmed AMS, Subramanian S, Griffin NW, Drewry LL, Petri WAJ et al. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 2014; 515: 423–426.
- Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012; 149: 1578–1593.
- Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol 2016; 70: 395–411.
- Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature 2016; 535: 75–84.
- Belkacem N, Serafini N, Wheeler R, Derrien M, Boucinha L, Couesnon A et al. Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS One 2017; 12: e0184976.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR et al. Enterotypes of the human gut microbiome. Nature 2011; 473: 174–180.
- Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012; 13: 260–270.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449: 804–810.
- Marathe N, Shetty S, Lanjekar V, Ranade D, Shouche Y. Changes in human gut flora with age: An Indian familial study. BMC Microbiol 2012; 12: 1.
- Dhotre D, Kumbhare S, Sinkar V, Shouche Y. Human gut microbiome research in India: A retrospect and future opportunities. Proc Indian Natl Sci Acad 2019. doi:10.16943/ptinsa/2019/49725.
- Bhute S, Pande P, Shetty SA, Shelar R, Mane S, Kumbhare S V. et al. Molecular characterization and meta-analysis of gut microbial communities illustrate enrichment of prevotella and megasphaera in Indian subjects. Front Microbiol 2016; 7: 1–14.
- Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014; 7: 17–44.
- De Filippis F, Vitaglione P, Cuomo R, Berni Canani R, Ercolini D. Dietary interventions to modulate the gut microbiome-how far away are we from precision medicine. Inflamm Bowel Dis 2018; 24: 2142–2154.
- Flint HJ. The impact of nutrition on the human microbiome. Nutr Rev 2012; 70 : S10-3.
- Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016; 65: 63–72.
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature 2011; 474: 327–336.
- Xu Z, Knight R. Dietary effects on human gut microbiome diversity. Br J Nutr 2015; 113 : S1-5.
- Mothershead AB. Dining Customs Around the World: With Occasional Recipes. Garrett Park Press, 1982.
- Kumar V, Reddy BM. Status of Austro-Asiatic groups in the peopling of India: An exploratory study based on the available prehistoric, linguistic and biological evidences. J Biosci 2003; 28: 507–522.
- Reich D, Thangaraj K, Patterson N, Price AL, Singh L. Reconstructing Indian population history. Nature 2009; 461: 489–494.
- Misra VN. Prehistoric human colonization of India. J Biosci 2001; 26: 491–531.
- Ramakrishna BS, Roediger WE. Bacterial short chain fatty acids: their role in gastrointestinal disease. Dig Dis 1990; 8: 337–345.
- Tandon D, Haque MM, R S, Shaikh S, P S, Dubey AK et al. A snapshot of gut microbiota of an adult urban population from Western region of India. PLoS One 2018; 13: e0195643.
- Ramadass B, Rani BS, Pugazhendhi S, John KR, Ramakrishna BS. Faecal microbiota of healthy adults in south India: Comparison of a tribal & a rural population. Indian J Med Res 2017; 145: 237–246.
- Okada H, Kuhn C, Feillet H, Bach J-F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010; 160: 1–9.
- Penders J, Gerhold K, Thijs C, Zimmermann K, Wahn U, Lau S et al. New insights into the hygiene hypothesis in allergic diseases: mediation of sibling and birth mode effects by the gut microbiota. Gut Microbes 2014; 5: 239–244.
- West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy 2015; 45: 43–53.
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005; 81: 341–354.
- Lindeberg S, Jönsson T, Granfeldt Y, Borgstrand E, Soffman J, Sjöström K et al. A Palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia 2007; 50: 1795–1807.
- Genoni A, Lyons-Wall P, Lo J, Devine A. Cardiovascular, metabolic effects and dietary composition of ad-libitum paleolithic vs. australian guide to healthy eating diets: a 4-week randomised trial. Nutrients 2016; 8. doi:10.3390/nu8050314.
- Barone M, Turroni S, Rampelli S, Soverini M, D’Amico F, Biagi E et al. Gut microbiome response to a modern Paleolithic diet in a Western lifestyle context. PLoS One 2019; 14: e0220619.
- Grotto D, Zied E. The Standard American Diet and its relationship to the health status of Americans. Nutr Clin Pract 2010; 25: 603–612.
- Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet 2016; 116: 302–10.e1.
- Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis 2014; 11: E62.
- Steele ME, Baraldi LG, Louzada ML da C, Moubarac J-C, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open 2016; 6: e009892.
- Sonnenburg ED, Sonnenburg JL. The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol 2019; 17: 383–390.
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563.
- Yu G, Gail MH, Consonni D, Carugno M, Humphrys M, Pesatori AC et al. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol 2016; 17: 163.
- Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu Rev Immunol 2015; 33: 227–256.
- Martin C, Burgel P-R, Lepage P, Andréjak C, Blic J de, Bourdin A et al. Host–microbe interactions in distal airways: relevance to chronic airway diseases. Eur Respir Rev 2015; 24: 78 LP – 91.
- Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 2011; 6: e16384.
- Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med 2012; 186: 536–545.
- Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink J V et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185: 1073–1080.
- Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med 2013; 187: 1067–1075.
- Segal LN, Alekseyenko A V, Clemente JC, Kulkarni R, Wu B, Chen H et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 2013; 1: 19.
- Einarsson GG, Comer DM, McIlreavey L, Parkhill J, Ennis M, Tunney MM et al. Community dynamics and the lower airway microbiota in stable chronic obstructive pulmonary disease, smokers and healthy non-smokers. Thorax 2016; 71: 795–803.
- Segal LN, Clemente JC, Tsay J-CJ, Koralov SB, Keller BC, Wu BG et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 2016; 1: 16031.
- Kim HJ, Kim Y-S, Kim K-H, Choi J-P, Kim Y-K, Yun S et al. The microbiome of the lung and its extracellular vesicles in nonsmokers, healthy smokers and COPD patients. Exp Mol Med 2017; 49: e316.
- de Steenhuijsen Piters WAA, Huijskens EGW, Wyllie AL, Biesbroek G, van den Bergh MR, Veenhoven RH et al. Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. ISME J 2016; 10: 97–108.
- Ingenito EP, Solway J, McFadden ERJ, Pichurko B, Bowman HF, Michaels D et al. Indirect assessment of mucosal surface temperatures in the airways: theory and tests. J Appl Physiol 1987; 63: 2075–2083.
- West JB. Regional differences in the lung. Chest 1978; 74: 426–437.
- Lanaspa M, Bassat Q, Medeiros MM, Munoz-Almagro C. Respiratory microbiota and lower respiratory tract disease. Expert Rev Anti Infect Ther 2017; 15: 703–711.
- Man WH, de Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 2017; 15: 259–270.
- Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016; 4: 59–72.
- Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 2007; 449: 811–818.
- Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch S V. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 2014; 52: 2813–2823.
- Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015; 17: 704–715.
- Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN et al. Analysis of culture-dependent versus culture-independent techniques for identification of bacteria in clinically obtained bronchoalveolar lavage fluid. J Clin Microbiol 2014; 52: 3605–3613.
- Twigg HL 3rd, Knox KS, Zhou J, Crothers KA, Nelson DE, Toh E et al. Effect of advanced HIV infection on the respiratory microbiome. Am J Respir Crit Care Med 2016; 194: 226–235.
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20: 159–166.
- Shukla SD, Budden KF, Neal R, Hansbro PM. Microbiome effects on immunity, health and disease in the lung. Clin Transl Immunol 2017; 6: e133.
- Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 2001; 107: 129–134.
- Looft T, Allen HK. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes 2012; 3: 463–467.
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 2011; 108: 5354–5359.
- Rossi GA, Colin AA. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J 2015; 45: 774–789.
- Openshaw PJM, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev 2005; 18: 541–555.
- Nagpal R, Kumar A, Kumar M, Behare P V., Jain S, Yadav H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol Lett 2012; 334: 1–15.
- Gill HS, Rutherfurd KJ. Viability and dose-response studies on the effects of the immunoenhancing lactic acid bacterium Lactobacillus rhamnosus in mice. Br J Nutr 2001; 86: 285–289.
- Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 2007; 23: 62–68.
- De Vrese M, Schrezenmeir J. Effect of probiotics on a defined immunologic challenge with Hepatitis A and B vaccine. Annu Reports 2000; : 59.
- Liaskovs’kyi TM, Rybalko SL, Pidhors’kyi VS, Kovalenko NK, Oleshchenko LT. [Effect of probiotic lactic acid bacteria strains on virus infection]. Mikrobiol Z 2007; 69: 55–63.
- Erdoğan Ö, Tanyeri B, Torun E, Gönüllü E, Arslan H, Erenberk U et al. The comparition of the efficacy of two different probiotics in rotavirus gastroenteritis in children. J Trop Med 2012; 2012: 787240.
- Irvine SL, Hummelen R, Hekmat S, Looman CWN, Habbema JDF, Reid G. Probiotic yogurt consumption is associated with an increase of CD4 count among people living with HIV/AIDS. J Clin Gastroenterol 2010; 44: e201-5.
- Ouwehand A, Leyer G, Carcano D. Probiotics reduce incidence and duration of respiratory tract infection symptoms in 3- to 5-year-old children. Pediatrics 2008; 121: S115 LP-S115.
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 2005; 102: 12891–12896.
- Anwar F, Altayb HN, Al-Abbasi FA, Al-Malki AL, Kamal MA, Kumar V. Antiviral effects of probiotic metabolites on COVID-19. J Biomol Struct Dyn 2020; : 1–10.
- Akour A. Probiotics and COVID-19: is there any link? Lett Appl Microbiol 2020; 71: 229–234.
- He F, Deng Y, Li W. Coronavirus disease 2019: what we know? J Med Virol 2020; : 1–7.
- Keidar S, Kaplan M, Gamliel-Lazarovich A. ACE2 of the heart: From angiotensin I to angiotensin (1-7). Cardiovasc Res 2007; 73: 463–469.
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11: 875–879.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H et al. Genomic characterisation and epidemiology of 2019 novel coronavirus : implications for virus origins and receptor binding. Lancet 2020; 395: 565–574.
- Khatiwada S, Subedi A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum Microbiome J 2020; 17: 100073.
- Rozga M, Cheng FW, Handu D. Effects of probiotics in conditions or infections similar to COVID-19 on health outcomes: an evidence analysis center scoping review. J Acad Nutr Diet 2020. doi:https://doi.org/10.1016/j.jand.2020.07.016.
- de Vrese M, Schrezenmeir J. Probiotics and non-intestinal infectious conditions. Br J Nutr 2002; 88 Suppl 1: S59-66.
- Zolnikova O, Komkova I, Potskherashvili N, Trukhmanov A, Ivashkin V. Application of probiotics for acute respiratory tract infections. Ital J Med 2018; 12: 32–38.
- Sembulingam K, Sembulingam P. Essentials of Medical Physiology. 6th ed. Jaypee Brothers Medical Publishers (P) Ltd, 2016.
- Gabryszewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB et al. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J Immunol 2011; 186: 1151–1161.
- Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr 2001; 74: 833–839.
- Azad MAK, Sarker M, Wan D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed Res Int 2018; 2018: 8063647.
- Cho SS, Finocchiaro T. Handbook of Prebiotics and Probiotics Ingredients: Health Benefits and Food Applications. CRC Press, 2009.
- Perdigón G, Maldonado Galdeano C, Valdez JC, Medici M. Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr 2002; 56: S21–S26.
- Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Vélez E, Perdigón G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 2019; 74: 115–124.
- Moeini H, Rahim RA, Omar AR, Shafee N, Yusoff K. Lactobacillus acidophilus as a live vehicle for oral immunization against chicken anemia virus. Appl Microbiol Biotechnol 2011; 90: 77–88.
- Tomosada Y, Chiba E, Zelaya H, Takahashi T, Tsukida K, Kitazawa H et al. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol 2013; 14: 40.
- Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr 2001; 73: 444s-450s.

This work is licensed under a Creative Commons Attribution 4.0 International License.





