How to Cite | Publication History | PlumX Article Matrix
Effect of Cadmium and Lead Stress on Seed Germination and Seedling Growth of Jatropha curcas L.
Department of Biotechnology, Faculty of Engineering and Technology, Manav Rachna International Institute of Research and Studies Sector-43, Delhi-Surajkund Road, Faridabad India.
Corresponding Author E-mail: aashourie@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3019
ABSTRACT:
Heavy metal pollution in the biosphere has become a worldwide problem. Metal industry effluents, mining sites, municipal and agricultural waste are important sources of metal dispersion in environment. Heavy metal imposed phytotoxicity affects seed germination, seedling growth, photosynthesis and other physiological processes. Exposure of seeds to cadmium (Cd) and lead (Pb) has deleterious effects resulting into inhibition of germination, delayed germination time and retardation of seedling growth due to toxicity. The aim of this research is to investigate the toxic effects of Cd and Pb on seed germination and seedling growth of Jatropha curcas L. and evaluate its tolerance for heavy metal stress. The experimental treatments included exposure to five concentrations of cadmium nitrate and lead acetate (ranging from 25 to 125 µM/L), under which the germination and seedling growth parameters were determined periodically. The germination and growth of J. curcas L. was affected by cadmium and lead supplemented at different concentrations and the toxicity effects were found to be concentration dependent. Tolerance indices declined sharply with increasing concentrations of lead and cadmium treatments. Germination was inhibited upto 50% by 100 μmol/L of Cd, 125 μmol/L of Pb. Mean germination time and seedling vigour index also gradually decreased with increase in Cd and Pb concentration. Cadmium proved to be more toxic than lead in all considerations.
KEYWORDS: Cadmium, Lead; Germination; Heavy Metal Stress; Jatropha curcas L; Seedling Growth
Download this article as:| Copy the following to cite this article: Shourie A. Effect of Cadmium and Lead Stress on Seed Germination and Seedling Growth of Jatropha curcas L. Biosci Biotech Res Asia 2022;19(3). |
| Copy the following to cite this URL: Shourie A. Effect of Cadmium and Lead Stress on Seed Germination and Seedling Growth of Jatropha curcas L. Biosci Biotech Res Asia 2022;19(3). Available from: https://bit.ly/3QLI6yM |
Introduction
Environmental contamination due to heavy metals have become a matter of great concern over few decades as it poses toxic effects on plants and animals inhabiting the affected region. These pollutants are added in soil and water through industrial discharge, mining, pesticides, fertilizers and automobile exhausts. High contamination of heavy metals in soil and water also pose a potential threat to ecosystem; while their toxic effects limit the agricultural yield, their uptake by plants incorporates them in the food chain causing hazardous health effects. Heavy metals such as lead, cadmium, arsenic, chromium and mercury are extremely toxic, causing oxidative burst and tissue damage, leading to acute or chronic poisoning in humans and animals.
Metal toxicity in plants has been reported by various authors1, 2. Trace amounts of heavy metals are potential enough to damage the vital functions of plants such as seed germination, seedling growth, photosynthesis and other physiological events. The plants respond to heavy metal stress through various mechanisms involving change in cell membrane permeability, oxidative burst, and excessive production of anti-oxidants. Growth inhibition is a general phenomenon associated with most of heavy metal toxicity3, 4. Severe impact on seed germination percentage and seedling growth parameters due to heavy metals like lead has been reported in Brassica pekinensis5, Cassia siamea6 and some tree species7,8. Heavy metal concentration levels between 10 to 200 ppm have been reported to affect the seed germination parameters like germination percentage, germination index, emergence and growth of plumule and radicle, root and shoot lengths, root and shoot dry matter, with variable impacts. Lead toxicity effects on cytological parameters of roots impacting their growth and architecture were studied in Allium cepa and Zea mays L.9,10.
Jatropha curcas L. belonging to family Euphorbiaceae, is a plant with multiple benefits. Its seeds contain approximately 40-45% oil and therefore it is a potential oilseed crop to be used as feedstock for biodiesel production11. It is also known for its ethno- pharmacological uses as an antimicrobial agent, a potential antioxidant and for its anti-inflammatory activity, due to the presence of large number of bioactive phytochemicals12. It is also being explored for its phytoremediation potential as it has shown high tolerance towards heavy metals when grown in contaminated soils13. J. curcus is a stress-resistant, perennial plant, growing well in marginal or poor soil, therefore there is special interest in its cultivation in barren and arid regions14. The aim of the present research was to investigate the effects of heavy metals Cd and Pb on seed germination and seedling growth of J. curcas L. and determine the tolerance of this plant towards these heavy metals.
Material and Method
Seed Collection and Preparation
Seeds of J. curcas L, which were 36 days old, were obtained from Jatropha Research Centre, Bawal, Haryana. Approximately 2000 seeds were screened for selection of healthy seeds. Aborted, damaged and infected seeds were discarded and healthy seeds of uniform weight were selected for the experiment.
Seed germination and growth conditions
The study was conducted in the green house in botanical garden of Manav Rachna International Institute of Research & Studies, Faridabad. Germination beds of 20 x 12 cm were prepared in polythene bags and filled with fertile soil collected from an agricultural field of Faridabad. It was mixed with organic compost in ratio 2:1 and tap water was added to each bag to bring the soil to field water capacity. Soil pH was maintained at7.2. Healthy and intact seeds were screened and surface sterilized using 1% sodium hypochlorite followed by washing thrice with distilled water before sowing. Soils in the bags were then raked using fingers and one seed was sown in each bag at a depth of 20-25 mm. Immediately after sowing soil was watered and thereafter watering was carried out on alternate days to keep the beds with adequate moisture.
Heavy Metal Treatment
Heavy metal treatment solutions were prepared using cadmium nitrate and lead acetate in concentrations of 25, 50, 75, 100 and 125 μmol/L. Each set of treatment consisted of 6 bags, each of which was supplied with 10 ml of respective treatment at every alternate day. There were 12 replicates per treatment. Control set was left untreated.
Seed germination and growth assessment
Germination was marked by the rupturing of seed coat and subsequent emergence of the radicle and plumule. Germination was recorded every day until complete emergence was achieved. Dry biomass of seedlings was determined after harvesting 10 random samples. Germination percentage and seedling vigor index (SVI) were calculated by applying the formula. First leaf emergence indicated the seedling growth. The seedlings were said to be established on emergence of the second leaf when the plantlets from each batch were uprooted and washed to remove soil particles adhered to roots. Dry matter and moisture content (mg g-1 dry weight) in all plant samples were also calculated. Tolerance index (TI) were determined on the basis of mean root length 15 using the formula given below-
TI (%) = (MLRtreatment / MLRcontrol) X 100
here,
MLRtreatment = mean length of the radicle in heavy metal treatment
MLRcontrol = mean length of the radicle in control
Statistical Analysis
The experiments were conducted in randomized block design in triplicate. The data was analyzed using one-way analysis of variance (ANOVA) to determine the effect of heavy metal treatments and least significant difference (LSD at P=0.05) tests were performed to determine the statistical significance of the differences between means of treatments.
Results and Discussion
The effect of Cd and Pb exposure to J. curcas L. seeds during germination and seedling growth was assessed on the basis of various growth parameters.
Effect on Germination
Germination frequencies of J. curcas L. seeds treated with different heavy metal solutions are presented in Table 1. A statistically significant decrease in germination rate, as compared with control seeds, was observed for seeds treated with 75 and 100μmol/L solutions of cadmium and lead salts respectively. After a prolonged exposure to high amounts of heavy metals some plants showed symptoms of phytotoxicity such as stunted growth, depletion of chlorophyll, shrunken and deformed leaves. Exposure to 100 μmol/L of Cd, 125 μmol/L of Pb resulted in approximately 50% inhibition of germination or early death of the majority of seedlings within 8 days (Figure-1, 2 and 3). The growth of seedlings was evidently affected by both the heavy metals.
Table 1: Effects of various concentrations of Cadmium and Lead on Seed Germination parameters of J. curcas L.
| Heavy Metal
Treatment (μmol/L) |
Germination
Percentage |
Mean Germination Time (Days) | Seedling Vigour Index | Dry Matter
(mg/gdw) |
Moisture
Content (%) |
|
| Cd | 25
|
77.08
+1.95 |
6.40
|
602.73
+10.0 |
38.58
+0.64 |
65
+1.55 |
| 50
|
73.96
+9.25 |
6.55
|
577.29
+10.94 |
37.81
+0.72 |
64
+1.11 |
|
| 75
|
68.75
+2.85 |
7.00
|
529.68
+14.59 |
37.08
+1.02 |
62
+1.80 |
|
| 100
|
56.25
+5.46 |
7.00
|
412.32
+12.82 |
28.86
+0.90 |
44
+5.28 |
|
| 125 | 40.63
+4.98 |
7.05
|
391.31
+11.77 |
27.59
+0.83 |
49
+1.50 |
|
| Pb | 25
|
87.50
+4.42 |
6.45
|
602.73
+7.12 |
38.58
+0.46 |
64.60
+1.33 |
| 50
|
86.46
+2.92 |
6.51
|
582.63
+18.97 |
38.16
+1.34 |
63.80
+1.96 |
|
| 75
|
81.25
+2.44 |
6.66
|
568.89
+16.12 |
38.10
+1.06 |
63.72
+1.74 |
|
| 100
|
72.92
+1.95 |
6.71
|
478.93
+18.68 |
33.53
+1.31 |
56.14
+2.38 |
|
| 125
|
58.33
3.29 |
6.96
|
430.50
+9.92 |
30.35
+0.70 |
50.79
+1.34 |
|
| Control
|
93.75
+2.44 |
5.12
|
648.97
+8.31 |
39.74
+0.51 |
65.14
+1.09 |
|
+ Standard Error, All values significant at P<0.05
The low percentage germination of the treated seeds, especially at the higher levels of the applied heavy metals, is comparable with the results of studies reporting that cadmium considerably inhibits the germination of Cassia siamea and Leucaena leucocephala16, 17. The elevated concentrations of heavy metals impact water uptake by germinating seeds that hamper nutrient mobilization, thereby retarding metabolic activities and inhibiting cell division. Concentration dependent decline in seedling vigour index was also noticed in all the cases. The inhibitory effects of lead on seed germination and seedling growth have also been reported in wheat18. Similar physiological effects were observed in this study where exposure to Cd and Pb not only caused inhibition of germination in J. curcas L. seedlings but also lead to chlorosis, necrosis and retardation of growth due to toxicity.
 |
Figure 1: Seed germination in J. curcas L. on exposure to Cd and Pb |
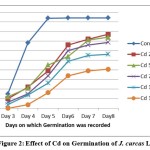 |
Figure 2: Effect of Cd on Germination of J. curcas L. |
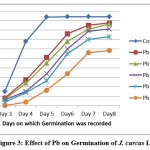 |
Figure 3: Effect of Pb on Germination of J. curcas L. |
Effect on Seedling growth
The root and shoot length of the treated seedlings were also found to be considerably reduced at all levels of the heavy metals applied, except at 25 μmol/L of Pb, where root length were more or less comparable with those of untreated seedlings (Figure-3). Root and shoot growth reduced to approximately 50% in J. curcas L. seedlings exposed to Cd and Pb concentrations > 50 μmol/L. The adverse effects on root and shoot growth were much more pronounced at the higher concentration of Cd and Pb, which may be one aspect of the role of metabolic inhibitors in the overall phenomenon of plants growth. The response of the test plants to the high levels of the Pb was reflected in overall decrease of growth. Lead is reported to adversely affect the growth and accumulate in aerial parts in Epipremnum aureum19. Adverse effects of Pb toxicity on seed germination and seedling growth have also been observed in Spartma alterniflora20. A very rapid reduction in root growth was observed when treated with various concentrations of lead in of Allium cepa9 and Zea mays10.
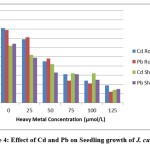 |
Figure 4: Effect of Cd and Pb on Seedling growth of J. curcas L. |
Effect on Moisture content and dry matter
The exposure to Cd and Pb decreased the moisture content and attenuated the dry matter significantly in experimental plants (Table-1). The considerable reduction in dry matter yields of the plants under the influence of high concentrations of heavy metals is in agreement with significant reduction reported in dry biomass of Albizzia lebbeck under the toxic effect of lead and cadmium21. The heavy metals Pb and Cd induced toxicity greatly reduced dry matter in Thespesia populnea L.22. Fresh weight, dry weight and relative water content were also found to be greatly decreased in mung bean on exposure to 200 ppm of Cd23. Exposure to 25 ppm of Cd and Pb caused significant decrease in dry weight of seedlings of Leucaena leucocephala17.
Effect on Tolerance Indices
The tolerance index was calculated on the basis of mean root length attained in cadmium and lead treatment groups with respect to control. The J. curcas L. seedlings were found to be more tolerant to cadmium as compared to lead. Cadmium concentration of 100 μmol/L and Pb concentration of 125μmol/L decreased the tolerance index to less than 50 percent, which is a direct indication of heavy metal toxicity in seedlings (Figure-4). Low tolerance indices have been attributed to the physiological alterations during growth in wheat under heavy metal stress24. Effect of cadmium, iron and zinc on wheat and bean species was evaluated and cadmium was found to be more toxic than iron and zinc. The metal tolerance of germinated seeds was also found to differ significantly between species and bean was more tolerant to metal stress than wheat25.
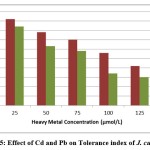 |
Figure 5: Effect of Cd and Pb on Tolerance index of J. curcas L. |
Conclusions
Heavy metal pollutants like Cd and Pb undoubtedly cause deleterious effects in plants. In this study, the tolerance limits and mechanisms of heavy metal tolerance of J. curcas L. were assessed against five levels each of Cd and Pb exposure, at seed germination and seedling development stage. Cd and Pb stress caused delay in germination and it was observed that the mean germination time in both the cases was higher as compared to the control. The germination percentage was seriously affected due to heavy metal stress. The Cd level 100 μmol/L and Pb concentration 125 μmol/L inhibited approximately 50% of germination due to severe toxicity leading to seed browning and tissue necrosis. All the growth parameters were affected at this concentration therefore, these levels were also determined as the tolerance limits for J. curcas L. These findings are relevant to evaluate the effect of heavy metal pollutants Cd and Pb on J. curcas L. seed germination and seedling growth and assess the tolerance levels of the plant. This plant is widely recognized for its potential to produce biodiesel, besides it is also reputed as a pharmacologically important plant. The results from this study would certainly be useful to find strategies to alleviate J. curcas L. from heavy metal stress and also to explore and enhance its phytoremediation potential.
Acknowledgement
The author acknowledges the support form Department of Biotechnology, Faculty of Engineering & Technology, Manav Rachna International Institute of Research and Studies, Faridabad, for providing all the resources to conduct the experimental work.
Conflict of Interest
The author declares that there is no conflict of interest.
Funding Source
This work was supported by Manav Rachna International Institute of Research and Studies, Faridabad
References
- Hediji, H., Kharbech, O., Massoud, M.B., Boukari, N., Debez, A., Chaibi, W., Chaoui, A. and Djebali, W. Salicylic acid mitigates cadmium toxicity in bean (Phaseolus vulgaris) seedlings by modulating cellular redox status. Environmental and Experimental Botany, 2021, 186, p.104432.
CrossRef - Joseph, L.U., L.C. Andrea and T.K. Mal. Effects of lead contamination on the growth of Lythrum salicaria. Environmental Pollution, 2002, 120(2): 319-323.
CrossRef - Peralta J. R., Gardea-Torresdey J. L., Tiemann K. J., Gomez E., Arteaga S., Rascon E., Parsons J. G. Study of the effects of heavy metals on seed germination and plant growth on alfalfa plant (Medicago sativa) grown in solid media. Proceedings of the 2000 conference on Hazardous Waste Research, Denver, Colorado, 2000, 135–140.
- Reichman S. M. The responses of plants to metal toxicity: a review focusing on copper, manganese and zinc. The Australian Minerals and Energy Environment Foundation. 2002, P. 54.
- Wierzbicka, M. and J. Obidzinska. The effect of lead on seed imbibition and germination in hyperaccumulator Brassica pekinensis Bull. Environ. Contam. Toxicol. 1998, (6): 258-291.
- Shafiq, M. and M.Z. Iqbal. The toxicity effects of heavy metals on germination and seedling growth of Cassia siamea. Lamark. Journal of New Seeds, 2005, 7: 95-105.
CrossRef - Goyal, D., Yadav, A., Prasad, M., Singh, T.B., Shrivastav, P., Ali, A., Dantu, P.K. and Mishra, S. Effect of heavy metals on plant growth: an overview. Contaminants in agriculture, 2020, pp.79-101.
CrossRef - Iqbal, M.Z. and D.A. Siddiqui. Effect of lead toxicity on seed germination and seedling growth of some tree species. Pakistan Journal of Scientific and Industrial Research, 1992, 35: 139-141. (8)
- Liu D., Jiang W., Zhao F. M., Lu C. Effects of lead on root growth, cell division and nucleolus of Allium cepa. Pollut,. 1994; Vol. 86. P. 1–4.
CrossRef - Jiang W., Liu D. Effects of Pb+2 on root growth, cell division, and nucleolus of Zea mays Bull. Environ. Contam. Toxicol. 2000, Vol. 65. pp. 786–793.
CrossRef - Mbako J., Ketlogetswe C. and Gandure J. Variation of Jatropha curcas seed oil content and fatty acid composition with fruit maturity stage. Heliyon, 2020, Vol. 6(1), pp. e03285.
CrossRef - Bastos, Santana E.M., Bispo da Silva A., Coelho P.L.C., Borges J.M.P., Amaral da Silva V.D., Moreau da Cunha V.H., and Costa S.L. Anti-inflammatory activity of Jatropha curcas in brain glial cells primary cultures. Journal of Ethnopharmacology, 2021, Vol. 264, pp. 113201.
CrossRef - Martín G., Francisco J., Caro M.C.G, Barrera M.C.L., García M.T., Barbin D. and Mateos P.A. Metal accumulation by Jatropha curcas adult plants grown on heavy metal-contaminated soil. Plants, 2020, Vol. 8(4), pp. 418.
CrossRef - Kashe, Keotshephile, Kgathi D.L., Murray-Hudson M. and Mfundisi K.B. Assessment of benefits and risks of growing Jatropha (Jatropha curcas) as a biofuel crop in sub-Saharan Africa: a contribution to agronomic and socio-economic policies. Journal of forestry research, 2018, Vol 29(1), pp.1-12.
CrossRef - Iqbal, M.Z. and K. Rahmati. Tolerance of Albizia lebbeck to Cu and Fe application. Ekologia (CSFR), 1992, 11: 427-430.
- Shafiq, M. and M.Z. Iqbal. The toxicity effects of heavy metals on germination and seedling growth of Cassia siamea. Lamark. Journal of New Seeds, 2005, 7: 95-105.
CrossRef - Shafiq M, Iqbal MZ, Mohammad A. Effect of lead and cadmium on germination and seedling growth of Leucaena leucocephala. Journal of Applied Sciences and Environmental Management. 2008, pp. 12(3).
CrossRef - Yang Y, Wei X, Lu J, You J, Wang W, Shi R. Lead-induced phytotoxicity mechanism involved in seed germination and seedling growth of wheat (Triticum aestivum). Ecotoxicology and environmental safety. 2010 Nov 1;73(8), pp.1982-7.
CrossRef - Kumari A, Shourie A, Vijayalakshmi U. Effect of Lead and Lead-EDTA on Growth and metal accumulation characteristics of Epipremnum aureum. International Journal of Applied Environmental Sciences. 2015;10(5), pp.1591-606.
- Morzeck, J.R.E. and N.A. Funicelli. Effect of zinc and lead on germination of Spartma alterniflora Loisel seeds at various salinities. Environmental and Experimental Botany, 1982, 22: 23-32.
CrossRef - Farooqi M, Zafar Iqbal, Kabir M and Shafiq M. Toxic effects of lead and cadmium on germination and seedling growth of Albizia lebbeck (L.), Benth, J. Bot., 2009,41(1): 27-33.
- Kabir M, Iqbal MZ, Shafiq M, Farooqi ZR. Reduction in germination and seedling growth of Thespesia populnea, caused by lead and cadmium treatments. Pak. J. Bot. 2008 Dec 1;40(6), pp. 2419-26.
- Shourie A. and Vijayalakshmi U. Role of Pseudomonas fluorescence in Cadmium stress alleviation in Vigna radiata (Mung Beans), International Journal of Pharma and Bioscience, 2018, Vol- 9(3): (B), pp. 61-65.
CrossRef - Khan, N.A., I. Ahmad, S. Singh and R. Nazar. Variation in growth, photosynthesis and yield of five wheat cultivars exposed to cadmium stress. World J. Agri. Sci., 2006, 2: 223-226.
- El Rasafi, T., Nouri, M., Bouda, S. and Haddioui, A. The effect of Cd, Zn and Fe on seed germination and early seedling growth of wheat and bean. Ekológia, 2016, 35(3), p.213.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






