How to Cite | Publication History | PlumX Article Matrix
Pooja J. Gupta* , Minal J. Trivedi
, Minal J. Trivedi and Harsha P. Soni
and Harsha P. Soni
Department of Biotechnology, Pramukh Swami Science and H.D. Patel Arts College, SV Campus, Kadi, Gujarat, India.
Corresponding Author E-mail: guptapooja0096@gmail.com
DOI : http://dx.doi.org//10.13005/bbra/3030
ABSTRACT: Chemical fertilizer use in agricultural areas causes a variety of issues, including pollution, health risks, disruption of natural ecological nutrient cycles, and the loss of biological communities. In this case, chemical fertilizers, herbicides, and other supplements are replaced by plant growth promoting bacteria for sustainable agriculture. The present research work focus on the isolation of the plant growth promoting bacteria from the Kadi vegetable market waste. Derived from morphological, biochemical, and 16S rRNA gene sequence analysis the strain was identified as Enterobacter cloacae PNE2. The antibiotic susceptibility test indicated that the isolate was sensitive to all 22 antibiotics tested. The isolate Enterobacter cloacae PNE2 has multiple growth-promoting activities like N2 fixation, phosphate, solubilization, potassium solubilization, phytohormone (Indole-3-acetic acid) production, EPS production, biopolymer degradation, and also possesses good seed germination ability. Quantitative analysis of nitrite production revealed the isolate Enterobacter cloacae PNE2 produced 0.15±0.01 µg/ml nitrite. The Phosphate Solubilization Index (PSI) of the isolate was recorded 3.58±0.08 and the isolate released 278.34±0.56 μg/ml phosphate in Pikovskaya’s broth. The isolate Enterobacter cloacae PNE2 solubilized 32.66 mg/l potassium. The isolate Enterobacter cloacae PNE2 possesses IAA (48.49±0.05µg/ml) in presence of tryptophan and EPS (19.1±0.2 g/l) production ability. The isolate Enterobacter cloacae PNE2 was also found to degrade Cellulose, Pectin, and Xylan. Furthermore, the isolate Enterobacter cloacae PNE2 enhances seedling growth of Trigonella foenum graceum (fenugreek). Thus, the isolate Enterobacter cloacae PNE2 has significant plant growth promoting characteristics and can be applied in a bio-fertilizer formulation for sustainable agriculture.
KEYWORDS: Enterobacter cloacae PNE2; Plant growth promoting bacteria; seed germination; Trigonella foenum graceum
Download this article as:| Copy the following to cite this article: Gupta P. J, Trivedi M. J, Soni H. P. Enterobacter cloacae PNE2 as Promising Plant Growth Promoting Bacterium, Isolated from the Kadi Vegetable Market Waste, Gujarat. Biosci Biotech Res Asia 2022;19(3). |
| Copy the following to cite this URL: Gupta P. J, Trivedi M. J, Soni H. P. Enterobacter cloacae PNE2 as Promising Plant Growth Promoting Bacterium, Isolated from the Kadi Vegetable Market Waste, Gujarat. Biosci Biotech Res Asia 2022;19(3). Available from: https://bit.ly/3UPPpbI |
Introduction
Agriculture plays a vital role in the economy in addition it is considered to be the backbone of the economic system for developing countries, but in the twenty-first century, the world’s agricultural system is facing new challenges, such as failing productivity and deterioration in the agroecosystem sustainability 1,39. The world’s population is currently around 7.9 billion, and it is expected to reach 8.5 billion by 2030 40. India’s population will also increase to 1403 million by 2025, as compared to the present 1391 million (2020-2021). Agriculture will play an essential role in supplying increased food demands for the rising human population as result in greater demand for to use of chemical fertilizers and pesticides. Moreover, the frequent use of these chemical fertilizers will cause air and groundwater pollution through the eutrophication of water bodies, will also negatively affect soil fertility, lower agricultural productivity, damage soil, and cause biodiversity loss. Therefore, using chemical fertilizer and pesticides poses a major risk to the ecosystem as well as health issues for people 2.
Plant growth promoting bacteria are used to replace these chemical fertilizers through a variety of mechanisms, including soil structure formation, organic matter decomposition, element recycling, mineral solubilization, plant growth regulator production, organic pollutant degradation, root growth stimulation, and soil fertility enhancement 3.
There are numerous genera in the Enterobacteriaceae family that have plant growth promoting abilities for e.g, Enterobacter, Erwinia, Klebsiella, Kluyvera, Pantoea, and Serratia 4.
Enterobacter cloacae is a gram-negative, short rod of the Enterobacteriaceae family 5. Several strains of Enterobacter cloacae have been reported as plant growth promoters due to their multiple growth promoting activities like phosphate solubilization, nitrogen fixation, phytohormone production, exopolysaccharides production, 1-Aminocyclopropane-1- carboxylate deaminase (ACC) production, etc 6. Thus, Enterobacter cloacae have the potential to contribute to the development of sustainable agricultural systems.
Nitrogen is the most vital element for plant growth and development. Approximately 78–79% of the available nitrogen (N) in the atmosphere do not directly absorb by the plants. Several plant growth promoting bacteria can able to fix atmospheric nitrogen dioxide into ammonia, which the plant may easily absorb7. Enterobacter cloacae HG-1 has nitrogen fixation ability7.
Phosphorus (P) is the second most important element for plant growth 8. It has a key role in several physiological processes in plants, particularly photosynthesis, carbon metabolism, and membrane production. Additionally, it is essential for root elongation, proliferation and its deficiency affects root structure 9. Approximately 95 to 99 percent of phosphorus is found in soil in an insoluble form along with Fe, Zn, Al, and ca. Only 1% to 2% of the phosphorus is taken by plants. Phosphate solubilizing microorganisms play a key role in phosphorus nutrition by transferring its availability to plants through solubilization and mineralization from inorganic and organic soil phosphorus. The majority of powerful phosphate solubilizing bacteria belong to bacteria such as Pseudomonas, Enterobacter, Enterobacter cloacae BAU3, and Bacillus 10.
Potassium (K) is the third most important element for a plant. it is important for photosynthesis, activation of enzymes, and synthesis of protein. As more than 90% of potassium exists in the form of insoluble rock and silicate minerals, the concentration of soluble potassium is usually very low in soil 1. Certain microorganisms use several biological processes to make potassium available from unavailable forms i.e Klebsiella variicola, Enterobacter cloacae, Enterobacter asburiae, Enterobacter aerogenes, Burkholderia cepacia, Microbacterium foliorum, and Pantoea agglomerans 11,2.
Furthermore, plant growth promoting bacteria are capable of synthesis phytohormones (Indole-3 acetic acid). Indole acetic acid has various functions like cell division, stimulation of seed germination, pigment production, and synthesis of metabolites. Numerous Enterobacter spp. having the potential to produce indole acetic acid (IAA) such as E. cloacae H3, E. cloacae NII-0931, E. cloacae MSR1, E. cloacae UW 5, E. asburiae, E. cancerogenus 13.
Several strains of plant growth promoting bacteria can able to produce exopolysaccharides (EPS). The EPS-producing plant growth promoting bacteria significantly enhance the volume of soil macropores and the rhizosphere soil aggregation, resulting in increased water and fertilizer availability to inoculated plants 14. Exopolysaccharides can be produced in significant quantities by the bacterial strain Enterobacter cloacae 15.
In the present research work, we have isolated the Enterobacter cloacae PNE2 from the vegetable market waste and characterized its plant growth promoting capability based on N2 fixation, phosphate solubilization, potassium solubilization, IAA production, EPS production, biopolymer degradation, and seedling growth of Trigonella foenum graceum (fenugreek) plant. Based on obtained results, we propose to use the indigenous isolate Enterobacter cloacae PNE2, as a biofertilizer.
Materials and Methods
Sample collection and isolation of the Enterobacter cloacae PNE2 from the vegetable market waste:
For the bacterial isolation mixed consolidated vegetable waste was collected from the Kadi vegetable market in Mehsana distinct from Gujarat. The sample was collected on a random basis and transferred into the laboratory. A serial dilution method was carried out to isolate bacteria from vegetable market waste. 1 ml of a separated liquid component collected from the vegetable market waste was added to 9 ml sterilized water and then serially diluted up to 106. Initially, the isolation was done using Ashby’s Mannitol agar. Spread 0.1 ml of each diluted sample onto Ashby’s Mannitol agar and the plate was incubated at 37±2 °C for 48 hours. The colonies that grow on Ashby’s Mannitol agar are thought to have the potential for nitrogen fixation. The colony was then isolated and purified depending on the distinctive colony morphology for further study 14.
Identification of the isolate
Morphological and biochemical identification
The Isolate was identified based on Gram-staining, morphological characteristics, and biochemical test using the HI-Assorted biochemical test kit (KB003) of HI-media Laboratories, Mumbai, India 14.
Molecular identification
Molecular identification of the isolate Enterobacter cloacae PNE2 was done by 16S rRNA partial gene sequencing analysis respectively. Fragment of 16S rRNA gene was amplified by PCR. Takara (EmeraldAmp® GT PCR Master Mix) was used for the amplification of DNA templates. The PCR amplicon was purified by column purification to remove contaminants. DNA sequencing reaction of PCR amplicon was carried out with 27 F & 1492 R universal primers using BigDye™ terminator v3.1 cycle sequencing kit 16. v 6.0 was used for sequence analysis from the electropherogram generated by the 3500XL Genetic Analyzer. The 16S rRNA sequence was used to carry out BLAST with the database of NCBI GenBank.
Phylogenetic Analysis
The Neighbor-Joining method was used to deduce the evolutionary history 17. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) was shown next to the branches 18. The evolutionary distances were computed using the greatest composite likelihood method, which is indicated the units of several base substitutions per site19. This analysis involved 8 nucleotide sequences. All positions containing gaps and missing data were eliminated (complete deletion option). There were a total of 842 positions in the final dataset. Evolutionary analyses were conducted in MEGA11 20.
Antibiotic Susceptibility test
The disc diffusion method was used to check the antibiotic susceptibility of the strain Enterobacter cloacae PNE2. The method involved spreading the 0.1ml of overnight grown culture (set the O.D 0.5 at 540 nm) 21 on a nutrient agar plate and placing antibiotic multidisc on the surface of the plate, incubating the plate at 37±2 °C for 24 hrs. Antibiotic susceptibility test was conducted using combi disk for Gram Negative bacteria from Hi Media Laboratories 14. The zone of inhibition was measured 22.
Plant growth-promoting characterization of the isolate Enterobacter cloacae PNE2
The Enterobacter cloacae PNE2 was further screened for plant growth promoting traits, like nitrogen fixation, phosphate solubilization, potassium solubilization, IAA production, EPS production, biopolymer degradation, and seed germination ability.
Determination of nitrite nitrogen
Quantitative determination of nitrite nitrogen was carried out by estimating nitrite nitrogen. 3 ml of 24 hrs old culture (O.D adjusted to 0.5 at 540 nm) was inoculated in 100 ml of Ashby’s N-free liquid medium. The inoculated flasks were incubated at room temperature in static conditions. Approximately 2ml broth was harvested at the interval of 24 hrs. Following the appropriate incubation period, the culture broth was centrifuged for 10 minutes at 10,000 rpm14. Nitrite nitrogen production in the broth was examined at an interval of 24 hrs of incubation by standard method 23.
Determination of phosphate solubilization activity
The isolate Enterobacter cloacae PNE2 was spot inoculated on Pikovskaya’s medium and the plate was incubated at 37±2°c for 48 hrs. After the incubation periods measured the Phosphate Solubilsation Index (PSI) by the following equation 22.
PSI = (colony diameter + halo zone) / colony diameter
Quantitative estimation of phosphate solubilization was carried out by using Pikovskaya’s liquid medium abundant with 0.5% tricalcium phosphate. Selected isolate Enterobacter cloacae PNE2 was inoculated in Pikovskaya’s broth and incubated for 7 to 9 days at 37+2°C under shaking conditions at 120 rpm. At an interval of 24 hrs, approximately 2ml of broth was harvested and centrifuged for 10 min at 10000 rpm. The vanadomolybdo phosphoric acid method was used to estimate the amount of phosphorous in the broth was estimated from the supernatant 24. A simultaneous change in the pH was also recorded from the supernatant by using eqiptronic digital pH meter.
Determination of Potassium Solubilization activity
Qualitative determination of Potassium Solubilization was carried out by using modified Aleksandrow agar medium + bromothymol blue. The isolate Enterobacter cloacae PNE2 was spot-inoculated onto the modified Aleksandrow agar medium, and incubate on the plate for 24 hrs at 37± °C 14. Khandeparkar’s selection ratio method was used to measure the formation of clear halos surrounding the colony 25.
D/d = Diameter of zone hydrolysis/ Diameter of growth
Quantitative estimation of Potassium Solubilization activity
Quantitative determination of potassium solubilization was carried out by estimation of K released from broth supplemented with 0.5 % Feldspar. Three ml of 24 hrs old culture (O.D adjusted to 0.5 at 540 nm) was inoculated in 100 ml of GYF (Glucose Yeast extract feldspar) broth 26. The inoculated flasks were incubated in an environmental shaker at 120 rpm. The amount of K released in the broth was examined at intervals of 7, 15, and 21 days of incubation. At the interval of the 7 days, approximately 5ml broth was harvested and centrifuged for 10 min at 10,000 rpm. Take supernatant from the centrifuge tube and estimate the potassium released from the broth by the flame photometric method. 14.
Determination of Indole acetic acid (IAA) production
The IAA production was determined qualitatively using Luria agar mixed with 0.06% sodium dodecyl sulphate and 1% glycerol.
Qualitative determination for the IAA production was detected using Luria agar supplemented with 0.06% sodium dodecyl sulphate and 1% glycerol. The overnight grown culture of the isolate was spot inoculated in each plate. The plates were layered immediately with a sterile disc of Whatman No.1 filter paper. After 48hrs, the filter paper disc was removed from the plates and treated with Salkowaski’s reagent (2% of 0.5M FeCl3 in 35% perchloric acid) by soaking in a petridish containing the reagent. The reaction was allowed to proceed until the adequate color was developed 27.
Quantitative estimation of IAA production
For the quantitative estimation of IAA production, three ml of 24 hrs old culture of the isolate Enterobacter cloacae PNE2 (O.D adjusted to 0.5 nm) was inoculated in 100 ml Luria broth supplement With 0.1% tryptophan or without tryptophan and the flask was incubated at 37+2°C for 7 days at under shaking condition at 120 rpm. Salkowski’s reagent was used to estimate the amount of IAA production in 1 ml of the supernatant after centrifuging 2 ml of broth for 10 min at 10,000 rpm 28.
Quantitative estimation of EPS production
The isolate Enterobacter cloacae PNE2 for the production of EPS was measured using the standard method. For quantitative estimation, the overnight grown culture of the isolate was inoculated in yeast extract medium supplemented with 5% sucrose and incubated at 37±2°C for 5 days on a shaker at 120 rpm. At the interval of 24 hours,10 ml of broth was taken in a centrifuge tube and centrifuged at 10,000 rpm for 10 min. The supernet was collected from each centrifuge tube and Three-time increased cooled acetone was added. A slimy precipitate that contained EPS was removed from the mixture and gathered on a pre-dried filter paper. At a temperature of 50 °C, the precipitates were left to dry overnight. After drying for the entire night, the filter paper was reweighed. The amount of EPS produced was determined by increasing the filter paper weight. 24 ,14.
Determination of potential for biopolymer degradation
The potential of the isolate Enterobacter cloacae PNE2 for degradation of cellulose, pectin, and xylan was carried out on a solid medium supplemented with 1% CMC, 1% Pectin, and 1% beech wood Xylan respectively as substrate with pH 6.8. The isolate was spot inoculated on basal mineral salt medium with CMC/Pectin/Xylan and plates were incubated at 37±2ºC till the development of colonies on the medium 29. The zone of solubilization was measured after flooding with 1% iodine solution on the medium. The Khandeparkar method was used to calculate the zone ratio. The Khandeparkar method was used to measure the zone ratio 25.
D/d = Diameter of zone of hydrolysis / Diameter of growth
Seed germination assay
Fenugreek seed was used for seed germination assay. Seed germination assay was performed by soil method 24. All of the chosen seeds were surface sterilized for 90 seconds with 1% NaOCl then immersed for 30 seconds in 70% ethanol, rinsed twice with sterile distilled water, and air drying under laminar airflow 16. A total of 50 Fenugreek seed was soaked for 15 minutes in 10 ml overnight grown bacterial culture Enterobacter cloacae PNE2 (adjust optical density 0.5 at measured at 540 nm which contain 108 cells /ml). Two controls were used for this study. i) Control 1st (primed control): 50 seeds were soaked in 10ml sterile distilled water for 15 minutes. ii) Control 2nd (unprimed control): 50 dry seeds were directly used in sowing without any treatment. The experiment was set up in black plastic bags which contain 300gm soil. Ten seeds were sown in each bag. The experiment was monitored for up to 7 days and the number of seeds germinated, germination index, seed vigour index, and plumule length were recorded. After recording the results for 7 days, the plants were uprooted, and measured the shoot lengths, root lengths, wet weight, and dry weight of the Trigonella foenum graceum (fenugreek) plant 24.
Statistical analyses
The result was shown as the mean± standard deviation (SD) for all experiments, which were carried out in triplicate 40. T-test two-sample with unequal variances was used to compare mean values, and significance was accepted at P≤ 0.05 level 42. Mean values were compared using a two-sample T-test with unequal variance, and significance was accepted at P≤ 0.05 level 43.
Results and Discussion
Isolation and identification of strain PNE2
The strain was isolated from the liquid content of vegetable market waste. The ability to fix atmospheric nitrogen was tested by inoculating the isolate on Ashby’s Mannitol agar. The isolates grow on nitrogen-deficient media, indicating that the isolate Enterobacter cloacae PNE2 was able to fix atmospheric nitrogen from the environment. The isolate was purified and preserved in the same medium for further study. Figure 1 depicts the data of cultural characteristics on Ashby’s Mannitol agar medium and Morphological characteristics of the selected isolate. On Ashby’s Mannitol agar, isolate formed non pigmented, medium, round, entire glossy, mucoid, opaque, convex colony. According to its morphology, the isolate was a Gram-negative short rod.
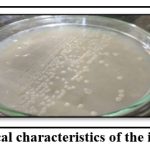 |
Figure 1: Cultural and morphological characteristics of the isolate Enterobacter cloacae PNE2. |
Biochemical characterization
Results depicted in Table 1 show the data of biochemical characterization of the isolate Enterobacter cloacae PNE2. Out of thirty biochemical tests performed, the isolate shows a positive result for 16 tests and a negative result for 9 tests. Out of 13 sugars the isolate fermented 10 sugars. Only 3 sugars melibiose, adonitol, and sorbitol are not utilized by the isolate. The isolate tested positive for ONPG, Urease production, Nitrate reduction, Citrate utilization, the Voges Proskauer test, and Esculin hydrolysis, showing that it has a wide metabolic range. In addition, lysine, ornithine utilization, phenylalanine, H2S generation, Methyl red, and Indole are all negative. The isolate was able to produce the enzymes like urease, lipase, protease, cellulase, pectinase, and xylanase indicating their biopolymer degradation ability. Out of 24 biochemical tests performed the isolate Enterobacter cloacae PGLO9 was shown to be 21 positive results i.e xylose, maltose, galactose, raffinose, trehalose, melibiose, L- arabinose, mannose, insulin, glycerol, inositol, sorbitol, mannitol, α-methyl-D-glucoside, glucoside, cellobiose, ONPG, esculin hydrolysis, D- arabinose, citrate utilization, and malonate utilization, three were shown negative results i.e. lactose, adinitol, rhamnose 30.
Table 1: Biochemical characterization of the isolate Enterobacter cloacae PNE2.
|
Biochemical Test |
Results |
Biochemical Test |
Results |
|
ONPG (O-Nitrophenyl-β-D-Galactopyranoside) |
+ |
Pectinase |
+ |
|
Lysine |
– |
Xylanase |
|
|
Ornithine utilization |
– |
Arabinose |
+ |
|
Phenylalanine |
– |
Xylose |
|
|
Nitrate reduction |
+ |
Adonitol |
– |
|
H2S production |
– |
Rhamnose |
+ |
|
Citrate utilization |
+ |
Cellobiose |
+ |
|
VP (Voges Proskauer) |
+ |
Melibiose |
– |
|
MR (Methyl Red) |
– |
Saccharose |
+ |
|
Indole |
– |
Raffinose |
+ |
|
Esculin hydrolysis |
+ |
Trehalose |
+ |
|
Urease |
+ |
Glucose |
+ |
|
Lipase |
+ |
Lactose |
+ |
|
Amylase |
+ |
Sorbitol |
– |
|
Cellulase |
+ |
Malonate |
+ |
Molecular identification of the PNE2
Molecular identification and 16S rRNA partial gene sequence analysis confirmed that the isolate is Enterobacter cloacae PNE2. The phylogenetic tree for the same is depicted in figure 2. The partial 16S rRNA gene sequence of Enterobacter cloacae PNE2 was summited at the NCBI Gene bank with Accession number ON945595.
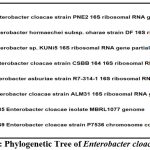 |
Figure 2: Phylogenetic Tree of Enterobacter cloacae PNE2 |
Antibiotic Susceptibility test
The isolate Enterobacter cloacae PNE2 was shown to be extremely sensitive to 22 antibiotics tested, as shown in Table 2. Compared to all 22 antibiotics tested, the isolate was most susceptible to two broad-spectrum antibiotics Carbenicillin (CB) (22 mm) and tobramycin (22 mm), followed by Ofloxacin (21). Antibiotics that inhibit protein synthesis (Tobramycin, Streptomycin, Gentamicin, Netllin, Tetracycline, Amikacin, and Kanamycin), as well as antibiotics that inhibit cell wall synthesis (Carbenicillin, Ceftriaxone, Ceftazidime, Cefepime, Cephalothin, Cefotaxime, Imipenem, Meropenem) 31, are more effective against the isolate Enterobacter cloacae PNE2.
Table 2: Antibiotic susceptibility test of Enterobacter cloacae PNE2.
|
Antibiotic Disk |
µg/ml |
Zone of inhibition (mm) |
Antibiotic Disk |
µg/ml |
Zone of inhibition (mm) |
|
Tetracycline |
30 |
5 |
Ceftazidime |
30 |
20 |
|
Streptomycin |
10 |
15 |
Cefepime |
30 |
12 |
|
Nitrofurantoin |
300 |
10 |
Cephalothin |
30 |
15 |
|
Kanamycin 30 |
30 |
9 |
Cefotaxime |
30 |
20 |
|
Co-Trimazine |
25 |
5 |
Imipenem |
10 |
15 |
|
Carbenicillin |
100 |
22 |
Meropenem |
10 |
18 |
|
Amikacin |
30 |
10 |
Ciprofloxacin |
5 |
20 |
|
Ofloxacin |
25 |
21 |
Tobramycin |
10 |
22 |
|
Co-Trimoxazole |
25 |
18 |
Moxifloxacin |
5 |
20 |
|
Gentamicin |
10 |
10 |
Sparfloxacin |
5 |
15 |
|
Ceftriaxone |
30 |
20 |
Netllin |
30 |
20 |
Plant growth promoting characterization by the isolate Enterobacter cloacae PNE2
Determination of nitrite nitrogen
The isolate Enterobacter cloacae PNE2 was found to grow well in N-free Ashby’s Mannitol media indicating that the isolate PNE2 was able to grow in nitrogen deficiency in media. The results in Table 3 show that isolated Enterobacter cloacae PNE2 produced 0.15 ±0.01 µg/ml nitrite after the 8th day of incubation. The isolate Klebsiella pneumoniae PNE1 produced 0.09 µg/ml nitrite after the 8th day of incubation 14.
Table 3: Characterization of nitrite nitrogen by Enterobacter cloacae PNE2
|
|
8th day |
9th day |
|
Nitrite |
0.15±0.01 µg/ml |
0.08 ±0.02 µg/ml |
Phosphate Solubilization activity
The isolate Enterobacter cloacae PNE2 produced a clear halo zone around its colony on Pikovskaya’s medium (figure 4). The phosphate solubilization index was recorded 3.58±0.08 after 5th day of incubation. 3.22 Phosphate Solubilization Index (PSI) was found by the isolate Enterobacter cloacae BAU3 after 10th days of incubation 10. The Phosphate Solubilization Index (PSI) of isolate Enterobacter cloacae PSB6 was 3.06 11.
 |
Figure 3: Zone of Phosphate solubilization by |
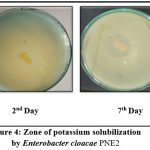 |
Figure 4: Zone of potassium solubilization |
Phosphate solubilization by the isolate and change in medium pH was shown in graph 1. The range of inorganic phosphate solubilization varied from 137.3±0.63 to 278.34±0.56 µg/ml. The highest solubilization of 55.66 % (278.34±0.56 µg/ml) was achieved on the 5th day of incubation by the isolate Enterobacter cloacae PNE2. The isolate Enterobacter cloacae PSB6 solubilized 96.86 µg/ml phosphorus after the 5th day of incubation, and also drop the pH from 6.90 to 4.90 after the 5th of incubation 11. Enterobacter cloacae NII-0931 solubilized 58.5±2 µg/ml phosphorus after the 15th day of incubation 32. Both strain Enterobacter cloacae PSB6 and Enterobacter cloacae NII-0931 are low amounts of phosphate solubilization as compared to Enterobacter cloacae PNE2.
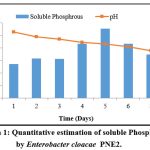 |
Graph 1: Quantitative estimation of soluble Phosphorus by Enterobacter cloacae PNE2. |
Determination of potassium solubilization activity
Table 4 showed the Potassium solubilization index of isolate Enterobacter cloacae PNE2 varies in ranges from 1.6 to 2.18 on Aleksandrov medium with bromothymol blue within 7th days of incubation. The isolate showed yellow color formation around the growth in the Aleksandrov medium with bromothymol blue its indicating that the isolate might be produced organic acid to solubilize the potassium from the medium (figure 4). The potassium solubilizing index by the isolate Enterobacter cloacae PSB6 was 3.05 11.
Table 4: Qualitative determination of soluble Potassium by Enterobacter cloacae PNE2.
|
Days |
Colony size (mm) |
Zone size (mm) |
Zone ratio (mm) |
|
1 |
6 |
10 |
1.6 |
|
2 |
8 |
15 |
1.8 |
|
4 |
10 |
21 |
2.1 |
|
7 |
12 |
23 |
1.76 |
The quantitative estimation amount of Potassium released from feldspar revealed the Potassium solubilization ability of the isolate Enterobacter cloacae PNE2 (graph 2). The range of inorganic Potassium solubilization varied from 24.53±0.06 mg/l to 32.69±0.39 mg/l. The maximum solubilization of 32.66 mg/l was achieved on the 21st day of incubation by the isolate Enterobacter cloacae PNE2. The isolate Enterobacter cloacae PSB6 solubilized 14.00 mg/l of potassium on the 5th day of incubation 11. The isolate Enterobacter cloacae GL7 solubilized less than 2 mg/l potassium 12. Enterobacter cloacae 38 solubilized 71.15 µg/ml potassium after the 5th day of incubation 33.
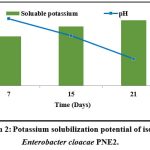 |
Graph 2: Potassium solubilization potential of isolate Enterobacter cloacae PNE2. |
Indole Acetic Acid (IAA) production
The qualitative determination of IAA production by the isolate Enterobacter PNE2 was shown in Figure 5. The isolate formed pink color on the filter paper after 72 hrs incubation, which confirmed that the isolate can produce IAA.
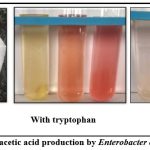 |
Figure 5: Indole acetic acid production by Enterobacter cloacae PNE2. |
As shown in graph 3, Enterobacter cloacae PNE2 produced a substantial amount of IAA both in the absence and presence of tryptophan. In presence of tryptophan, IAA production ranges from 23.5±0.45 to 48.49±0.05µg/ml. Maximum IAA production was recorded 48.49±0.05 µg/ml in presence of tryptophan after 5th day of incubation. In absence of tryptophan, IAA production ranges from 15.18±0.03 to 22.41±0.01 µg/ml. Maximum IAA production was recorded 22.41±0.01 µg/ml in absence of tryptophan after 3rd day of incubation. Enterobacter cloacae PNE2 showed maximum IAA producing ability in presence of tryptophan indicating that it might be the precursor for IAA biosynthesis. The isolate E. cloacae JAS7 produced 46.6±0.5 μg/ml IAA34. The isolate Enterobacter cloacae produced 2.443 μg/ml of IAA35. The isolate Enterobacter cloacae H3 produced 12.28 μg/ml IAA after 5th day of incubation 13.
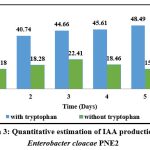 |
Graph 3: Quantitative estimation of IAA production by Enterobacter cloacae PNE2 |
Quantitative estimation of EPS production
Data presented in graph 4 depicts EPS Production by Enterobacter cloacae PNE2 using 5% sucrose as substrate. The amount of EPS produced ranged from 6.83±0.11 to 19.1±0.2 g/l. The EPS production increased till the third day and thereafter it continuously decreased. Thus, maximum production could have occurred around the 3rd day. The decrease in the number of EPS after the 4th day may be due to the utilization of own EPS as substrate by the producing organism or EPS yield may probably decrease because of the action of glycohydrolases produced in the culture that catalyzed the degradation of polysaccharides, resulting in decreased EPS yields 37. After the fourth day, EPS production may have been reduced because the isolate began to utilize its own EPS as a substrate. EPS yield may also have dropped as a result of the activity of glycohydrolases created in the culture, which catalyzed the breakdown of polysaccharides37. 38 reported 8.83 g/l of EPS yield of the isolate Enterobacter cloacae. 15 reported 12.95 g/L of EPS yield by E. cloacae Z0206. 36 reported 18.1 g/l of EPS yield by Enterobacter sp. strain ACD 2 after the 3rd day of incubation
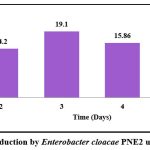 |
Graph 4: EPS Production by Enterobacter cloacae PNE2 using 5% sucrose. |
Determination of Potential for biopolymer degradation
As shown in Table 5, the isolate Enterobacter cloacae PNE2 could degrade cellulose, pectin, and xylan. Based on the zone ratio the ability of the isolate to degrade biopolymers was best for pectin, good for cellulose, and least for xylan. The zone ratio for cellulose degradation was 5.5 mm. The zone ratio for Pectin degradation was 9.2 mm and the zone ratio for Xylan degradation was 1.6 mm. The isolate Klebsiella pneumoniae PNE1 had a good ability to degrade cellulose, pectin, and xylan 14.
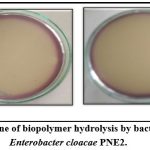 |
Figure 6: Zone of biopolymer hydrolysis by bacterial isolate |
Table 5: Potential for biopolymer degradation
|
Zone of hydrolysis (mm) |
||||||||
|
Cellulose |
Pectin |
Xylan |
||||||
|
Colony size |
Zone size |
Zone ratio |
Colony size |
Zone size |
Zone ratio |
Colony size |
Zone size |
Zone ratio |
|
10 |
55 |
5.5 |
7 |
65 |
9.2 |
12 |
20 |
1.6 |
Seed germination assay
Figure: 7 showed the effects of Enterobacter cloacae PNE2 on Trigonella foenum graceum (fenugreek). Table 6 showed the seed germination and seedling growth of Trigonella foenum graceum (fenugreek) plant till 7th day of observation. When the Trigonella foenum graceum (fenugreek) seed was treated with the plant growth promoting isolate Enterobacter cloacae PNE2, significantly improved (P<0.05) in growth of Trigonella foenum graceum (fenugreek) plant was observed as compared to unprimed control in terms percentage germination (73.33±5.77), germination index (13.33±0.20), plumules length (1±0.1), shoot length (10.43±0.11), vigour index (808.43), wet weight (0.13±0.01), dry weight (0.083±0.002) and root length and also compare to primed control in terms germination index (13.33±0.20), plumules length (1±0.1), root length, vigour index (808.43), wet weight (0.13±0.01), dry weight (0.083±0.002) and root length. No significant difference was found between primed control (C2) and T1 (Enterobacter cloacae PNE2) in terms of percentage germination, shoot length and dry weight. 11 reported that the isolate Enterobacter cloacae use in seedling growth of fennel (Foeniculum vulgare Mill) crop. 33 reported Enterobacter spp. significantly improved overall germination parameters and vigour index of okra seed.
 |
Figure 7: Effects of Enterobacter cloacae PNE2 on Trigonella foenum graceum (fenugreek) plant up to 7th days: A. (C2): Primed control, B. (C1): Unprimed control C. T1: Enterobacter cloacae PNE2. |
Table 6: Effect of Enterobacter cloacae PNE2 on seedling growth of fenugreek plant up to 7th days
|
|
C1 |
C2 |
T1 |
|
% Germination |
58.33±2.88 |
66.66±5.77 |
73.33±5.77 |
|
Germination index (GI) |
6.62±0.07 |
8.4±0.1 |
13.33±0.20 |
|
Plumule length (cm) |
0.73±0.05 |
0.76±0.05 |
1±0.1 |
|
Shoot length (cm) |
8.86±0.23 |
10.23±0.25 |
10.43±0.11 |
|
Root length (cm) |
9.8±0.26 |
10.16±0.15 |
10.9±0.36 |
|
Vigour index (VI) |
580.36 |
687.56 |
808.43 |
|
Wet weight (gm) |
0.08±0.02 |
0.105±0.05 |
0.13±0.01 |
|
Dry weight (gm) |
0.045±0.03 |
0.066±0.005 |
0.083±0.002 |
Mean ± SD = Mean + standard deviation
(C1): Unprimed control, (C2): Primed control T1: Enterobacter cloacae PNE2
Conclusion
Based on morphological, biochemical, and 16S rRNA partial gene sequencing analysis, the isolate obtained from vegetable market waste has been identified as Enterobacter cloacae PNE2. The antibiotic susceptibility test indicated that the isolate was found to be sensitive to all 22 antibiotics tested. Plant growth promoting characterization revealed that the isolate was capable of producing nitrite and solubilizing phosphate and potassium through the biological process from unavailable to readily available forms. The ability of the isolate Enterobacter cloacae PNE2 to produce nitrite and solubilized potassium and phosphorous will enhance the nutrient status of soil as these are essential for the growth of the plants. Furthermore, the isolate Enterobacter cloacae PNE2 can also produce considerable amounts of phytohormones (Indole acetic acid) and exopolysaccharides (EPS), which will enhance survival and promote plant growth. Moreover the isolate Enterobacter cloacae PNE2 was also found capable of degrading biopolymers viz., cellulose, pectin, and xylan. The isolate Enterobacter cloacae PNE2 to enhance the seedling growth of Trigonella foenum graceum (fenugreek) plant as compared to the control. Thus, the current study demonstrates that the isolates have a variety of plant growth promoting traits, which are beneficial to crops when employed as biofertilizers in agricultural fields.
Acknowledgment
We are thankful to the Gujarat State Biotechnology Mission, and the Government of Gujarat for providing funds for the research work as a Major Research Project grant. We are also thankful to Pramukh Swami Science and H.D. Patel Arts College, Kadi for all the facilities and support.
Conflict of interest
The authors would hereby like to declare that there is no conflict of interest.
Funding sources
We are thankful to the Gujarat State Biotechnology Mission (GSBTM) providing funds for the research work.
References
- Prasad, M., Srinivasan, R., & Chaudhary, M. Plant growth promoting rhizobacteria ( PGPR ) for sustainable Agriculture : Perspectives and Challenges. In PGPR amelioration in sustainable agriculture: Elsevier 2019; pp 129-157. https://doi.org/10.1016/B978-0-12-815879-1.00007-0.
CrossRef - Mazumdar, D., Saha, S. P., & Ghosh, S. Klebsiella pneumoniae rs26 as a potent PGPR isolated from chickpea (Cicer arietinum) rhizosphere.201. The Pharma Innovation Journal. 2018; 7(11), 56–62.
- Chandra Dinesh, Pallavi, Barh Anupam Barh, S. I. P. S. Plant Growth Promoting Bacteria : A Gateway to Sustainable Agriculture. Chapter 20. 2018; 318–338. https://doi.org/10.4018/978-1-5225-3126-5.
CrossRef - Chaitanya Kumar Jha, Abhinav Aeron, Baldev V.Patel, M. S. Enterobacter : Role in Plant Growth Promotion In : Bacteria in Agrobiology : Plant Growth Responses. 2011; https://www.researchgate.net/publication/232262661.
- Liu, W. Y., Wong, C. F., Chung, K. M. K., Jiang, J. W., and Leung, F. C. C. Comparative Genome Analysis of Enterobacter cloacae. PLOS ONE. 2013; 8(9), 1–15.
CrossRef - Khalifa, A. Y. Z., Alsyeeh, A. M., Almalki, M. A., & Saleh, F. A. Characterization of the plant growth promoting bacterium, Enterobacter cloacae MSR1, isolated from roots of non-nodulating Medicago sativa. Saudi Journal of Biological Sciences. 2016; 23(1), 79–86.
CrossRef - Ji, C., Liu, Z., Hao, L., Song, X., Wang, C., & Liu, Y. Effects of Enterobacter cloacae HG-1 on the Nitrogen-Fixing Community Structure of Wheat Rhizosphere Soil and on Salt Tolerance. Frontiers in Plant Science. 2020;, 1–17.
CrossRef - Tarek M. Abdel-Ghany, a, b, and M. M. A. a, & High. com Molecular Identification of Rhizospheric Thermo- halotolerant Aspergillus terreus and its Correlation to Sustainable Agriculture. Bioresources. 2018; 13, 8012–8023.
CrossRef - Kumari Baby, Anand Kumar, and Mallick, M. A. Phosphate solubilizing microbes: an effective and alternative approach as biofertilizers. International Journal of Pharmacy and Pharmaceutical Sciences. 2016; 8(2), 37–40.
- Singh, M. Isolation and characterization of insoluble inorganic phosphate solubilizer rice rhizosphere strain Enterobacter cloacae Journal of Applied and Natural Science. 2018; 10(4), 1204–1209.
CrossRef - Elhamed, R. S. A., & Hassanein, W. A. Possibility of decreasing consumption of chemical fertilizers with using phosphorous and potassium solubilizing bacteria inoculation on fennel. Plant Archives. 2007; 20, 3159–3169.
- Zhang, C., & Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Applied Soil Ecology. 2014; 82, 18–25.
CrossRef - T Widowati, Nuriyanah, and H. S. Production of indole acetic acid by Enterobacter cloacae H3 isolated from Mungbean ( Vigna radiata ) and it’s potential supporting the growth of soybean seedling. IOP Conf. Series: Earth and Environmental Science. 2018; 308, 1–6.
CrossRef - Gupta, P., Trivedi, M., & Soni, H. Isolation, Identification and Evaluation of Indigenous Plant Growth Promoting Bacterium Klebsiella pneumoniae International Journal for Research in Applied Sciences and Biotechnology. 2021; 6(6), 47–56.
CrossRef - L.Jin, K.Zaho, Q. S. H. and P. S. Preparation, Structure and Biological Activities of Exopolysaccharides Produced by Enterobacter cloacae. Asian Journal of Chemistry. 2013; 25(16), 9101–9104.
CrossRef - Patel, P., Shah, R., and Modi, K. Isolation and characterization of plant growth promoting potential of Acinetobacter RSC7 isolated from Saccharum officinarum cultivar Co 671. Journal of Experimental Biology and Agricultural Sciences. 2017; 5(4), 483–491.
CrossRef - Naruya, M. Nei. The neighbor-joining method: A new method for reconstructing phylogenetic trees. 1987; http://test.scripts.psu.edu/nxm2/1987 Publications/1987-saitou-nei.pdf.
- Felsenstein, J. Confidence Limits on Phylogenies : An Approach Using the Bootstrap. Society for the Study of Evolution. 2014; 39(4), 783–791.
CrossRef - Tamura, K., Nei, M., and Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. PNAS. 2004; 101, 11030–11035.
CrossRef - Tamura, K., Stecher, G., & Kumar, S. MEGA11 : Molecular Evolutionary Genetics Analysis Version. 2021; 11.38(7), 3022–3027.
CrossRef - Sachdev, D. P., Chaudhari, H. G., Kasture, V. M., Dhavale, D. D., & Chopade, B. A. Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumoniae strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian Journal of Experimental Biology. 2009; 47(12), 993–1000.
- Bhardwaj, G., Shah, R., Joshi, B., & Patel, P. Klebsiella pneumoniae VRE36 as a PGPR isolated from Saccharum officinarum cultivar Co99004. Journal of Applied Biology and Biotechnology. 2017; 5(01), 047–052.
CrossRef - Nerdy, N., & Putra, E. D. L. Spectrophotometric method for determination of nitrite and nitrate Levels in broccoli and cauliflower with different fertilization treatment. Oriental Journal of Chemistry. 2018; Vol. 34, N, 2983–2991.
CrossRef - Ansari Mohammed Faisal. Evaluation and improvements of selected liquid biofertilizers available in Gujarat.Thesis (Issue December). Gujarat University. 2014.
- Gayal, S. and Khandeparkar, V . Production of Amylase by Bacillus subtillus. Indian Journal of Microbiology. 1979; 9:226-231.
- B. Prajapati and H.A.Modi. Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil. CIB Tech Journal of Microbiology. 2012; Vol. 1 (2-3), 8-14.
- Bric, J. M., Bostock, R. M., & Silverstonet, S. E. Rapid In situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Applied and Environmental Microbiology. 1991; 1, 535–538.
CrossRef - Sarker, A. Analytical Protocol for determination of Indole 3 acetic acid ( IAA ) production by Plant Growth Promoting Bacteria ( PGPB ). 2013; 3–5.
CrossRef - Gupta, P., Trivedi, M., & Soni, H. Screening for diverse biopolymer degrading microbes from vegetable market waste of kadi. Life Sciences Leaflets, 2020; 4297, 21–30.
- Verma, P., & Shahi, S. K. Isolation and Characterization of Bacterial Isolates from potato Rhizosphere as Potent Plant Growth Promoters. International Journal of Current Microbiology and Applied Sciences. 2015; 4(3), 521–528.
- Tortora, J. G., Funke, B. & Case, C. L. Microbiology: An Introduction, eleventh addition .2013; (Vol. 53, Issue 11). 2013; pp 567-572
- K. Deepa., Syed G. Dastager., Ashok Pandey. Isolation and characterization of plant growth promoting bacteria from non-rhizospheric soil and their effect on cowpea ( Vigna unguiculata ( L .) Walp .) seedling growth. World J Microbiol Biotechnol. 2010; 26:1233-1240.
CrossRef - Roslan, M. A. M., Zulkifli, N. N., Sobri, Z. M., Zuan, A. T. K., Cheak, S. C., and Rahman, N. A. A. Seed biopriming with P- A nd K-solubilizing Enterobacter hormaechei Improves the early vegetative growth and the P and K uptake of okra (Abelmoschu sesculentus) seedling. 2020; 1–38.
CrossRef - Abraham, J., and Silambarasan, S. Plant Growth Promoting Bacteria Enterobacter asburiae JAS5 and Enterobacter cloacae JAS7 in Mineralization of Endosulfan. Applied Biochemistry and Biotechnology. 2015; 175(7), 3336–3348.
CrossRef - Andriani, L. T., Aini, L. Q., and Hadiastono, T. Glyphosate biodegradation by plant growth promoting bacteria and their effect to paddy germination in glyphosate contaminated soil. Journal of Degraded and Mining Lands Management. 2017; 5(1), 995–1000.
CrossRef - Almutairi, M. H., and Helal, M. M. I. Exopolysaccharide production from isolated Enterobacter sp . strain ACD2 from the northwest of Saudi Arabia. Journal of King Saud University – Science. 2021; 33(2), 101318, 129-158
CrossRef - Moghannem, S. A. M., Farag, M. M. S., Shehab, A. M., and Azab, M. S. Media Optimization for exopolysaccharide Producing Klebsiella oxytoca KY498625 under varying cultural conditions. International Journal of Advanced Research in Biological Sciences. 2017; 4, 16–30.
CrossRef - Sun, S., Zhang, Z., Luo, Y., Zhong, W., Xiao, M., Yi, W., and Yu, L. Exopolysaccharide production by a genetically engineered Enterobacter cloaca strain for microbial enhanced oil recovery. Bioresource Technology. 2011; 102, 6153–6158.
CrossRef - Abd El-Latif Hesham, Tanvir Kaur, Rubee Devi, Divjot KourPrasad, S., Yadav, N., & Singh, C . Current Trends in Microbial Biotechnology for Agricultural Sustainability: Conclusion and Future Challenges. Springer Science and Business Media LLC. 2021; 555–572. https://doi.org/10.1007/978-981-15-6949-4
CrossRef - https://www.un.org/en/observances/world-population-day
- Paul, D., & Sinha, S. N. Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river. Annals of Agrarian Sciences.2017; 15(1), 130–136.
CrossRef - Chatepa, L., Resources, N., Masamba, K. G., & Resources, N. Proximate composition , physical characteristics and mineral content of fruit , pulp and seeds of Parinari curatellifolia ( Maula ) from Central Malawi. African Journal of Food Science.2018; 12(9) pp 238–245. https://doi.org/10.5897/AJFS2017.1662
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





