How to Cite | Publication History | PlumX Article Matrix
Alankar Shrivastav1* , Arun Kumar Mishra1
, Arun Kumar Mishra1 and Ashessh Kumar Gupta2
and Ashessh Kumar Gupta2
1Department of Pharmacology, Pharmacy Academy, IFTM University, Moradabad, India
2School of Pharmaceutical Sciences, IFTM University, Moradabad, India
Corresponding Author E-mail: alankar1994.ss@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3046
ABSTRACT: To prepare an herbal ointment using an ethanolic extract of Berberis aristata and assess the anti-psoriatic effectiveness of the finished product. We were able to make multiple ointments that were each categorized as F-1, F-2, and F-3 by adding varying concentrations of stearic acid and cetyl alcohol by learning about various formulation types, such as oil in water. The analysis of many factors, including pH, viscosity, spreadability, and stability, was used to evaluate all formulations. A formulation of ethanolic extract (F-3) showed antipsoriatic action. No evidence of a separate phase or ease of removal was found in any of the formations, but they all demonstrated good spreadability, consistency, appearance, and pH. Additionally, during irritancy trials, the formulation F-3 did not cause redness, oedema, erythema, or irritation. The use of this formulation on the skin is safe. As a result, the study implies that the extract's and the ointment's base's compositions are safer and more stable, however, they might also have synergistic effects.
KEYWORDS: Antipsoriatic; Berberis aristata; Ethanolic extract; Formulation; Ointment
Download this article as:| Copy the following to cite this article: Shrivastav A, Mishra A. K, Gupta A. K. Formulation and Evaluation of Herbal Ointment of Berberis aristata for Inhibition of Psoriasis-like Symptoms. Biosci Biotech Res Asia 2022;19(4). |
| Copy the following to cite this URL: Shrivastav A, Mishra A. K, Gupta A. K. Formulation and Evaluation of Herbal Ointment of Berberis aristata for Inhibition of Psoriasis-like Symptoms. Biosci Biotech Res Asia 2022;19(4). Available from: https://bit.ly/3MJeMbt |
Introduction
In some groups, psoriasis is infrequent or uncommon, probably because of genetic causes. In a survey at a teaching hospital in Nigeria, very few psoriatic patients were identified, and the condition is reportedly uncommon among Eskimos. Indians from North or South America hardly ever exhibit it. The prevalence of psoriasis is equal in males and females, according to the current consensus, despite numerous research in the past showing a varying sex incidence. The age of onset and mean age of affected males and females varied, according to Steinberg and colleagues; the clinical disease manifested in females at a younger age, and the mean age of statistically studied groups of females with psoriasis was three to four years lower than that of comparable males. Ointments, which are topical formulations, are more well-liked by patients since they have higher patient compliance (1). Ointment are the semisolid dosage preparations intended for topically infected diseases which carries water, waxes, hydrocarbons and volatile oils as a semisolid (2). Protection, antibacterial, emollient, antipruritic, keratolytic, and astringent characteristics are all uses for ointments (3). Plants were being used as medicine much before recorded history. According to a World health organisation (WHO) report, almost 25% of human prescription drugs are made from plants, while 80% of people still use the conventional medical system (4). The WHO has created guidelines to aid its members in creating national old-style medicine policies. WHO recognises the value of medicinal plants for the provision of public health services in developing countries (5). Herbalism has traditionally been practised outside of the realm of mainstream medicine, but it is growing in popularity as new studies and research demonstrate how successful they are at diagnosing, treating, and preventing disease (6). Because of the skin’s accessibility, size and exposure area medication delivery system through the skin has been considered a promising approach. The primary objective of this study was to formulate and evaluate anti-psoriatic by combining ethanol extract of Berberis aristata with additional components to provide a variety of skin advantages, including antibacterial and antiseptic properties.
Materials and Methods
Identification, collection and authentication of plant material
Plants of the Berberis aristata Linn. species were harvested in March 2021 from the Garhwal district of Uttarakhand. The root bark was cleaned and allowed to air dry. The scientist In-charge of the Botanical Survey of India, Allahabad (Central region), India, performed the authentication. The department of botany received an herbarium for the future reference (Accession No.: 104540).
Preparation of Extract from Root-barks
The fresh root barks of Berberis aristata was collected in March 2021, the root barks was washed and dried at RT (Room temperature), then converted into a coarse powder by the help of grinder and passes through a sieve number 18 to get the uniform size. To get rid of fat and other pigments, powdered root bark was defatted using petroleum ether (40–60%). Using a Soxhlet device, the defatted dry root barks were further extracted with ethyl acetate and subsequently with ethanol. The extracts were dried completely in a vacuum oven until all traces of ethanol were eliminated. In a refrigerator set between 2-8 °C, the extracts were stored (7).
Formulation of Herbal Ointment
The topical ointment was prepared by using an ethanolic extract of the Berberis aristata (EEBA). A combination of appropriate preservatives (Methylparaben and Propylparaben) were used to create the EEBA in concentrations of 1%, 2%, and 4%, and the ointment was kept in the refrigerator until further usage. Weighed and melted the required ingredient given in the table below. A sufficient amount (i.e. 1grm for 1%, 2 grm for 2% and 4 grm for 4%) of ethanolic extract of the root bark was added to this and thoroughly mixed to create a homogenous material (8). Table 1 represents the different polyherbal ointments’ components.
Table 1: Composition of Berberis aristata extract-based ointment
|
Ingredients |
F1 (g) |
F2 (g) |
F3 (g) |
|
Ethanolic Extract |
1.000 |
2.000 |
3.000 |
|
Stearic acid |
1.200 |
1.200 |
1.200 |
|
Triethanolamine |
0.165 |
0.165 |
0.165 |
|
Rose water |
0.400 |
0.400 |
0.400 |
|
Paraffin oil |
0.300 |
0.300 |
0.300 |
|
Moisturizer |
1.200 |
1.200 |
1.200 |
|
Cetyl alcohol |
0.100 |
0.100 |
0.100 |
|
Methylparaben |
0.018 |
0.018 |
0.018 |
|
Propylparaben |
0.002 |
0.002 |
0.002 |
|
EDTA |
0.010 |
0.010 |
0.010 |
|
Water |
q.s. |
q.s. |
q.s. |
 |
Figure 1: Simple Ointment Base. |
Physicochemical evaluation parameters
The following preliminary testing of formulations at various doses was done: (9).
Colour and aroma
Visual inspection was used to assess colour and smell or aroma.
pH
Using a digital pH metre, the pH of several formulations was determined. 100 millilitres of distilled water were used to dissolve one gramme of ointment, which was allowed to stand for two hours. Each formulation’s pH was measured triple times, and the average results are shown in the table.
Spreadability
Spreadability of the formulation was determined by an apparatus suggested by Multimer with some modifications. It consists of a wooden block having a pulley at one end with fixed glass slide on block. An excess of ointment (3 g) placed on ground plate. The ointment was sandwiched between this plate and another glass plate having the dimension of fixed ground plate and provided with the hook. A 1 kg weight was placed on the top of the two plates for 5 min to expel air and to provide a uniform film of the ointment between the plates. Excess of ointment was scrapped off from the edges. The top plate was then subjected to pull of 240 g. With the help of spring attached to the hook and time required by the top plate to cover a distance of 10 cm was noted.
Extrudability
A straightforward methodology was used to carry out this study. Collapsible tube was used to evaluate the extrudability of the formulated ointment. The formulated onement filled into the hopper and tubes were filled with ointment carefully. The extrudability of each ointment formulation was measured by the weight in grammes required to extrude an ointment ribbon of 0.5 cm in length in 10 seconds.
Consistency
The consistency of the formulations and the presence of coarse particles were used to evaluate the texture and homogeneity of the formulations. Immediate skin feel (including stiffness, grittiness, and greasiness) was also evaluated.
Diffusion study
The agar nutritional medium was made to conduct the diffusion investigation. The ointment was put inside a board with a hole in the middle of it. It was noticed and evaluate, how long it took the ointment to diffuse.
LOD
The LOD was calculated by putting the formulation in a petri dish and drying it at 105ºC.
Solubility
Solubility was determined by dissolving a required amounts of prepared ointments were tried to solubilizes in 10 ml of solvent such as water, boiling water, ether, alcohol etc.
Washability
For evaluating the washability 0.5 g ointment were applied to the skin and then after some time ointments were wash with running water and then the result was analyzed by manual inspection.
Non-irritancy Test
For evaluating the irritancy, 0.5g of formulated ointment was applied to the human skin and wait for some time, then the results were predicted.
Stability study
The developed ointment formulations were subjected to stability study as per the International Conference on Harmonization (ICH) guidelines. The formulated ointment was filled in the collapsible tubes and stored at different temperatures and humidity conditions, namely, 25°C±2°C /60%±5% Relative Humidity (RH), 30°C±2°C /65%±5% RH, and 40°C±2°C /75%±5% RH for a period of 1 months.
Evaluation of Antipsoriatic activity
Antipsoriatic Activity
Animal ethical work was approved by the Institutional Animal Ethical Committee (IAEC). CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) examined all experimental protocols and procedures utilized in this work. Wistar rats of either sex, weighing 180–220 g, were used for the experiment when they were 8–11 weeks old. Until the completion of the 23-day experiment, animals had unrestricted access to water and a conventional meal.
There were five groupings created from the animals. In each group, there was six rats. Using a delicate hair removal treatment, the backs of all the animals were carefully shaved. All animals, had their backs shaved after 24 hours and then specific amount of imiquimod cream applied using an applicator brush for 7 days in a row (10).
Group-I: Rats in the control group received normal feeding and a basic ointment base. Imiquimod 5% cream at a dose of 62.5 mg (equivalent to 3.125 mg API) was applied topically to the shaved backs of this group of animals for 7 days in a row to induce the condition. For seven days, rats were given acetate in their water to increase the skin inflammation caused by imiquimod (11).
Group-II: Rats in the standard control group received the same psoriasis treatment as Group I animals. Retino-A 0.025% was administered topically for the following 16 days starting on the eighth day (12)
Group-III: Similar to the animals in Group II, this group also had psoriasis induced. 1% w/w of a herbal preparation was applied topically for 16 days starting on the 8th day.
Group-IV: Similar to the animals in Group II, this group also had psoriasis induced. In-house herbal preparation 2% w/w was administered topically for 16 days starting on the 8th day.
Group-V: Similar to the animals in Group II, this group also had psoriasis induced. 4% w/w in-house herbal preparation was applied topically for 16 days starting on the 8th day.
A digital micrometre was used to measure the thickness of the skin every other day (SLB Works). Skin irritation was measured by the increase in skin thickness (13).
Scoring Severity of Inflammation
Based on the Psoriasis Area and Severity Index, scoring system was created to assess the degree of inflammation on the back skin. On a scale from 0 to 4, erythema, induration, and desquamation were rated separately: ‘0’ mean: none; ‘1’ mean: faint; ‘2’ mean: moderate; ‘3’ mean: marked; ‘4’ mean: very marked (14).
Histopathological Examination of Skin
10% formalin in saline was used to preserve the isolated skin in its containers. Animal skin was cut into longitudinal histological slices and stained with hematoxylin-eosin (15).
Effect of Imiquimod (IMQ) induced psoriasis in rats
Morphological discoveries Imiquimod treatment resulted in scales and redness on the skin of mice.
Results and Discussions
Physiochemical evaluation of formulated formulation
The emulsifying ointment was used as the substrate to create herbal ointments in the current investigation. The formulations’ physical characteristics were then assessed. These physicochemical characteristics were acceptable. The formulations appeared to be stable based on the stability analyses that were conducted. In tabular form, the maximum dosages of compounded ointments are displayed (Table No. 2)
Table 2: Physiochemical evaluation of formulated formulation
|
Physiochemical Parameters |
F1 (1% w/w) |
F2 (2% w/w) |
F3 (4% w/w) |
|
Colour |
Off Brown |
Light Brown |
Dark Brown |
|
Odour |
None |
None |
None |
|
Consistency |
Slightly dry |
Smooth |
Smooth |
|
pH |
7.02 |
6.88 |
6.70 |
|
Spreadability (Seconds) |
4.26 |
4.33 |
4.26 |
|
Extrudability |
3.60 g |
3.40 g |
3.65 g |
|
Diffusion study (after 60 min) |
0.7cm |
0.72cm |
0.65cm |
|
LOD |
30% |
30% |
28% |
|
Solubility |
Boiling water |
Boiling water |
Boiling water |
|
Washability |
Easy to wash |
Good |
Good |
|
Non-irritancy |
None |
None |
None |
|
Stability (2ºC, 25 ºC, 37 ºC) |
Stable |
Stable |
Stable |
 |
Figure 2: Different Concentrations of Ointments |
Psoriasis Area and Severity Index Assessment (PASI)
Throughout the trial, all rats’ morphological characteristics and health states, including food and water consumption, behavioral indicators, b.w., cardiovascular indicators and respiratory patterns, were normal. The dorsal skin was seen to have erythema, scaling, and thickening two to three days after the IMQ treatment began (Figure 3). After that, the severity of the ill rats’ psoriasis-like symptoms gradually worsened until 16th day or end of the experiment. The dorsal skin of rats in Group I treated daily with a ointment base, however, does not exhibit any symptoms of inflammation (Figure 3). Before starting treatment with a retinoid-A and extract-based ointment formulation, the independent PASI scores after IMQ application from days 1 to 7 showed a continuous rise in inflammation levels. The intensity of PASI scores peaked seven days after IMQ therapy, indicating that the mice treated with the drug successfully induced psoriasis-like dermatitis.
However, starting on day 9, which is equivalent to the second day of treatment, there was a statistically significant reduction in psoriasis-like symptoms. On the 2nd day following the start of the retino-A and F-1, F-2, and F-3 formulation treatment. Up until day 16, these symptoms continuously got better. Figure 3 shows the individual PASI scores and the total scores of all groups from days 1 to 16. The formulation treated groups shows a substantial inhibitory effect on IMQ-induced psoriasis like dermatitis as compared to the IMQ-treated group. Group IV (F-3 formulation) of rats showed the greatest improvement in the PASI inflammatory symptoms. The Retino-A treatment group’s significant decrease in PASI scores was equivalent to the 4% w/w EEBA treated group.
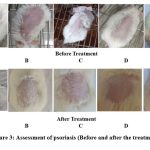 |
Figure 3: Assessment of psoriasis (Before and after the treatment). |
Histological Slides
The evaluation of several parameters, including inflammatory cells, neutrophils, etc., was made easier by the histology slides [Figure 4] (16). A degenerative change in skin tissue was seen in the skin section treated or induced with imiquimod. This alteration includes the thickness of the epidermis, increase the no. of keratinization, decline the portion of infection and orthokeratosis, how much extension of ridges, vessels dilation with microabcess and vacuolization, they all shows the severity of the skin tissue. The skin tissue cytoarchitecture of animals given retinol and 4% w/w EEBA ointment was normal, indicating degenerative changes.
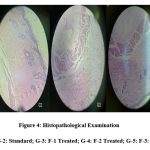 |
Figure 4: Histopathological Examination. |
Conclusion
Berberis has been utilised for its several therapeutic characteristics, including antibacterial, antifungal, and anti-inflammatory ones, since ancient times. As a result, this ointment may be used as a simple dose form to make effective use of these medicinal characteristics. The goal of the current study was to formulate and assess herbal ointment. To achieve a good yield of extract for this, the herbal extracts were made using a straightforward extraction technique using Soxhlet, with no harm to the chemical contents or their activity. The ointment was made using the fusion method, which ensured that the herbal extract and ointment base were mixed uniformly and remained stable throughout storage. The physicochemical properties have been investigated, and the findings for spreadability, extrudability, washability, solubility, loss on drying, and other features are satisfactory. Additionally, the formulation was left for four weeks to undergo stability testing at various temperatures, including 2, 25, and 37 degrees Celsius. In terms of spreading ability, diffusion research, or irritating effect, there were no alterations found. Beginning on day 9, there was a statistically significant reduction in psoriasis-like symptoms in the Antipsoriatic trial. The graph shows the individual PASI scores as well as the total scores for every group from days 1 through 16. The IMQ-induced psoriasis-like dermatitis was significantly inhibited when compared to the IMQ-treated group and the EEBA-treated groups. The 4% EEBA (F-3) treated group of rats showed a decrease in the PASI inflammatory signs. The Retino-A treatment group’s significant decrease in PASI scores was equivalent to the 4% w/w EEBA treated group. Berberis has become an effective antipsoriatic drug over the years (17). Berberis aristata has a huge antioxidant effect and is suitable for the treatment of various diseases, such as diabetes, diarrhea, and hormone disorders (18). However, the pro-apoptotic effect of this alkaloid is terrible. Berberis aristata contains alkaloids like berberine, berberine inhibits the proliferation and metastasis of a variety of psoriasis, such as leukemia, colorectal, prostate, lung, glioma and ovarian psoriasis (19), although there are certain obstacles that greatly affect the anti-infective properties of berberine. The main reason for this limited apoptotic effect is its poor absorption and limited biological activity. However, with the advancement of nanotechnology, these problems can be overcome (20).
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding sources.
Reference
- Rajasree, P. H., Vishwanad, V., Cherian, M., Eldhose, J., & Singh, R. (2012). Formulation and evaluation of antiseptic polyherbal ointment. International Journal of Pharmacy & Life Sciences, 3(10).
- Khandelwal, K. (2008). Practical pharmacognosy. Pragati Books Pvt. Ltd..
- Elsaied, E. H., Dawaba, H. M., Ibrahim, E. A., & Afouna, M. I. (2016). Investigation of proniosomes gel as a promising carrier for transdermal delivery of Glimepiride. Universal Journal of Pharmaceutical Research, 1(2), 1-10.
CrossRef - Verma, A. R., Vijayakumar, M., Mathela, C. S., & Rao, C. V. (2009). In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food and Chemical Toxicology, 47(9), 2196-2201.
CrossRef - Mishra, S. P., Singh, P., & Singh, S. (2011). Nutritional and medicinal value of Moringa oleifera leaves: Potential and Prospects. Forestry Bulletin, 11(1), 46-58.
- Duke, J. A. (1987). Moringaceae: Horseradish-tree, benzolive-tree, drumstick-tree, sohnja, moringa, murunga-kai, malunggay. Moringa: A multipurpose vegetable and tree that purifies water. Sci. & Technol./For., Environ., & Natural Resources Agro-Forestation Tech. Ser, 27, 19-28.
- Kolhe, S. S. (2018). Evaluation of polyherbal ointment for wound healing activity in Wistar rats. Journal of Drug Delivery and Therapeutics, 8(6-s), 26-31.
CrossRef - Chhetri, H. P., Yogol, N. S., Sherchan, J., Anupa, K. C., Mansoor, S., & Thapa, P. (2010). Formulation and evaluation of antimicrobial herbal ointment. Kathmandu university journal of science, engineering and technology, 6(1), 102-107.
CrossRef - Van Der Fits, L., Mourits, S., Voerman, J. S., Kant, M., Boon, L., Laman, J. D., Lubberts, E. Shim, S.K., Kim, H., Kwak, C.D. (2009). Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. The Journal of Immunology, 182(9), 5836-5845.
CrossRef - Muruganantham, N., Basavaraj, K. H., Dhanabal, S. P., Praveen, T. K., Shamasundar, N. M., & Rao, K. S. (2011). Screening of Caesalpinia bonduc leaves for antipsoriatic activity. Journal of ethnopharmacology, 133(2), 897-901.
CrossRef - Shrivastav, S., Sindhu, R., Kumar, S., & Kumar, P. (2009). Anti-psoriatic and phytochemical evaluation of Thespesia populnea bark extracts. Int J Pharm Pharm Sci, 1(1).
- Nadeem, A., Ahmad, S. F., Al-Harbi, N. O., El-Sherbeeny, A. M., Al-Harbi, M. M., & Almukhlafi, T. S. (2017). GPR43 activation enhances psoriasis-like inflammation through epidermal upregulation of IL-6 and dual oxidase 2 signaling in a murine model. Cellular Signalling, 33, 59-68.
CrossRef - Swindell, W. R., Michaels, K. A., Sutter, A. J., Diaconu, D., Fritz, Y., Xing, X., Liu, J., Xing, X., Theros, J., Ward, N. L. (2017). Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome medicine, 9(1), 1-21.
CrossRef - Nakaguma, H., Kambara, T., & Yamamoto, T. (1995). Rat ultraviolet ray B photodermatitis: an experimental model of psoriasis vulgaris. International journal of experimental pathology, 76(1), 65.
- Geetha, M. (2014). Anti-psoriatic activity of flavonoids from Cassia tora leaves using the rat ultraviolet B ray photodermatitis model. Revista Brasileira de Farmacognosia, 24, 322-329.
CrossRef - Loo, Y.S., Madheswaran, T., Rajendran, R., Bose, R.J. (2020). Encapsulation of berberine into liquid crystalline nanoparticles to enhance its solubility and anticancer activity in MCF7 human breast cancer cells. Journal of Drug Delivery Science and Technology, 57.
CrossRef - Zhong, L., Luo, N., Zhong, X., Xu, T., & Hao, P. (2022). The immunoregulatory effects of natural products on psoriasis via its action on Th17 cells versus regulatory T cells balance. International Immunopharmacology, 110, 109032.
CrossRef - Chaudhary, A., & Mittal, S. (2022). A study on ethosomes as mode for transdermal delivery of an antipsoriatic drug. Chinese journal of medical genetics, 31(3).
- Balkrishna, A., Sakat, S., Joshi, K., Singh, R., Verma, S., Nain, P., … & Varshney, A. (2022). Modulation of psoriatic-like skin inflammation by traditional Indian medicine Divya-Kayakalp-Vati and Oil through attenuation of pro-inflammatory cytokines. Journal of traditional and complementary medicine, 12(4), 335-344.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





