How to Cite | Publication History | PlumX Article Matrix
Production And Characterization Of Fructan From Acetobacter Species
Sherif M. A. S. Keshk
Biological and Physical Sciences Department, Institute of Environmental Studies and Research, Ain Shams University, Abassiya, Cairo, (Egypt)
ABSTRACT: In a screening program for new microbial producers of fructan. Acetobacter species have been isolated from Egyptian soil sample, which produce fructan, when cultured on sucrose containing medium. The structure of a water insoluble fructan produced by this species has been determined by means of various spectroscopic methods. Comparison of the 13C-spectrum of the extracted fructan and natural inulin shows that former has a close structural similarity with the natural inulin backbone (C-1-C-6). Moreover, the extracted inulin differs from plant inulin with respect to its high crystallinity and purity. Acetobacter species could be therefore added as new microbial source for inulin.
KEYWORDS: Acetobacter sp; crystallinity; 13C-NMR; Fructan; Inulin
Download this article as:| Copy the following to cite this article: Keshk S. M. A. S. Production And Characterization Of Fructan From Acetobacter Species. Biosci Biotechnol Res Asia 2003;1(1) |
| Copy the following to cite this URL: Keshk S. M. A. S. Production And Characterization Of Fructan From Acetobacter Species. Biosci Biotechnol Res Asia 2003;1(1). Available from: https://www.biotech-asia.org/?p=3366 |
Introduction
Fructans are polysaccharides consisting of a sucrose molecule that is elongated by a chain of fructosyl units connected through β -(2-1) or β -(2-6) linkages. Depending on the linkage type they are called inulin and levan, respectively (Carpita et al, 1989). In nature, fructans occur in bacteria, fungi and plants, serving different functions. Inulin is not simply one molecule but a polydisperse β -(2-1) fructan (Niness, 1999). The unique aspect of its structure refers to this β -(2-1) linkage rendering it a prebiotic fiber non-digestible by human intestinal enzymes and hence a non-metabolizable low caloric food additive (Jenkins, et al, 1999). It has been used successfully to replace fats in many foods, deserts, fillings and dressings without contributing deleterious organoleptic effects (Crittendent et al, 1996 and Coussement, 1997). Recent researches found no influence of inulin on serum glucose with no stimulation of insulin and/or glucagon secretion which recommends its suitability for consumption by diabetics (Beringer & Wenger, 1995). Moreover, inulin is a health-promoting fiber, it nourishes and stimulates the growth of useful intestinal lactobacilli and bifidobacteria (Gibson et. al., 1995, Bouhink et al, 1996 and Roberfroid et al, 1998). Which outcompete detrimental organisms and hence it prevents many intestinal diseases, stimulates immune system and aids the adsorption of certain ions (Butel et al, 1997 and Ohta et al, 1997). In fact it has been estimated that Americans
consume on average 1-4 g/day of inulin and oligofructose, while European’s average is 3-10g/ day (Vanhaastrecht et al, 1995). Therefore, it is logic to expect unquestionably the increasing demands for inulin in the forth coming few years due to its worldwide new medical and nutritional applications specially in prevention and/or protection against colon and breast cancers (Kim et al, 2001 and Reddy et al, 1997). Since most of inulin and oligofructose commercially available in the “industrial food ingredient market” today is either synthesized from sucrose or extracted from chicory roots, it is necessary to search for new durable inulin producers other than plants (Pazola & Cieslak, 1979 and Deleenheer & Hoebregs, 1994). Based on the idea that some bacterial species produce biopolymers such as cellulose by Acetobacter xylinum (Kai & Keshk, 1998, 1999 and Keshk, 2002) and levan by Bacillus or Streptococcus species (Marchel et al, 1980). This study aimed to screen new bacterial species for their capability to produce reliable and convenient yields of fructan inulin with much easier fermentation and least expensive extraction techniques.
Material and Methods
Organisms isolation and maintainance
Thirty-two soil samples from various governorates of Egypt were initially used as sources for isolation of many bacterial colonies on nutrient agar-glucose medium. A total of bacterial isolates of both Gram-positive and Gram-negative species were isolated in pure forms on slants of the same sterile medium and aseptically transferred regularly every two weeks for maintaince during the screening period.
Screening
All bacterial isolate were subjected to screening program by fermenting their colonies on nutrient broth medium (pH =7) for 12-16 days at 37°C under submerged conditions and agitated conditions (180 rpm). Their 250-ml cultures were centrifuged under cooling condition (4°C), and then the obtained fermentation broth was examined for existence of perceptible fructan inulin.
Strain identification
The organism under investigation was subjected to the well-accepted battery of investigations for its biological activities viz. hydrolysis of specific substrates and extracted fructanion of certain enzymes. The different physiologic and fermentation capabilities of the organism are summarized in Table I.
Cultivation
The isolated Acetobacter species (Figure 1) was submerged grown on sterile synthetic medium containing (g/l): Peptone, 10.0; beef extract, 3.0; sucrose, 7.5; yeast extract, 3.0 and sodium chloride, 5.0 for 14 day at 37°C.
Fructan extraction and purification.
Broth of the fermentation culture was centrifuged (6000 rpm at 4°C) for 20 min to remove cells. Acetone was added (1:2) to clear solution. The precipitate is washed by ethanol then petroleum ether. Then the pure precipitated is kept under vacuum till complete dryness.
Thin-layer chromatography
Fructose and extracted fructan were analyzed by TLC on silica gel F1500 (Schleicher and Schuell, Dassel, Germany). 2µL of both fructose and extracted fructan were spotted on the plates and developed in 87:13 acetone-water and stained with a fructose-specific urea phosphoric acid spray.
Methods for the Measurements
For structure identification and characterization of the extracted fructan from Acetobacter species. Microanalyses were performed in the Unit of Microanalyses (Central Laboratories, Cairo University, Cairo, Egypt). It’s optical rotation was measured with a JASCO DIP-
Table 1 : A Summary of the Morphological, Physiological and Biochemical
Properties of the Acetobacter Species.
Character Acetobacter species
| Cell-shape | Short rods |
| Gram reaction | – |
| Oxidase | – |
| Catalase | + |
| KOH test | + |
| Nitrate reduction | – |
| M.R. test | – |
| V-P test | – |
| Nitrite reduction | – |
| KCN test | + |
| H2S extracted fructanion | – |
| Motility | + |
| Amylase | – |
| Protease | – |
| Cellulase | ± |
| MacConkey agar | + |
| Citrate utilization | – |
| Spore strain | – |
| Fermentation of: | |
| Glucose | – |
| Galactose | – |
| Fructose | + |
| Mannose | + |
| Sucrose | + |
| Lactose | – |
| Maltose | – |
| Raffinose | – |
| Trehalose | ± |
| Rhamnose | – |
| Xylose | – |
| Mannitol | ± |
| Pectinase | – |
| Cellulase | ± |
| Lipase | – |
| Urease | + |
| Blood haemolysis | α |
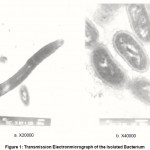 |
Figure 1: Transmission Electronmicrograph of the Isolated Bacterium |
1000 digital polarimeter. The UV spectrum was measured on PYE-Unicam SP-1800 spectrophotometer in hot water. IR spectrum was recorded on Horiba FT-210 infrared spectrometer. 1H-NMR spectrum was recorded on JMS 60011 instrument operated at 400 MHz and the solvent was DMSO-d6. 13C-NMR spectrum was recorded on JEOL α -400 (399.65 and 100.40 MHz) NMR spectrometers. Chemical shifts are given on the δ scale with TMS as internal standard.
Results and Discussion
The elemental analysis of the extracted fructan was % C 40.80, %H 5.7 with absence of both nitrogen and sulfur. From its empirical formula we have CH2O which deiced it is polysaccharides (fructan). The extracted fructan is a white powder; its melting point ranged between 224°C and 226°C, which indicates the good purity of the compound. It is insoluble in cold water yet in hot water and it forms a colloidal solution that does not form gel on cooling. Moreover, The extracted fructan does not give iodine test.
Spectroscopic studies of the extracted fructan were used in the conformation of its structure. From UV measurement, no peaks in the region 200-400 nm was observed, assignment that it has no double bond or aromatic groups. A weak absorption band at 894 cm-1 in IR spectrum of the extracted fructan is pointed to bending vibration of C1-H (axial), which is specific to β -linkage (Nakanishi, 1962). A strong band at 928 cm-1, which is corresponds to β – pyranose ring vibration (Nakanishi, 1962). The ether linkage (C-O-C anti-symmetric stretching) appears as weak band at 1132 cm-1, while a strong band at 1657 cm-1 was observed, due to the presence carbonyl group of β -keto structure (Barker et al., 1954). The stretching vibration of C-H (for aliphatic C-H) appeared at 2941 cm-1 and the stretching vibration of O-H appeared as broad band at 3439 cm-1. O-H frequency indicated that, the presence of strong hydrogen bond. On the other hand, the crystallinity index of the natural inulin and extracted fructan have been measured according to Conner et al. (1958). The bands at 1430 cm-1 and 900cm-1 assigned to crystalline and amorphous regions, respectively. So, the crystallinity index for natural inulin and extracted fructan are 1.26 and 0.97, respectively. Therefore, the crystallinity of extracted fructan is higher than natural inulin.
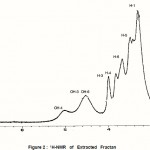 |
Figure 2 : 1H-NMR of Extracted Fractan |
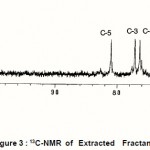 |
Figure 3 : 13C-NMR of Extracted Fractan |
The 1H and 13C NMR spectra of the extracted fructan dissolved in Me2SO-d6 are shown in Figures and 3 respectively. Comparison of the 13C-spectrum of the extracted fructan and natural inulin shows that they have a close structural similarity with the natural inulin backbone (C-1-C-6) (Baumgartner et al, 2000). The most significant difference between the extracted fructan and natural inulin is the relative intensity of the C-2 inulin backbone signal at 103.5 ppm and extracted fructan at 104.5 ppm (Baumgartner et al, 2000). This difference indicates a significantly lower degree of branching and high crystallinity index of extracted fructan as compared with natural inulin. This result confirmed by the high melting point of the extracted fructan rather than natural inulin. The significant difference in 13C NMR between leaven and inulin the chemical-shift of C-1 that appears at 60.2 and 3 respectively (Simms et al, 1990 and Baumgartner et al, 2000). In addition to these differences bacterial levan are more extensively branched and water soluble (Simms et al, 1990). However, comparison between the extracted fructan and inulin by the various relative signals positions and intensities indicates a close structural similarity evident between them. Moreover, 13C NMR chemical shift of extracted fructan of C-1 appear at 61.6 and it is water insoluble.
Conclusion
From these results, we can confirm the structure of extracted fructan as bacterial inulin. So, in addition to plants, bacteria such as Acetobacter species is able to produce inulin, which differs from plant inulin with respect to its high crystallinity and purity. Moreover, their rapid growth and ability to be maintained under controlled conditions, bacteria often offer unique possibility in the isolation of mutants for biochemical and genetic analysis.
Acknowledgement
The authors wish to thank Prof. Dr. M. Elkady, Chemistry Department and Prof. Dr. N. Salah, Biochemistry Department, Faculty of Science, Ain Shams University for their valuable criticism and constructive prepositions.
References
- Barker S, Bourne E, Whiffen D, eds. Methods of biochemical analysis. Interscience, 213- 245 (1956)
- Baumgartner S, Dax T, Praznik W, Falk H Characterization of the high molecular weight fructan isolated from garlic. Carbohydr.Res. 328; 177-183 (2000)
- Beringer A, Wenger R., Inulin in deren hrung des diabetikers. Verdauungs stofwechsel-krankh (In Germany), 15, 268-272 (1995)
- Bouhink Y, Flourie B, Ouarne F, Riottot M, Bisetti N, Bornet F, Rambaud J Effects of prolonged ingestion of fructooligo-saccharides on colonic bifidobacteria, fecal enzymes and bile acids in humans. Gastroenterology 106; 598-603 (1994)
- Butel M, Roland N, Hibert A, Tessedre A, Bensaada M, Rimbault A, Szylit O, Clostridial pathogenicity in experimental necrotising enterocolitis in Gnotobiotic quails andprotective role of bifidobacteria. J.Med. Microbiol., 47; 391-399 (1997)
- Carpita N, Kanabus J, Housley L., Linkage structure of fructans and fructan oligomers from Triticum aestivum and Festuca arundinacea leaves. Plant Physiol. 134; 162-68 (1989)
- Crittendent R, Planyne M., Production, properties and application of food grade Oligosaccharides. Trend Food Sci.Technol., 7; 357-362 (1996)
- Coussement P., Powerful products. World , 8; 12-17 (1997)
- Deleenheer L, Hoebregs H., Progress in the elucidation of the composition of chicory inulin. 46; 193-198 (1994)
- Gibson R, Beatty R, Wang X., Cummings H Selective stimulation of bifidobacterria in the human colon by oligofructose and inulin. Gastroenterology, 108; 975-982 (1995)
- Jenkins D., Kendall C., Vuksan V., Inulin, oligofructose and intestinal function. Nutr. 129; 1431-1433 (1999)
- Kai A, Keshk S Structure of nascent microbial cellulose I. J. 30; 996-1002 (1998)
- Kai A, Keshk S., Structure of nascent microbial cellulose
- Kim Y, Faqih M, Wang S., Factors affecting gel formation of inulin. Carbohydr. Poly. 46; 135-145 (2001)
- Marshall K., Weigel H., Extracellular β -D-fructofuranosidase elaborated by Streptococcus salivarius strain 51: Prepartion and mode of action on levan and on homologues of inulobiose. Carbohydr. Res., 83; 315-320 (1980)
- Nada A, Shabaka A, Yousef M, Nour A., Infrared spectroscopies and dielectric studies of swollen cellulose. Appl. Polym. Sci., 40; 731-739 (1990)
- Nakanishi K., Infrared absorption spectroscopy. In: Djerassi, C, ed. Nankodo company limited,Tokyo, pp. 151-155 (1962)
- Niness K., Inulin and Oligofructose. What Are They. J.Nutr., 129; 1402-1406 (1999
- Ohta A, Baba S, Ohtsuki M, Takizawa T, Adachi T, Hara H., In vivo absorption of calcium carbonate and magnesium oxide from the large intestine in rats. J.Nutr.Sci. Vitaminol.43; 35-46 (1997)
- Pazola Z, Cieslak J., Changes in carbohydrates during the production of coffee substitute extracts especially in the roasting processes. Food Chem., 4; 41-46 (1979)
- Reddy D, Hamid R, Rao V., Effect off dietary oligofructose and inulin on colonic preneoplastic aberrant crypt foci inhibition. Carcinogenesis 18; 1371-1374 (1997)
- Roberfroid M, Van J, Gibson G., The bifidogenic nature of chicory inulin and its hydrolysis products. J.Nutr. 128; 11-19 (1998)
- Simms P, Boyko W, Edwards J., The structural analysis of a levan produced by Streptococcus salivarius ss2. Carbohydr. Res. 208; 193-198 (1990)
- VanHaastrecht J., Promising performers; Oligosaccharides present new product development opportunities for a wide range of processed foods. Food Ingredients, 1; 23-27 (1995)

This work is licensed under a Creative Commons Attribution 4.0 International License.





