How to Cite | Publication History | PlumX Article Matrix
Sulfonamides : A Novel Approach For Antimicrobial Chemotherapy
N. P. Shukla
N. P. Shukla Biotechnology Application Centre, M. P. Council of Science and Technology, Bhopal - 462 003 (India)
ABSTRACT: The discovery of the sulphonamides marked the beginning of the chemotherapeutic era making possible a direct attack on microbial infections. Sulfonamides antibacterials, now in their fifth decade, continue to be used because they are effective, inexpensive and free of the superinfection problem of the broad spectrum abntibiotics. They have been quite safe even when widely used in ambututory patients, an application in which they are often preferred. After 40 years most of the original problems of toxicity have been taken care of and during the last three decades the sulfonamides have withstood critical evaluation along side the antibiotics, which have problem of their own. During this period active compounds with a vide range of choice in all the pertinent pharmacological properties and with lower toxicity have been synthesized and a wide spectrum of activity has been established. Thus, the activity of compounds acting as p-aminobenzoic acid antagonists extends from use against acute and chronic Gram negative Gram positive bacterial infections (Such as meningitis, intestinal tract infection or preoperative treatment of urinary tract infections and respiratory infections) through tuberculosis and leprosy to malaria and coccidiosis with certain structural specificities for each1-4,6,9,13,34,45,68,85,89,91. The mechanically related diamondiphenylfulfonae is the well established drug of choice. The antibacterial action of these agents is by way of interference with a bacterial biochemical reaction lacking in man and represents the first magic bullet the sense visualized by Ehrlich.
KEYWORDS: Sulfonamides; chemotherapy; antimicrobial activity
Download this article as:| Copy the following to cite this article: Shukla N. P. Sulfonamides : A Novel Approach For Antimicrobial Chemotherapy. Biosci Biotechnol Res Asia 2003;1(1) |
| Copy the following to cite this URL: Shukla N. P. Sulfonamides : A Novel Approach For Antimicrobial Chemotherapy. Biosci Biotechnol Res Asia 2003;1(1). Available from: https://www.biotech-asia.org/?p=3378 |
Introduction
A major advance in sulfonamides therapy came with the proper appreciation of the role of pharmacokinetic studies in determining the dosage schedule of these drugs. The era of newer long acting sulfonamides started in 1956 with the introduction of sulfamethoxypyridazine having a half life of 37 hours which had to be administered only once a day12,16,17,56,86.
A number of new sulfonamides have been described recently. An isomer of sulphadi-methoxine. RO 4-4394 (4-sulphanilamido-5,6-dimethoxypirimidine) is characterized by an extremely long persistence inthe blood and other body fluids20,21,46. Its activity against Gram positive and Gram negative organisms in comparable to that of sulphadiazine and sulphmethoxydiazine. A remarkably constant effect of a single oral dose in infections with both Gram positive and Gram negative bacteria is reported23,25,30,31,36,54. Barbotin and Nguyen Trung-Luong successfully treated meningococcal meningitis with a single intramuscular injection of the drug. RO 4-4393 seems to exert remarkable action in the treatment of trachoma. Milano19 obtained a cure rate of 83%. Barclay and his associated8 obtained favourable results in leprosy patients within a relatively short period of treatment with RO 4-4393. Similarly, number of investigators reported the therapeutic value of this sulfonamide against various types of
leprosy24,27,39,41-44.
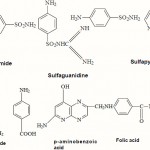 |
Scheme 1 |
The sulfonamides also succeeded in greatly reducing the number of deaths due to epidemic meningitis, thus in the city of Hamburg, mortality dropped from 43% in 1936 to 12% in 194115. Similarly, it became possible for the first time to employ casual therapy of bacillary dysentery and numberous other infectious diseases81.
Since, long back Dapsone (DDS) is the drug of choice for the prophylaxis and treatment of human leprosy17,18,50,51,58,59. Shephard75 has shown that acedapsone (DADDS, 4-4diacetylamide diphenyl sulphone), a repsoitory sulphone was effective in preventing growth of Mycobacterium leprae in mouse foot pad. Its value as an antileprosy drug has been demonstrated in Philippines76,77. New Guinea69,70 and Micronesia71,72. Administration of the drug to leprosy patients in dose of 225 mg (1.5 ml) deep in at intervals of eleven weeks, produce release of DDS at a steady rate after the second injection53,79. During regular treatment with acadapsons, plasma levels of DDS is maintained well above the MIC of DDS for M. leprae35. Recently, a controlled study on chemorprophylaxis against leprosy with acadepsone was carried out by the central leprosy teaching and Research Institute, South India between 1976 and 1980. This study establish the fact that the chemoprophylactic and therapeutic value of acedepsone is more than that of dapsone52,60,61,63,64.
 |
Scheme 2 |
Nomenclature & Classification
The general term “Sulfonamides” have been used for derivatives of p-aminobenzene sulfonamide (sulfanilamide), whereas specific compounds are described as N¹ and N4 substituted sulfanilamides depending on whether the substitution is on the amino or aromatic amino group, respectively. Most of the sulfonamides used currently are N¹ derivatives. The generic name of a sulfonamide is built up by adding the prefix “sulfa-” to an abbreviated form of the chemical name of the N’-residue. This is done in two ways, either the amid nitrogen is taken as a part of the “sulfa” residue (e.g. Sulfapyridine) or the amid nitrogen is taken as a part of the N¹-residue as in sulfaguanidine. The length of time that a sulfonamide remains in the body is normally expressed in terms of its half life i.e. the time required for half of the amount of drug introduced to be eliminated from the organism. There have been numerous attempts to classify the sulfonamides15,29,82. One based on absorption and half life appears to be the most logical and clinically relevant. Sulfonamide that have a half life of less than 10 hr are termed short acting between 10 and 24 hr are considered to be medium acting and longer than 24 hr are long acting.
Mode of action
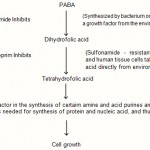
The mode of action of the sulfonamides is characterized by a competitive antagonism of certain vital factors in the metabolism of many bacteria. This antagonism, namely between p-aminobenzoic acid (PABA) and p-aminobenzene sulfonamide, was first mentioned in 1940 by Woods and Fildes. With regard to this antagonistic mode of action, a distinction can be drawn between the two classes of bacteria81
This class accounts for the great majority of bacteria that require PABA as a nutrient or that can themselves synthesize PABA from the nutrient medium. The bacterium is so say, deceived by the structural similarity between the sulfonamide and PABA as a result of the latter is incorporated into the cell of the bacterium but does not play its expected part in the metabolism of the cell. Until 1946, no nutrient substance was known that contained PABA, it was only with the discovery of the vitamin, folic acid by American workers and with the identification of its structure that the first useful information on PABA metabolism became available.
Bacteria that can neither synthesize PABA nor utilize it but must rely on a supply of folic acid. This group includes for example, enterococci, which like the cells of the human organism, must rely on a supply of vitamins of the folic acid group and are accordingly unaffected by sulfonamides19,65-67,95.
More specifically the action of the sulfonamides is generally understood by the following:
The sulfonamides permeate is non-ionized from into the bacterial cell. Once dissociation equilibrium has been established, the ionized form of the sulfonamide antagonizes the biosynthesis of folic acid which is necessary as a coenzyme for the formation of purines such as adenine and guanine, the pyrimidine base thymine, and also the amino acids methionine, serine and histidine. The sulfonamide ions inhibit the enzyme that condenses PABA with dihydropteridine. This inhibition has the practical effect that the dihydrofolic acid synthesis does not take place. Dihydrofolic acid is a preliminary stage in the formation of a coenzyme. Under normal conditions the penultimate stage in the formation of this coenzyme is the reduction of dihydrofolic acid to tetrahydrofolic acid. This reduction is accomplished with the aid of the enzyme dihydrofolic acid reductase37,74,89.
The last stage in the formation of the complete coenzmye consists in the condensation of the latter with one carbon compound to give one of the carbon tetrahydrofolic acids. The function of the holoenzyme consists in a one-carbon transferase action. The enzyme, in other words cause the incorporation of a single carbon atom in the substrates i.e. uracil, serine etc26,34.
The lack of transferase activity inhibits the synthesis of thymine from uracil which is an essential constituent of DNA. This inhibition of DNA synthesis, finally explains the action of the sulfonamides. Since no DNA synthesis takes place in the quiescent stage of the bacteria, the sulfonamides have no effect during this stage even when used in very high doses. The sulfonamides exert their bacteriostatic action during the multiplication stage after a given “log period”. This log period is dependenent on the quantity of stored PABA73,80,81.
If sulfonamides are compared with other antibacterial substances, e.g. penicillin, an important difference becomes apparent, whereasthe bactericidal effect of the antibiotic depends primarily on high concentrations rather than on the contact time, the opposite is true of the bacteriostatic concentrations for a period of time sufficient for the normal defence mechanism of the organism to become active against the bacteria38
Synergism and Combination
Examination of mixtures in vitro suggests that sulfonamides and bacteriostatic drugs have an additive effect and that sulfonamides and bactericidal antibiotics have an antagonistic or a supra-additive effect on bacteria. Thus, it can be said that combinations of sulfonamides and certain antibiotics and other anti-infective drugs have a wide antibacterial spectrum and that such mixtures might be more effective than either constituent alone. One well authenticated case of an additive effect is the combined treatment of H. influenzae meningitis with a mixture of sulphadizine and chloramphenical. Polymyxin acts synergically with sulfonamides. A considerable amount of work has been carried out to potentiate the sulfanilamides, bacteriostasis by using them in combination with a compound that blocks a later step in the sequence of folic coenzyme syntheses. Sequential blocking has been achieved with “antibiotics” which have a
4 diamino 1,3-diazo structure and a bulky aromatic group at the 5 position. Such compounds are trimethoprim and the earlier pyrimethamine (2,4-diamine-4-ethyl-5-p-chlorophenyl pyrimidine) and 2,4-diamino-5-diamethey,6-p-chlorophenyl-5, 6-dihydro-5-triazine.
In vitro tests show that the bacteriostatic drugs, trimethoprim and sulphamethoxazole combination (1:5) acts synergistically to produce a bactericidal effect at most levels achieved in clinical therapy. Both components exert their antimicrobial action by blocking folate metabolism. Trimethorpim inhibits the enzyme dihydrofolate reductase and thereby prevents the reduction of dihydrolate to tetrahydrofolate. This leads to an inhibition of the synthesis of bacterial DNA. Sulphamethoxazole acts as a typical sulfonamide and inhibits the process whereby bacteria from dihydrofolate from para-amino benzoate and from pteroate. Both of these bacterial actions have a high therapeutic index relative to folate metabolism in man. The combination is active against a broad spectrum of infecting organisms which include Streptococci, Staphylococci (including those resistant to penicillin), Salmonellae, Shigellae, Coliform bacteria. Neisseriae some strans of Proteus and of Brucella, Diplococcus pneumoniae, H. influenzae and Klebsiella pneumonaie.
Drug Resistance
The development of resistance is considered to arise because of over production of PABA88. Altered permeability of the organisms to sulfonamides62, or altered sensitivity to H2-pteroate synthetase enzyme from resistant cells (which can bind PABA more tightly and sulfonamide less tightly than the corresponding enzyme from the sensitive cells) are also possible cause of resistance96,92,93. Difference sulfonamides show cross resistance, but there is no cross resistance to other antibacterial. It has been found that sulfonamide resistant strains are also resistant to pyrimethamine, that presumably can utilize the reduced forms of folic acid available in the host erythrocytes10,11.
It was noticed that multiple drug resistance involving Streptomycin, Chloramphenicol, Tetracycline and Sulfonamides could be transferred between Shigella and E. coli in mixed cultivation. Now this fact is established that the transfer of R-factors is carried out through conjugation by plasmids this transfer is noticed in both conditions i.e. in vitro as well as in the alimentary tract. Drug resistance acquired in this manner can be transferred to other sensitive strains indefinitely. It has been shown that in E. coli R-plamid transmitted resistance is the most common mechanism of sulfonamide resistance51,83,90,91.
Toxicity and side effects
Sulfonamides should be administered with caution to pregnant woman near term, and to premature and new born infants since detoxifying mechanisms do not develop fully until later in infancy. The sulfonamides carry the risk of numerous toxic effects, which are particularly severe in the case of the long acting drugs. The problem of crytalluria with the possible consequence of hematuria and oliguria, was common with the older preparations and is still a risk with sulfadiazine and sulfamethoxazole. Precautions should be taken to avoid crystalluria, particularly in dehydrated patients, concomitant alkalinization of the urine is advisable22,96. Sulfonamides should be used with great care or avoided in patients with impaired renal functions. Acute haemolytic anameia is a rare but serious complication sometimes occur early in treatment. it may occur in subjects with a glucose-6-phosphate dehydrogenase deficiency. the foetus and premature infants normally have low levels of this enzyme. A secondary anaemia accompanied by fatigue and mental depression often follows the administration of sulfonamides over a long period.
References
- Akimov, A. A. and Filov, V.A., Eksperiment Onkolog, 16(2-3), 102-108 (1994)
- Albala, D.M., Prein, E.L. and Galal, A.H., J Endurolog, 8(6), 401-403 (1994)
- Almasi, I., Howarth, E. and Ternak, G., Orvasi Hetilap, 136(5), 239-243 (1995)
- Amara, A., Zaini, Z. and Bouzoubaa, K., Veterinary Microbiol., 43(4), (1995)
- Anderson, E.S., In Ecology and Epidemology of transferable Drug Resistance Bacterial Episomes and Plasmids (Ciba Foundation Symposium, Churchhill, London) 102 (1969)
- Atawodi, S.E., Mendi P., Pfundestein, B., Preussmann, R. and Spiegel-Halder, B., Food and Chem. Toxicol., 33(8), 625-63028 (1995)
- Barbotin, M. and Nguyen Trung-Luong, Press Med., 2923 (1964)
- Barclay, C.A., Willanson, F.F. and Faciano Leprologia (Buenos), 8, 71 (1963)
- Baure, A.W., Chemotherapia, 10, 152 (1966)
- Bishop, A., Biol. Rev., 34, 445 (1959)
- Bishop, A., In Drug Parasite and Hisb. (Little Brown, Boston), 98 (1962)
- Bleganowska, M.L., Droczyns, A. and Petruczynek, A., J. Planar Chromato 122-128 (1995)
- Brezjanin, R. and Mikov, M., Abst. 5th Int Cong. Chemothep., Vienna, 881 (1967)
- Brown, G.M., J. Biol. Chem., 237, 536 (1962)
- Bunger, P., Hamburger Arztablatt., 18, 349 (1964)
- Burri, K., Breu, F.V., Cassal, J., Clozel, M., Fischli, W., Graw, G.A., Hirth, G., LoefflersB., Muller, M., Neidhart, W., Ramuz, H. and Trzeciak, A., Europe J. Med. Chem., 30, 385 389 (1994)
- Chegwidden, W.R. and Spencer, I.M., Inflammopharmacol., 3(3), 231-239 (1995)
- Cheng, Y. amd Minjun, C., Antimicrobial Agents and Chemotherapy, 38(12), 2838-2842 (1994)
- Davis, B.D., In Principia of Chemotherapy (Lippincot Camp., Philadelphia), (1965)
- Fayer, R., J. Parasitol., 78(3), 534-537 (1992)
- Fermer, C., Kristian, B.E., Scold, O. and Swedberg G., J. Bacteril., 177(16), 4669- 1695 (1995)
- Finegold, S.M. and Ziment, I., In Antimicrobial Therapy (Savnders, W.B., Comp. Philadelphia), 102 (1994)
- Fogg, A.G., Rahim, A., Yusoff, H.M., Moreira, J.C. and Zaho R., Analyt. Proceed., 32(3) 95-97 (1994)
- Gaind, M.L. and Soli, A.S., U.S. Armed Forces Med. J., 20, 12 (1964)
- Garcua, A., Coque, M.C., SImoalfonso, E., Ramus, G. and Esteve-Romero, J.S., J. Pharmaceut. Biomed. Analyt., 13(3), 237-245 89 (1995)
- Garrod, C.P. and Waterworth, P.M., 5th Intern. Cong. Chemother., Vienna (Verlag Wien Med. Acad.) 747 (1967)
- Ghosh, S. and Chakraborty B.K., Preliminary report Bull. Calcutta Sch. Trop. Med., 28 (1963)
- Gordon, G.R., Murray J.C.Jr., Peters, J.H.,Russel, D.A. and Vincin, D.R., Inter. J. Lepr.,42, 125 (1974)
- G Sell, O., Arstl. Prax., 18, 651 (1966)
- Heinze, W. and Wachtel, J., Monasch Veterina Ermed., 47(5), 279-283 (1992)
- Hirai, H., Ikeuchi, Y. and Okada Y., Neuroscience Letters, 182, 33-36 32.
- Hitchings, G.H. and Burchall, J.J., Adv. Enzymol., 27, 417 (1965)
- Ho, R. and Cormen, L., Antimicrob. Agent Chem., 5, 388 (1974)
- Hoechne, C. and Patsch, R., Zbl. Bacteriol,Parasitenk. Infection Skr., 202-220 (1967)
- Hotchkiss, R.D. and Evans, A.H., Fed. Proc., 19, 912 (1960) 36. Hugo, P.G. and Okonkwo, J.O., J. Infec.
Control., 20(3), 126-130 - Huligol, S.C. and Black M.M., Clinic Experimental Dermatol., 20(3), 189-220 (1995)
- Isliker, H., Sch. Weiz. Med. Wschr., 38, 127(1978)
- Izawa, T. and Komabayashi, T., J. Appl.phycol., 77(4), 2618-2624 (1994)
- Knzett, H., 5th Intern. Cong. Chemother., Vienna (Verlag Wein Med. Akad), 225 (1967)
- Lanctot, K.L., Ghajar, B.M., Shear N.M., and Naranjo, C.A., J. Clinic. Pharmacol., 34(12), 1228-1233 (1994)
- Laungillon, J. and Clary, J., Presse. Med., 72, 2804 (1964)
- Laungillon, J., Med. Trop., 24, 522 (1964)
- Lopes, C.F. and Deniz, O., Abst. 3rd Intern. Cong. Leprology, Rio-dejaneiro, 12, 33 (1964)
- Lyght, C.E., Keefer, C.S., RIchards, D.W. and Sebrell, W.H., The Merck Manual Ed., 10, (Merck, Rahway, N.J.) (1961)
- Madsen, S.T., Abst. 3rd Inter. Cong.Chemotherapy, Stuttgar, 86, (1963)
- Margileth, A.M., Pradiatr. Infec. Dis. J., 11(6) 474-478 (1992)
- Meekins, C.V., Sullivan, T.J. and Gruchalla, A.H., J. Endurolog., 8(6) 401-403 (1994)
- Milano, C., Bull. Oculist, 43, 499 (1964)
- Nakanishi, K., Nadai, T., Masada, M. and Miyajima, K., Chem. Pharmacol. Bull., (Tokyo), 40(5), 1252-1256 (1992)
- Naqui, S.H., Bhutta Z.A. and Farooqui, B.J.,
- Scand. J. Infec. Dis., 24(2), 175-179 (1992)
- Neelan, P.N., Noorden, S.K. and Shivprasad, N., Indian J. Med. Res., 78, 307 (1983)
- Neglec, T., Hajsig, D., Halavaty, H., Gmajniki,B., Bilick, V. and Vakalovic, M., Alpe Adra Microbiol. J., 4(1), 45-56 (1995)
- Neipp, L., In Experimental Chemotherapy Vol.IV (Academic Press, New York), 515 (1966)
- Nichols, R.L., Jones, W.F. and Finland, M.,Proc. Soc. Exp. Biol. Med., 92, 637 (1956)
- Nishimura, H. and Nakajima, K., Ann. Rept.Suonogi Res. Lab., 13, 19 (1963)
- Nitya Anand, In Burger’s Medicinal Chemistry, 4th Ed. Part II (John Wiley and Sons, New York), 1 (1979)
- Noordeen, S.K. and Neelan, P.N., Lepr. India,48, 635 (1976)59. Noordeen, S.K. and Neelan, P.N., Indian J.
Med. Res., 67, 515 (1978) 60. Opermicale, E.M., Scales, D.K. and Sharpe, M.R., Opthalmol., 99(6), 920-925 (1992) - Pandey, P., Bhattacharya, S. and Dave A., J.Liq. Chromatogr., 15(10), 1665-1671 (1992)
- Pato, M.C. and Brown, G.M., Arch. Biochem.Biophys., 103, 443 (1963)
- Patoux, P. Hirel, M.B. Chesne, C. Waterex,E., Breton, J.C. and Guillouzo, A., Toxicol.,9(4), 493-497 (1995)
- Paul, J. Bargrie, C. and Parums, D.V., J. Chin.Pathol., (Lond.), 45(6) 528-530 (1992)
- Pingarroh, C.J.L., Dominguez, R.A. and PoloDiez, L.M., Talanta, 39(6), 631-635 (1992)
- Punchard, N.A., Diane, J.B., Greenfield, S.M. and Thompson, R.P.J., Biochem. Pharmacol.,
43(11), 2369-2376 (1992) - Riley, R.J. and Leeder, J.S., ClinicalExperiment. Immunol., 99 1-6 (1995)
- Roedy, J., Can. Med. Assoc. J., 92, 1125(1965)
- Russel, D.A., Shepard, C.C., McRae, D.H.,Scoh, G.C. and Vincin, D.R., Ann. J. Trop. Med.,Hyg., 20, 495 (1971)
- Russel, D.A., Worth, R.M., Scott, G.C., Vincin,D.R., Jano, B., Fasal, P. and Shepard, C.C.,Intern. J. Lepr., 44, 170 (1976)
- Russel, D.A., Worth, R.M., Jano, B., Fasal, P.and Shepard, C.C., Abstr. Xi Intern. Lepr.Cong., Mexico, Intern. J. Lepr., 47, 329 (1979)
- Russel, D.A., Worth, R.M., Jano, B., Fasal, P.and Shepard, C.C., Ann. J. Trop. Med. Hyg.,28, 559 (1979)
- Scholtan, W., 5th Intern. Cong. Chemo.,Vienna (Verlag Wien Med. Akad.), 223 (1967)
- Sharma, V.D., Dixit, V.P. and Joshi, R.K., J.Food. Sci. Technol., 32(3) 221-223 (1967)
- Shepard, C.C., Proc. Soc. Exp. Biol. Med.,48, 635 (1967)
- Shepard, C., Tolentino, J.G. and McRae, D.H.,Ann. J. Trop. Med. Hyg., 17, 192 (1968)
- Shepard, C.C., Levy, L. and Fasal, F., Ann. J.Trop. Med. Hyg., 21, 440 (1992)
- Shepard, R.G., In Med. Chem., 3rd Ed. (Wiley Inter-Science, New York) 255-304 (1977)
- Sloan, N.R., Worth, R.M., Jano B., Fasal, P.and Shepard, C.C., Intern. J. Lepr. 40, 40
(1972) - Spillane, W.J., Morini, G. and Birch, Food.Chem., 47(5), 337-342 (1992)
- Strullr, T., In Progress in Drug Research, 12,(E. Jucker Basal Ed.) 389 (1968)
- Walter, A.M. and Heilmeyer, L., In AntibiotikaFible (Thieme Stottgart), (1965)
- Watambe, T., Bacteriol. Rev., 27, 87 (1963)
- Watambe, T., and Futkasawa, T., J. Bacteriol.,82, 202 (1961)
- Weinstein, L., Medoff, M.A. and Sumet, C.M.,New Engl. J. Med., 263, 793 (1960)
- Weinstein, L., In Pharmacological basis of therapeutics, L.S. Goodman and A. Gilman Eds, (Macmilan, New York) 1144-1171
- Weisman, R. and Brown, G.M., J. Biol. Chem.,239, 326 (1964)
- White, P.J. and Woods, D.D., J. Gen.Microbiol., 40, 243 (1963)
- Williams, J.D. and Leigh, D.A., Brit. J. Clin.Prac., 20, 177 (1966)
- Wise, E.M. and Abon-Donia, M.M., Proc. Nat. Acad. Sci., USA, 72, 2621 (1975)
- Witzgall, H., Theraplewoche, 15, 977 (1965)
- Wokenstein, P., Dominique, C., Laurent, P.,Revuz, J., Claude-Roujeau, J. and Bagot,M., Arch. Dermatol., 131(5), 544-551 (1995)
- Wolf, B. and Hotchkiss, R.D., Biochemistry,2, 145 (1963)
- Wolkenstien, P. Carriere, V., Chaure, D. Bastuji, S.G., Revuz, J. Roujeau, J.C., Beaune, P. and Bagot, M.,
Pharmacogenetics, 5(4), 255-258 (1995) - Woods D.D., J.Gen.Microbiol., 29, 687 (1962)
- Yu, C.J. and Zhu, L., Chin. J. Phar., 22(4),
173-174 (1991)

This work is licensed under a Creative Commons Attribution 4.0 International License.





