How to Cite | Publication History | PlumX Article Matrix
Bacteriological Analysis Of "Ready - To - Eat" Foods Obtained In Akure Markets
A. O. Ojokoh1 and B. A. Ojokoh2
1Department of Microbiolgoy, Federal University of Technology Akure (Nigeria). 2Department of Industrial Maths and Computer Science, Federal University of Technology, Akure (Nigeria)
ABSTRACT: Bacteriological analysis of some "ready-to-eat" food sample collected from Akure makets were carried out to determine the bacteria load. Analysis of the food samples steamed-bean pudding(moinmoin), fried bean paste (akare),cooked white rice, vegetable stew and cooked eko or pap which were cold were contaminated with some bacteria species. Akara, rice and eko had higher average total aerobic bacteria counts of 3.1x109 cfu/g; 3.0X109 cuf/g and 2.9x109 cfu/g, respectively. The average bacteria counts for stew
KEYWORDS: Bacteriological analysis; Akure; Health
Download this article as:| Copy the following to cite this article: A.O. Ojokoh A. O, Ojokoh B. A. Bacteriological Analysis Of "Ready - To - Eat" Foods Obtained In Akure Markets. Biosci Biotechnol Res Asia 2003;2(1) |
| Copy the following to cite this URL: A.O. Ojokoh A. O, Ojokoh B. A. Bacteriological Analysis Of "Ready - To - Eat" Foods Obtained In Akure Markets. Biosci Biotechnol Res Asia 2003;2(1). Available from: https://www.biotech-asia.org/?p=3407 |
Introduction
The food eaten has a direct influence on health, it is therefore an important task for food inspectors, food manufactures and food handlers to keep food safe from pathogenic microorganisms, especially when such food are to be consumed without further processing, that is “ready-to-eat” foods or fast foods (Bertram, 1975).
Several types of microorganism have been known to affect the quality of food, thereby constituting health hazards when foods contaminated with these organisms are consumed. High viable count often indicates contaminated (except in fermented foods) which may be due to contaminated raw food materials. It may also be as a result of inappropriate time and temperatures exposure during production/processing of foods or storage of the finished food products or the combination of these (Bryan and Bartleson, 1985).
Table 1: Bacteria species isolated from “Ready-to-eat” foods in Akure markets
| Samples | ||||||
| Bacteria species | Cooked white | Stew | “Monimoin” | “Akara” | Eko(Agidi) | |
| rice | (Vegetable) | |||||
| Bacillus cereus | – | – | 5(70) | 6(70) | – | |
| Corynebacterium sp | – | 4(60) | 7(60) | 8(60) | 5(70) | |
| Lactobacillus acidophilus | 3(20) | – | 5(20) | – | – | |
| L. plantarum | 3(20) | – | 5(20) | – | – | |
| L.brevis | – | 6(70) | – | 6(40) | – | |
| Micrococcus roseus | – | 3(20) | 3(40) | 6(70) | – | |
| Staphylococcus aureus | 2(30) | 7(70) | 8(20) | 7(60) | 5(50) | |
| Staph epidermis | 6(60) | – | – | 5(70) | – | |
| Flavobacterium sp | 6(40) | 8(70) | – | – | 5(40) | |
| Gemella sp | 7(40) | – | 5(60) | 5(60) | 5(40) | |
| Streptococcus sp | 4(50) | 5(40) | 2(40) | 2(40) | – | |
| Necromonas sp | – | 7(60) | 6(70) | 5(20) | – | |
| Bacillus sp | – | – | – | 6(60) | 5(50) | |
| Salmonella sp | – | – | 5(60) | 7(60) | – | |
Numbers of samples from which the organisms was obtained is shown with the determined percentage of occurrence in brackets Not detected O. Ojokoh & B. A. Ojokoh, Biosci, Biotech. Res. Asia, Vol. 01(2) 79-85 (2003)
Table 2 : Chemotaxonomic results of selected bacteria isolates
| Tests | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Gram’s staining | + | + | + | + | – | + | – | ±a | – | + | + | + | + | + |
| Shape of cells | R | R | S | R | R | S | R | S | R | R | R | S | R | S |
| Motility | – | – | – | + | – | – | + | – | – | – | + | – | – | – |
| Catalase | + | – | + | + | + | + | + | – | + | – | + | – | – | + |
| Oxidase | – | – | – | – | + | – | – | – | + | – | – | – | – | – |
| Spores | – | – | . | UX . | – | . | . | . | – | + | . | – | – | |
| Glucose | – | A | A | A | A | A | A | A | A | + | A | A | A | – |
| Coagulase | . | . | + | . | . | . | . | . | . | . | . | . | . | . |
| Carbohydrates acid from : | ||||||||||||||
| Lactose | – | + | + | – | – | + | – | – | – | + | + | + | + | + |
| Maltose | + | + | + | + | – | + | + | + | + | + | + | + | + | – |
| Sucrose | – | + | + | + | – | + | ± | . | . | + | – | + | + | + |
| Arabinose | + | – | + | – | – | + | + | ± | + | + | + | + | – | + |
| Fructose | – | – | + | + | – | + | + | – | – | + | – | + | – | – |
| Growth in 65% NaCl | . | . | + | . | . | . | . | . | . | . | . | . | . | . |
| Mannoitol | – | – | . | – | . | – | + | ± | + | + | + | – | – | – |
a = gram positive but easily decolourized, Ux = Centre spore (oral shape),S = Cocci, G =Gas, R = Rod, + = Positive, – = Negative, ± = Depend on the medium size, A = acid, = Test not carried out.
It has been reported that the presence of mesophilic microorganism in foods is an indication that pathogenic microbes are likely to be present in such foods. A number of food items sold locally have been shown to be highly contaminated with Bacillus species, Staphylococcus and other bacteria species (Owhe-ureghe et al., 1993).
In Nigeria, widespread occurrence of pathogenic microorganism in both animals and humans have been observed (Adekeye, 1981; Adesiun and Usman, 1983). This may be the result of the close association of animals (pets and livestock) to human population in the environment (Adesiyun and Usman, 1983). The poor sanitary condition in most of the local markets and environment being highly polluted and charged with spoilage and pathogenic flora is likely to be source of contamination to food items sold in such markets (Okodugha and Obanu, 1989).
It is believed that for a proper consumer protection service, the quality of food must be ensured at all level of production and marketing. Quality determination at different points of sale, would reflect to a large extent, the quality of food purchased by the consumer (Ofuya and Patrick 1987).
This study reports the micro-biological quality of some “ready-to-eat” foods hawked in Akure markets to evaluate their suitability for consumption.
Materials and Methods
Collection of Samples
A total of 25 samples of five types of “ready-to-eat” food (akara, moinmoin, white rice, vegetable stew and cooked maize pulp (agidi) which are sold cold were obtained from Oja-Oba (location A), Odo-Ikoyi market (location B), Oluwatuyi market (location C), Ondo road/Isinkan market (location D) and NEPA junction market (location E) of Akure. The food samples collected were wrapped aseptically in sterile containers. All samples were carried immediately to the laboratory for examination.
Microbiological Analysis
0g of the food samples was aseptically weighed using analytical meter balance (Model AC-100) into a sterile laboratory mortar. The samples were crushed with the aid of a sterile pestle in the mortar and diluted with distilled water mixed thoroughly to give homogenous suspension with a final concentration of 1g/ml (Harrigan and Macance, 1982).For the stew samples, 1 ml of it was aseptically withdraw and added to 9ml of sterile distilled water in a sterile test tube to provide 10-1 dilution which was used for further dilutions up to 10-7.
The diluted sample (1ml each) was inoculated into nutrient agar and MacConkey agar plates by the pour plate method to determine the total viable counts and coliform level respectively. The petri plates were incubated aerobically at 37°c for 24 hours in duplicate after this period of incubation bacterial colonies were counted. The various bacterial isolates were characterized based on their colonial morphology, cellular morphology and biochemical reaction that included catalase, coagulase, oxidase, motility, oxidative/fermentative (°/F) utilization of sugar and other carbohydrates as described by MacFaddin (1980) and Cruickshank et al (1975). The ability of the isolates to grow in 6.5% NaCl was also determined.
 |
Figure 1 : Total Aerobic Bacterial Population in Different Types of “Ready-to-eat” Food |
 |
Figure 2 : Coliform Population in Different Types of “Ready-to-eat” Food |
Results
A total of fourteen different bacteria species were isolated from five food types, their percentage of occurrence and their chemotaxonomic properties are presented in Tabes 1 and 2 respectively. Mean of Total Plate Count/g/ml (Log 10)
The arithmetic means of the total bacteria and coliform plates counts per gram (or per milliliter) of food samples examined are presented in Figures 1 and 2.
 |
Figure 3: Relationship between the Total Plate Counts/g/ml Log 10 and Coliform counts/g/ml obtained from Oja-Oba Market (location A) |
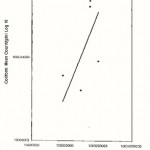 |
Figure 4: Relationship between the Total Plate Counts/g/ml (log 10) and the Coliform counts/g/ml obtained from Odo Ikoyl market (location B) |
In the (location A) Oja-Oba market, the mean total bacteria plate counts varied from 8.4×107 cfu/g for rice to 2.0 x 109 cfu/g for stew while the mean coliform counts ranged from 3.9×107 cfu/ml for moinmoin to 4.5 x 108 cfu/g for rice.
For (location B ) Odo-lkoyi market, the mean total bacteria plate counts varied from 8.5×107 cfu/g for eko to 1.0×109 cfu/g for moinmoin while the mean coliform counts ranged from 4.0×107 cfu/g for rice and stew to 4.7×108 cfu/g for akara.
For (location C) Oluwatuyi market, the mean total bacteria plate counts varied from 1.2×108 cfu/g for moinmoin to 1.5×109 cfu/g for eko while the mean coliform counts ranged from 4.0×107 cfu/g for stew to 6.0×108 cfu/g for akara.
For (location D) Ondo road/Isinkan market, the mean total bacteria plate counts varied from 1.2×107 cfu/ml for stew to 1.0×109 cfu/g for rice, while the mean coliform counts ranged from 3.0×107 cfu/g for rice to 5.0×108 cfu/g for moinmoin.
For (location E) NEPA junction market, the mean total bacteria plate counts varied from 4.0×107 cfu/g for eko to 7.2×108 cfu/g for rice, while the mean coliform count ranged from 4.0×107 cfu/ml for stew to 4.0×108 cfu/g for rice.
The results showed that the location did had significant influence on the total bacteria mean counts and coloform mean plate counts as there was a trending line relationship between coliform counts and total counts (Fig. – 3-7) which was trending either towards negative or positive.
Discussion
The International microbiological standards recommended limits of bacteria contaminated for foods which fall into the range of 101 and 102 cfu/g of food for coliform organism and less than 105 cfu/g of food for total bacteria plate counts (Owhe-Ureghe et al. 1993).
The result revealed that all the “ready-to-eat” food had a total bacteria and coliform plant counts which ranged from 1.2×107 cfu/ ml to 1.5×109 cfu/g and 5.0×107 cfu/g to 6.0×108 cfu/g. Akara had the highest microbial load 3.1×109 cfu/g. This was followed by rice with a total load of 3.0×109 cfu/g and eko which had 2.9×109 cfu/g. The least contaminated were stew and moinmoin samples with total bateria load of 2.5 x 109 cfu/ml and 1.5×109 cfu/g respectively.
Jawetz et al. (1982) stated in his work that any count greater than 1.0×106 cfu/g of food for mean total aerobic count be incriminated in food poisoning or infection outbreaks. The high level of coliform bacteria could be the result of the failure of food handlers to observed basic sanitary rules or may be due to faulty heat process. All food samples examined thus are high above the acceptable limits and are therefore microbiologically unacceptable.
The high bacteria count obtained from akara, rice and eko which range from 2.9 x 109 cfu/ml – 3.1×109 cfu/g may be due to the relatively, high moisture content of these intermediate moisture foods.
The possible sources(s) of these organisms in the food samples could be from nose where it is commonly found, hands, skin and clothing of handlers (Hobbs and Gilbert, 1982),Coughing, talking and sneezing produced droplets which could settle on food during transportation, storage and retailing, from water used for washing utensils and wrapping materials, the exposure of the products to high temperature storage, reheating of kept food and open market which are heavily polluted by various microorganism (Salle, 1985).
 |
Figure 5: Relationship between the Total Plate Counts/g/ml (log 10) and the Coliform counts/g/ml obtained from Oluwatuyl Market (location C)
Click here to View Figure |
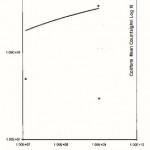 |
Figure 6: Relationship between the Total Plate Counts/g/ml (log 10) and the Coliform counts/g/ml obtained from Ondo/Isinkan Market (location D) |
The present of the organisms likes Streptococcus sp., Bacillus cereus, Staphylococcus aureus, Samonella sp. and Corynebacterium sp. Occurring in high numbers is of health significance as some of them are know to be associated with food poisoning, infection and intoxication capable of causing various ailments in adults and children which may be fatal.
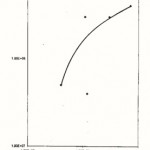 |
Figure 7: Relationship between the Total Plate Counts/g/ml (log 10) and the Coliform counts/g/ml obtained from Nepa-Junction Market (location E) |
In conclusion, in order to reduce the incidence of food poisoning, infection and intoxication measure such as thorough cooking, the provision or clean glass boxes to prevent excessive environmental contaminated of cooked foods, using clean wrapping materials and cutleries, serving the food hot to reduce their microbial load as much as possible and the practice of basic sanitary rules in preparing foods, personal hygiene should be employed. There is need for continuous monitoring by the public Health officers who should inspect and monitor the sales of safely “ready-to-eat” foods sold in markets and other catering establishments on a regular basis.
References
- Adekeye, J.O. Epidemiological study of human and caine staphylococcus aureus in Nigeria by serological means. In Staphylococcal infection. Ed. J. Jeljaszewicz 991-995. Guster Fischer Vertag, Stuttgart. (1981)
- Adesiyun, A.A. and Usman, B. Isolation of enterotoxigenic strains of staphylococcal from dogs. Microbiol. 8, 459-468. (1983)
- Bertram, P. Fast Food Operations. Barrie and Jenkins Limited London, 69-70. (1975)
- Bryan, F.L. and Bartleson, C.A. Mexican – style food service operations. Hazard analysis, critical control points and monitoring. Food Protect., 48, 509-524. (1985)
- Cruickshank, R.C., Duguid, J.P., Marmion, B.P. and Swain, R.H.A. Medical Microbiology. The Vol. 11,12th Churchill Livingstone Medical division of Longman Group Limited. 587 (1975)
- Harrigan, W.F. and McCance, M.E. Laboratory Methods in Food and Dairy microbiology. Academic press Inc. London limited, 452. (1982)
- Hobbs, B.C. and Golbert, J.R. Food poisoning and food Hygiene 4th edition Edward Arnold Limited London, 366. (1982)
- Jawetz, E. Melnick, J.L. and Adelberg, E.A. Review of Medical Microbiology 15th Edition, Lange medical publication California, 530. (1982)
- Ofuya, O.C. and Patrick, A.I. Quality of soft drinks found in port Harcourt. Nigeria Journal of Microbiology 7(1-2) 116-120. (1987)
- Okudugha, S.A. and Obanu, A. Effects of description processing on microflora of raw beef. Food J., 7, 39-49. (1989)
- Owhe-ureghe, U.B., Ekundayo, A.O. Agbonlahor, D.E., Oboh, P.A. and Orhue, P., Bacteriological examination of some “ready-to-eat” foods marked in Ekpoma, Edo state of Nigeria. Food J. 11, 45-52. (1993)
- Salle, A.J. Fundamental Principle of Bacteriology 6th Edition McGraw Hill Book Co. 433. (1985)

This work is licensed under a Creative Commons Attribution 4.0 International License.





