How to Cite | Publication History | PlumX Article Matrix
Farid A. Badria*1, Wael E. Houssen1, Eman M. El-Nashar2 and Shehta A. Said3
1Pharmacognosy1 Departments, Faculty of Pharmacy, Mansoura University, Mansoura.
2Histology and Cytology Department, Benha Faculty of Medicine, Zagazig (Egypt)
3Pharmacology Departments, Faculty of Pharmacy, Mansoura University, Mansoura.
ABSTRACT: The hepatoprotective effect of glycyrrhizin (GL) and ethanolic extract of Boswellia carterii (BC) rat liver injury induced by CCl4 was studied. Rats were administered orally with CCL4 (once a week for 4 weeks with the following doses; 0.16, 0.24, 0.32 and 0.4 ml.kg-1 for first, second, third and fourth week respectively. Two CCl4 challenged groups were concomitantly administered orally with GL (100 mg.kg-1, once daily for 4 weeks) and BC (50 mg.kg-1, once daily for 4 weeks). Serum activities of alanine aminotransferase (ALT) and alkaline phosphatase (AP) and serum concentrations of total bilirubin and albumin were measured and histopathological changes in livers were examined. The elevation of serum ALT, AP and bilirubin was delayed and attenuated and hepatic parenchymal swelling and necrosis produced by CCl4 were ameliorated by both GL and BC. The results showed that both oleanene-type triterpenes (GL and BC) can protect rats against CCl4-induced hepatotoxicity in subchronic CCl4 exposure, and the protection may be partly related to decrease of CCL4–induced inflammation and additional deposition of collagen in target organs.
KEYWORDS: Glycyrrhizin; Boswellia carterii; Oleanene triterpenes; Liver injury
Download this article as:| Copy the following to cite this article: Badria F. A, Houssen W. E, El-Nashar E. M, Said S. A. Biochemical and Histopathological Evaluation Glycyrrhizin and Boswellia carterii Extract on Rat Liver Injury. Biosci Biotechnol Res Asia 2003;1(2) |
| Copy the following to cite this URL: Badria F. A, Houssen W. E, El-Nashar E. M, Said S. A. Biochemical and Histopathological Evaluation Glycyrrhizin and Boswellia carterii Extract on Rat Liver Injury. Biosci Biotechnol Res Asia 2003;1(2). Available from: https://www.biotech-asia.org/?p=3395 |
Introduction
Although hepatotoxins-induced liver lesions may be reversed in the early stages, they can not be healed only by removal of the toxin after critical periods, highlighting the need of effective remedies for liver diseases. In our search for plant constituents with antihepatotoxic potential, both glycyrrhizin and ethanolic extract of Boswellia carterii (BC) proved to exhibit a protective effect against (CCl4)-induced hepatotoxicity. Glycyrrhizin (Glz), an Oleanene triterpenoid glycoside obtained from the roots of Glycyrrhiza glabra, was known with its preventive effect against several forms of experimental liver injury in animals¹. Glzis widely used to treat hepatocellular injury especially hepatitis2-4. It inhibits the activity of 11-beta-hydroxysteroid dehydrogenase, PGE2 production by macrophages and modifies arachidonic acid metabolism. It also has antioxidant activity². Moreover, it was noticed that glycyrrhizin treatment blunts ALT elevations and impedes fibrosis in animals 3,4. Boswellic acid (BA) and its analogues were identified as the active principles of Boswellia species5. Safayhi and Sailer showed that boswellic acid might be a rich natural source as anti-inflammatory drug development[?]. It was reported that BA showed a significant protectionagainst galactosamine/endotoxin induced hepatitis in mice Due to the structure similarity between boswellic acid and glycyrrhizin (Fig. 1), we suggest that boswellic acid may have a potential antihepatotoxic activity. So in this work, the hepatoprotective effects of glycyrrhizin and ethanolic extract of BC were biochemically and histpathologically evaluated.
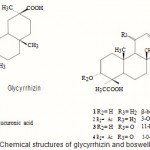 |
Figure 1
Click here to View figure |
Materials and Methods
Reagents
Glycyrrhizin solution (2.5%) was prepared by dissolving glycyrrhizin sodium salt (Aldrich, USA) in distilled water. Ethanolic extract of BC was prepared in our lab and given as o/w emulsion using cremophore (2%) as emulsifier. Cremophore (2%) was previously tested on a separate group of animals and proved to have no effect on liver function (Unpublished data). All other reagents were of analytical grade.
Animals
Six groups of adult male albino rats (150-200 g) were obtained from????, each consisting of 10 rats, were used to perform this experiment. One group is taken as control and others received CCl4, GL, GL+CCl4, BC and BC+CCl4. BC extract and GL were given orally once daily for four weeks at 50 mg.kg-1 and 100 mg.kg-1 respectively. CCl4 was administered orally once a week for four weeks with the following doses (0.16, 0.24, 0.32 and 0.4 ml.kg-1 for first, second, third and forth week respectively) [6]. All CCl4 doses were mixed with corn oil (1:1 v/v) before administration to alleviate irritation.
Biochemical evaluation of liver function
After 4 weeks experiment, rats were sacrificed and blood was collected for biochemical analysis. Serum was separated for estimation of alanine aminotransferase (ALT) activity7, alkaline phosphatase (AP) activity8, total bilirubin9 and albumin10.
Histopathological studies
Liver specimens of all groups were fixed in neutral buffered formalin, conventionally processed, paraffin embedded and sectioned at 4 microns11. The obtained slides were stained with Haematoxylin and Eosin (Hx. & E.) and Masson trichrome stains.
Statistical analysis
Values are expressed as means ± S.E. Means were compared using Sudent’s (t) test. Differences were considered significant when p < 0.05.
Results
A- Biochemical results
Effect on alanine aminotransferase (ALT) and alkaline phosphatase (AP) activities:
Table 1 : Effect of CCl4 alone and in combination with BC and GL on serum ALT, AP, total bilirubin and albumin
| Treatment | ALT | AP | Bilirubin | Albumin | |
| (units/ml) | (Kind and king units %) | (mg %) | (g %) | ||
| Control | 33.5 | ± 02.51 | 21.5 ± 6.04 | 0.22 ± 0.09 | 3.6 ± 0.18 |
| CCl4 | 129.0 | ± 12.88 * | 51.4 ± 6.24 * | 0.72 ± 0.16 ** | 3.5 ± 0.15 |
| BC (50 mg.kg-1) | 35.3 | ± 03.71 | 33.1 ± 7.31 | 0.38 ± 0.12 | 3.18 ± 0.17 |
| BC + CCl4 | |||||
| (50 mg.kg-1) | 43.0 ± 02.84 *** | 29.6 ± 3.60 *** | 0.43 ± 0.07 | 3.4 ± 0.01 | |
| GL (100 mg.kg-1) | 29.8 | ± 01.32 | 37.8 ± 8.52 | 0.41 ± 0.05 | 3.3 ± 0.15 |
| GL + CCl4 | |||||
| (100 mg.kg-1) | 63.6 ± 08.34 *** | 27.4 ± 0.03 *** | 0.82 ± 0.12 ** | 3.6 ± 0.18 | |
*Significantly different from control group (p < 0.01) using Student’s (t) test.
**Significantly different from control group (p < 0.05) using Student’s (t) test.
***Significantly lower than that of CCl4-treated group (p < 0.01) using Student’s (t) test.
CCl4 group showed a significant (p < 0.01) increase in serum activities of ALT and AP compared to the control group. Administration of BC extract or GL showed no significant alteration in ALT and AP activities from the control values. Co-administration of either BC extract or GL with CCl4 resulted in a significant decrease in ALT and AP activities compared to CCL4-treated group (Table 1).
Effect on total bilirubin concentration
CCl4 group showed a significant (p < 0.05) increase in bilirubin level compared to the control group. Neither BC extract nor GL significantly altered total bilirubin level from the control value. Only BC extract showed a marked reduction in total bilirubin level.
Effect on serum albumin level
Neither CCl4 administration nor treatments with BC extract or GL significantly altered albumin levels.
B- Histopathological observations
Rats of CCl4 group showed cirrhotic changes in their livers manifested by doubling of central veins and dilatation of blood sinusoids. Also, cirrhotic nodules, periportal fibrosis and extensive degeneration of hepatocytes were markedly distinct. On the other hand, those pretreated with either BC extract or GL showed a marked decrease of these cirrhotic changes. Animals treated with either BC extract (50 mg.kg-1) or GL (100 mg.kg-1) alone showed a normal liver architecture.
Discussion and Conclusion
The ability of a hepatoprotective compounds to reduce the injurious effects or to preserve the normal hepatic physiological mechanisms, which have been disturbed by a hepatotoxin, is the index of its protective effect. Carbon tetrachloride (CCl4) is long known to produce liver injury. The mechanism by which CCl4 induces hepatotoxicity was attributed to the release of free radicals, which could interact with other lipid- rich cells producing alteration in the structure and function of the liver cells12. In 1982, Proctor and Chatamra6 stated that intragastric CCl4 administration is preferred than the subcutaneous or the inhalation routes to induce liver toxicity in rats. This is because, the inhalation method suffers from the fact that CCl4 will pass from the lung to the left atrium with subsequent high peak concentration of CCl4 in the arterial blood. This high concentration is much more likely to produce extrahepatic (e.g. renal and cerebral) effects before the CCl4 is sufficiently extracted and concentrated in the liver while subcutaneous route of administering CCl4 is a very slow and unreliable method of producing cirrhosis. On the other hand, intragastric administration of CCl4 assures that the major part of it goes to the liver through the portal vein before entering the arterial system as rat liver selectively concentrates CCl4 in ratio of 13:1 with respect to the blood6 . Moreover, the same authors6 observed a variable response in rats toward CCl4 toxicity with respect to time. This is due to two factors – the increasing age of rats which reduces the sensitivity to CCl415, and the increasing damage of the liver with each dose of CCl4, which reduces the amount of cytochrome P450/CCl4 “toxin” effect16.
Bilirubin is one of the most useful biochemical clues to the severity of necrosis18, and its accumulation is a measure of binding, conjugation and excretory capacity of hepatocytes relative to the erythrocytes degradation rate19.
In this study, both GL and BC extract were tested for their hepatoprotective effects against CCl4-induced liver injury. Many investigators have reported on the hepatoprotective effects of GL4,20,21. In 1984, Kiso et al.22 have shown that GL inhibits cytotoxicity caused by carbon tetrachloride (CCl4) in primary cultured rat hepatocytes in vitro22. Due to the close similarity in chemical structure between GL and Boswellic acids (the main active constituents of Boswellia species), we have investigated the hepatoprotective effect of BC extract. GL and BC extract were administered orally once daily for four weeks at 100 mg.kg-1 and 50 mg.kg-1 respectively with or without CCl4 hepatotoxin.
The obtained results indicated significant protective effects of GL and BC against CCl4-induced hepatotoxicity at dose levels of 100 and 50 mg.kg-1 respectively.
Under given route of application, GL and BC seem to preserve the integrity of liver cell membrane as proved by the significant reduction of the CCl4-induced rise of ALT and AP levels. They also have the ability to prevent CCl4-induced hepatocellular necrosis and to maintain the normal functional status of the liver as proved by the significant reduction of the CCl4-induced rise in serum bilirubin and by the histopathological study. Astonishingly, in the present study, no change was observed in the serum albumin levels in all groups. This might probably be due to the long biological half life time of albumin as it is not altered in acute and sub-chronic liver damage19.
This study demonstrated that GL and BC under the given route and schedule of treatment are hepatoprotective drugs in rats and can significantly reduce the hepatic damage induced by CCl4 intoxication.
References
- Van Rossum TG, Vulto AG, De Man RA,Brouwer JT, Schalm SW. Review article: Glycyrrhizin as a Potential Treatment for Chronic Hepatitis C. Aliment PharmacolTher 2: 199-205 (1998)
- Shaikh ZA, Vu TT, Zaman K. Oxidative Stress as a Mechanism of Chronic Cadmium- induced Hepatotoxicity and Renal Toxicity and Protection by Antioxidants. Toxicol Appl Pharmacol 154: 256-263 (1999)
- Wang JY, Guo JS, Li H, Liu SL, Zern MA. Inhibitory Effect of Glycyrrhizin on NF-kappa B Binding Activity in CCl4-plus Ethanol- induced Liver Cirrhosis in Rats. Liver 18, 180-185 (1998)
- Nose M, Ito M, Kamimura K, Shimizu M, Ogihara Y. A Comparison of the Antihepatotoxic Activity between Glycyrrhizin and Glycyrrhetinic. Acid. Planta Med., 60, 136-139 (1994)
- Shao Y, Ho C, Chin C, Badmaev V, Ma W, Huang M. Inhibitory Activity of Boswellic Acids from Boswellia serrata against Human Leukemia HL-60 Cells in Culture. Planta Med., 64: 328-331 (1998)
- Proctor E, Chatamra K. High Yield Micronodular Cirrhosis in the Rat. Gastroentrol, 83, 1183-1190 (1982)
- Reitman S, Frankel S., Am J Clin Path.,56 (1957)
- Belfield A, Goldberg DM. Revised Assay for Serum Phenyl Phosphatase Activity Using 4-amino-antipyrine. Enzyme 12, 561-573 (1971)
- Jendrassik L, et al. Biochem., 7297, 81 (1938)
- Doumas B, Warson W, Biggs H., Clin Chem Acta., 31, 87 (1971)
- Banchroft JD, Stevens A, Turner DR. Theory and Practice of Histological techniques, Fourth Ed., New York, Edinburgh, London, Madrid, Melbourne, San Francisco, Tokyo, 101 & 129 (1996)
- Recknagel RO, Ghoshal AK. Lab Investig 15, 132 (1966)
- Andrews L, Synder R. Toxic Effects of Solvents and Vapours. In: Amdur MO, Doull I, Klassen CD eds. Toxicology, the Basic Science of Poisons. 4th ed., Pergamon Press, 963-965 (1991)
- Rubinstein D., Am J Physiol., 203, 1033 (1962)
- Cameron GR, Karunaratne WAE. Carbon tetrachloride Cirrhosis in Relation to Liver Regeneration. J. Pathol. Bacteriol., 42, 1-21 (1936)
- McLean EK, McLean AEM, Sutton PM. Instant Cirrhosis. Br J. Exp. Pathol., 50, 502-506 (1969)
- Klingensmith JS, Mehendale HM. Toxicol Lett 11, 149 (1982)
- Zimmerman HJ. In: Gall EA, Mostofi FK eds. The Liver. William and Wilkins Co., Baltimore, 384-405 (1973)
- Edmondson HA, Peters RL. In: Kissane JH ed. Andersons Pathology. CV-Mosby Co., St. Louis, Trontro, 1097-1101 (1985)
- Kiso Y, Tohkin M, Hikino H. Planta Med., 49,222-225 (1983)
- Kiso Y, Tohkin M, Hikino H. J Nat Prod., 46:841-847 (1983)
- Kiso Y, Tohkin M, Hattori M, Sakamoto T, Namba T. Planta Med., 50: 298-302 (1984)

This work is licensed under a Creative Commons Attribution 4.0 International License.





