How to Cite | Publication History | PlumX Article Matrix
Waykar Bhalchandra
Department of Zoology, Reshtriya Arts, Science and Commerce College, Chalisgaon, District Jalgaon - 424 101 (India)
ABSTRACT: Cypermethrin induced alternations in neuroendocrine cell of fresh water bivalve, parreysia cylindrical, has been studied after acute(0.499 ppm) upto 96 hours and chronic (0.0988 ppm) upto 21 days exposure. Pesticidal stress caused, vacuolization, acute cellular degeneration, clumping of chromatin material, undulation of cell envelope, damage of neuropile, pronounced alternation in C/N ratio, among the neurosectretory cells. In the initial stage of poisoning NSCs of cerebral, visceral and pedal ganglia showed decrease in C/N ratio after pesticidal stress indicating increased synthetic activity, this may be an initial response to the emergency caused by pesticidal stress. As the exposure period was increased, the C/N ratio was also increased, indicating decreased synthetic activity. Pesticidal stress, affected the pyriform cells [A-cells] as compare to oval cells [B-cells].
KEYWORDS: Cypermethrin; Neuroendrocrine; Parreysia cylindrica
Download this article as:| Copy the following to cite this article: Bhalchandra W. Cypermethrin Induced Alterations In Neuroendocrine Cells Of The Freshwater Bivalve, Parreysia Cylindrica. Biosci Biotechnol Res Asia 2003;1(2) |
| Copy the following to cite this URL: Bhalchandra W. Cypermethrin Induced Alterations In Neuroendocrine Cells Of The Freshwater Bivalve, Parreysia Cylindrica. Biosci Biotechnol Res Asia 2003;1(2). Available from: https://www.biotech-asia.org/?p=3401 |
Introduction
The insecticides cause violent physiological action upon nervous, digestive and reproductive function of the animals. It is well known that organophosphate and carbamates are the powerful inhibitors of acetylcholine esterage and work by interfering with the passage of impulse in the nervous system1 and also cause histopathological changes in the neurosecretory cells of the animal2. Sabesan and Ramalingam3 reported various histopathological changes in the neurosecretory cells of the Odontopus vericornis after pesticidal exposure. Akarte et al.4 studied the effect of commercial and technical grade malathion on cerebral ganglia of the fresh water bivalve, Indonaia caerulus. It was further substantiated in bivalve, Lamellidens marginalis and L.corrians5 , Parreysia corrugeta6,7 and P. favidence8 upon exposure to pollutant.
Inspite of all the advance in our knowledge of insecticides, practically there is not much work done on the mode of action of these chemicals at the cellular level and the histopathological changes caused by them in the neurosecretory cells of mollusc. Hence the detailed study of the effect of the insecticide cypermethrin on the neurosecretory cells of the fresh water bivalve, Parreysia cylindrica was carried out.
Material and Methods
Medium sized bivalve, were collected from Girna Dam reservoirs. The animals were brought in laboratory, were acclimatized for 4-5 days to dechlorinated tap water.
Healthy active medium sized bivalves were taken, 20 each in 4 plastic troughs. One trough was maintained as control, animals of second trough were exposed to acute dose to cypermethrin [0.4992 PPM] for 96 hours. The bivalves of third trough were used as control for chronic and those of 4th were exposed to chronic dose [0.09986 PPM] for 21 days.
The cerebral, visceral and pedal ganglia from all groups of animal were removed after, 24 hours and 96 hours interval from trough 1st and 2nd, after 7 days and 21 days interval from trough 3rd and 4th and were fixed in aqueous Bouin’s fluid for 24 hours.
The ganglia from control and experimental groups were processesed as per usual method. The serial sections were cut at 5µ and were stained with Mallory’s triple stain. Nuclear as well as cell diameters from control and experimental animals were measured. Then the average of cell and nuclear diameter was calculated and the ratio of cell diameter to nuclear diameter [C/N] was calculated9.
Results
Control neurosecretory cells (Plate I Fig ‘a’ & ‘f’; Plate II-Fig.’k’):-
Cytological examinations of the section of cerebral visceral and pedal ganglia stained with Mallory’s triple stain revealed the presence of groups of cells which are cytologically different and larger than ordinary ganglions cells. The chromatin material of control NSCs was intact. They were provided with definite regular envelope and neuropile. Most of the large cells possess large nuclei and abundant cytoplasm their perikarya and axons are filled with fine granules and showed moderate synthetic activity, which stained conspicuously. Two types of NSCs are distinguished as cell type-I (A) or pyriform cell and cell type-II (B) or oval cells, in Parreysia cylindrica. Pyriform cells ranging from 12.08 to 20µ in length and 7.96 to 12.75µ in width. The nucleus is round or oval, 3.68 to 8.3 m in diameter and contains a large nucleolus. Fine granules of secetory material are present in the cytoplasm. Type II(B) cells are oval or round measuring about 8.12µ in diameter their nucleus being large, vesicular and measuring about 3.69 to 6.47µ in diameter. Normally one large nucleus, occasionally 3 or 4 nucleoli are present in the pedal ganglia.
Effect of Cypermethrin on Neurosecretory cells
Cerebral ganglia :- (Plate I -Fig ‘b’ to ‘e’): Neurosecretory cells of cerebral ganglia showed many cytomorphic alternations after pesticidal stress as compared to control. After 24 hrs. exposure the size of pyriform cell and its nuclear diameter were increased, the C/N ratio was decreased and also intensity of neurosecretory material was increased. This showed the enhancement of synthetic activity. At this stage the rate of transport of secretory material along with axons must be very less compared to the rate of synthesis, which caused accumulation of secretory material in axon hillock in pyriform cells. For 96 hrs. exposure, the pyriform cell diameter was increased, the nuclear diameter was decreased, the C/N ratio was increased and also neurosecretory material intensity was decreased, thus synthetic activity was lowered and cell become empty. This shows that, synthetic activity was very low as compared to the rate of transport which caused complete drainage of neurosecretory material. Along with this, the changes like shrinkage of nuclei, clumping of chromatin material, undulation of cell wall were observed.
After chronic exposure, the pyriform cell diameter was increased and the nuclear diameter was decreased, the C/N ratio was increased the cells showed lowered synthetic activity. The pesticidal stress caused shrinkage of nuclei, vacuolization and loss of chromatin material. After acute and chronic exposures, the nuclear diameter was increased, the C/N ratio was decreased, showing increased synthetic activity in oval cells.
Visceral ganglia (Plate 1 Fig. ‘g’ to ‘j’) : neurosecretory cells of visceral ganglia showed many cytomorphic alternation after pesticidal stress in pyriform and oval cells. When subjected for 24 hrs. exposure, the pyriform cell and its nuclear diameter was increased, the C/N ratio was decreased as compare to that of control and also neurosecretory material intensity was increased, thus showing increased synthetic activity in pyriform cells. Along with these changes, neurosecretory cells showed, damaged neuropile and vacuolization. Neurosecretory material was accumulated in axon hillock. After 96 hrs. exposure pyriform cells became elongated, the cell and nuclear diameter were decreased, nuclei became shrunken, the C/N ratio was increased, thus synthetic activity was very low as compared to the rate of transport which is caused complete drinage of secretory material. Changes like undulation of cell wall, vacuolization and damage of chromatin material and neuropile were also observed in neurosecretory cells. However after acute exposure, the oval cells became enlarged, the nuclear diameter was increased, as a result the C/N ratio was decreased, showing enhanced synthetic activity.
After 7 days exposure, the pyriform cells became narrower and taller,the nuclear diameter was increased, as a result the C/N ratio was decreased and also neurosecretory material intensity was increased. This synthetic activity was increased in pyriform and oval cells. Neurosecretory material was condensed in cytoplasm. However, after 21 days exposure, the nuclear diameter was decreased, the C/N ratio was increased,showing decreased synthetic activity in pyriform andoval cells.
Pedal ganglia (Plate II – Fig. ‘I’ to ‘o’) : Cypermethrin exposed neurosecretory cells of pedal ganglia showed some morphological changes in pyriform an oval cells. When subjected for acute exposure, pyriform cells became narrower and taller; Oval cells became shrunken, the cell and nuclear diameter were decreased, the C/N ratio was increased, indicating decreased synthetic activity in pyriform and oval cells. The neurosecretory material was accumulated in cytoplasm. The nucleus became oval in shape, nucleous became smaller, and chromatin material was less.
After chronic exposure,the cell diameter was increased with decrease in nuclear diameter hence the C/N ratio was increased and also the intensity of neurosecretory material was decreased in the cells. This shows that synthetic activity was very low as compared to the rate of transport which caused complete drainage of neurosecretory material and cells became empty. Along with these changes, the change like, vacuolization in cytoplasm, smaller nucleolus, less chromatin material with vacuoles appearing in the nucleus were observed.
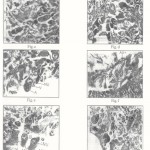 |
Figure 1 |
 |
Figure 2 |
Discussion
In the present investigation the effect of cypermethrin of the neurosecretory cells of Parreysia cylindrica were studied. Cell type I(A) cell type II (B) NSCs from cerebral, visceral and pedal ganglia were studied in relation to pesticidal stress and the results are summarized in Table 1 and 2 plate I & II.
Gundevia and Ramamurhti10 have first of all postulated various histopathological changes like vacuolization in the perikarya, undulation of the cell bondaries, nad clumping of the chromatin material in the neurosecretory cells of Hydrophilus olivaceous when exposed to pesticides. The pesticides endrin and sumithion caused impairment in both inter and intracellular structure of the neurosecretory cells and also alters the various grades of disturbances in the compactness of neurosecretory elements and undulation in the periphery of cell bounderies along with the occurrence of small to large number of vacuoles inside the perikarya in Periplanata americana11 similar changes were also recorded in a snail Indoplanoribis exustus12 under pesticidal stress. The degeneration changes in the neurosecretory cells and the neuropilar tissue, such as undulation of the cell bounderies, loss of compactness and necrosis were reported in the cerebral ganglia of fresh water prawn, Caridina welberi after pesticidal exposue13. Sarojini and Mirajkar9 reported different histopathological changes like vacuolization in cytoplasm, undulation of cell wall and clumping of chromatin material in the neuroprofile (brain) of fresh water prawn, Macrobranchium kistnesis after organophoshorus exposure.Thoat6 studied the impact of pesticides and heavy metals on the bivalve, Parreysia corrugata and reported various grades of histopathological changes such as vacuolization, cellular degeneration, alternation in cytoplasmic and nuclear areas and altered stining properties and neurosecretory activity of NSCs. These alternations are in agreement with the present study.
In present study, after short treatment enhanced synthetic activity with accumulation of neurosecretory material and after prolonged treatment inhibitions of synthetic activity and the depletion of neurosecretory material in the cells were observed. These results are in agreements of with the previous findings6,7,1,13.
The study of C/N ratio of NSCs is the best parameter to know the responses of the cells to the pesticidal stress.In the present study, it was observed that in the initial stage of poisoning, the C/N ratio was decreased, indicating increase in synthetic activity. An enhanced synthetic activity may be correlated to extent of pollution stress and maintenance of homeostasis in the internal environment. However, as exposure period was increased, the C/N ratio was increased, over the control NSCs. This indicated that long duration of pesticides hampered the synthetic activity of neurosecretory cells.
| P | 6.2142 | ± | 0.4272 | 4.0102 | ± | 0.1244 | 1.5495 | 5.1672 | ± | 0.3216 | 3.8212 | ± | 0.1126 | 1.3522 | |||||
| Table1:Cypermethrininducedalterationsinneuroendocrinecellsoffreshwaterbivalve,Parreysiacylindrica,after24hoursand96hoursexposure | Parameter Control After24hoursExposure After96hoursexposure | C V P C V P C V | 0666 738 52376 9235Cell8.8.03567.9.8.23336.89938. 7.5892 | diameter ± ± ± ± ± ± ± ± | u 8013 6712 8417 26260.0.66700.0.0.68350.64011. 1.6366 | Nuclear 6261 0991 1428 33715.5.15715.7.5.35714.12085. 4.4642 | diameter ± ± ± ± ± ± ± ± | u 0. 0.61876175 4468 9259 26260.0.0.12620.65871. 0.7651 | Celldiameter/ 1.433 1.5581 5175 333 5368 67291.1.1.1.67421. 11.700 | Nucleardiameter(C/N) | Cell 6. 6.4886 6. 7.4566014064405 84516.58475.62496. 7.1825 | diamter ± ± ± ± ± ± ± ± | u 1. 0.4472 1. 1. 0.6214 0.6973126612482271 42090. 0.8911 | Nuclear 4. 4.2628 4. 5. 4.5200 4.37757404501427015 71425. 4.9666 | diameter ± ± ± ± ± ± ± ± | u 0. 0.1434 0. 1. 1. 0. 0. 0.719725946755200945278868 | Celldiameter/ 1.3620 1.5221 1. 1. 1.4567 1.2849 1.498130501979 1.4461 | Nucleardiameter(C/N) | ganglia,V=Visceralganglia,P=Pedalganglia |
| CellType | Cell | TypeI | Cell | TypeII | C=Cerebral |
Table 2
| P | 9.3782 | ± | 0.7290 | 4.1672 | ± | 0.1683 | 2.250 | 7.1428 | ± | 0.6354 | 4.006 | ± | 0.8748 | 1.7830 | |||||
| Table2:Cypermethrininducedalterationsinneuroendocrinecellsoffreshwaterbivalve,Parreysiacylindrica,after7daysand21daysexposure | Parameter Control After7daysExposure After21daysexposure | C V P C V P C V | 0666 0356 738 2256 4394 9235Cell8.8.7.8.9.22619.8. 8.1246 | diameter ± ± ± ± ± ± ± ± | 8013 6712 2672 2264u0.0.6670.1.1.11361. 02961. 0.4412 | 6261 0991 2143 2499Nuclear5.5.15715.5.6.69644. 5. 4.52162671 | diameter ± ± ± ± ± ± ± ± | 6175 4468 6274 1786u0.0.61870.0.0.76530. 0. 0.22466512 | 5581 5175 5775 2210Celldiameter/1.4331.1.1.1.37772. 1. 1.79686951 | Nuclear | diameter(C/N) | 4566 01406 5121Cell6.6.48866.6. 6.2499 6. 6. 6.14256078035 | diamter ± ± ± ± ± ± ± ± | 1266 1248 2672u1.0.44721.0. 0.8928 1. 0. 0.465233624464 | 74045 0142 1467Nuclear4.4.26284.5. 5.1338 3. 5. 3.96429003571 | diameter ± ± ± ± ± ± ± ± | 2594 6755 4266u0.0.14340.0. 0.6696 0.6428 0. 0.02163346 | 4981 265Celldiameter/1.36201.52211.1. 1. 1.6941 1. 1.549521742699 | ganglia,V=Visceralganglia,P=Pedalganglia |
| CellType | Cell | TypeI | Cell | TypeII | C=Cerebral |
In the present investigation, it was observed that in the initial stage of poisoning there was a gradual synthesis of secretory material but as the incubation period was prolonged a gradual release of secretory material was started. This discharge became more vigorous and finally the entire secretory material got drained off. Sabesan and Ramalingam3 reported the accelerated synthesis after short duration and release of secretory material after long duration of the endosulphan intoxication. It was shown that short exposure period to insecticides trigger the synthetic activity10 while decreased after longer exposure9. The initiation of synthesis, its gradual acceleration and ultimately accumulation of secretory material by pesticides is indicative of the fact that the accelerated rate of synthesis may be an initial response to the emergency caused by the pesticidal action.
The functional status of the neurosecretory element is linked with change in the size of nucleus and nucleolus and may be considered the index of cell activity 14,15. In the present investigation, it was noted that due to pesticidal stess the areas of nucleus and nucleolus is altered. The chromatin material in the nuclei of the neurosecretory cells, treated with pesticides, became so immobilized after clumping, that it was unable to act with other cellular constituents and it was possible that in this state the DNA content of such nuclei became quite diminished leading to a loss in production on an optimum amount of RNA. In this way the inhibition of synthetic activity after prolonged incubation period may be assumed as a failure of the RNA synthetic machinery which inhibits the further synthesis of secretory material15.
It was shown16 that the activity of neurosecretory cellis affected by three factors, rate of synthesis of secretory material, rate of transport of secretory material along with axons and, the rate of release of transported material into the circulatory system. In present study it was noted that cypermethrin intoxication caused discharged of neurosecretory material from the perikarya of the neurosecretory cells of Parreysia cylindrica. The pesticides act on neurosecretory cell and other neurons by rendering the plasma membrane very permeable to Ca++7. The role of Ca++ is linked with stimulus18. In the present study pesticides might be causing hormonal release due to excessive entry of Ca++ inside the neurosecretory cells. Thus it can be concluded that pesticides might be exterting their effects on the neurotransmitters which inturn give message of the NSCs for the release of neurhormones.
References
- Coppage D.L.; Motheus, E; Cook, G.H; and Knight,E.J. Pestic Biochem. Physiol. 5:536. (1975)
- Padmasheela, N.C. and S. Krishnan : J.Env. Zool. 12:181-186 (1998)
- Sabesan and Ramalingam : Pyrrhocorentomen, 4(3) : 223-228 (1979)
- Akarte, S.R.; Muley, D.V., Hiwale, V.V. and Mane, U.H., of the all India sym. Held ata Aurangabad,117-123 (1982)
- Muley D.V. :- Ph.D. Thesis, Marathwada University, Aurangabad (1988)
- Thorat, D.H. : Ph.D. Thesis, Marathwada University, Aurangabad (1990)
- Deshmukh Menakshi, B: Ph.D. Thesis, Dr. B.A. Marathwada University, Aurangabad (1995)
- Bhambre, P.R. : Ph.D. Thesis, Marathwada University, Aurangabad. [M.S.] India (1993)
- Sarojini R. and S.S. Mirajkar : Proc. of the India sym. Held at Aurangabad (1982)
- Gundevia, H.S. and P.S. Ramamurthi : Tiere. 71:355-375 (1972)
- Nanda, D.K. : D.C. Nature Wissenchiften. 61(10) 451-452 (1974)
- Hanumante, M.M.; Farrooqui U.M. and Nagabhushanam, R.C., Exp. Biol, 17(9). 964-995 (1974)
- Nagabhushanam, R.T.; S.N. Reddy and Sarojini : of the all India sym. held at Aurangabad, 134-138 (1982)
- Ortman, R.C.: American physiology society, Washington. D.C. 12: 1039-1061 (1960)
- Ghosh, J.J.; Ghosh, S; Chanda, S; Sikdar K. and Bhaduri, S.: Science and Culture 34-62 (1968)
- Highnam, K.C. and Hill, L : The compara-tive endocrinology of the invertebrates II edition prints Williams clowes and sons Ltd. London Beccler and Colchester (1978)
- Cooke, T.M. : Ed. Horold Gainer, 345 (1977)
- Fingerman, S.W. and Fingerman, M.: Comp. Physiology. 55-8. (1977)

This work is licensed under a Creative Commons Attribution 4.0 International License.





