How to Cite | Publication History | PlumX Article Matrix
Effect of Environmental Lead on Human Health
Meena Iqbal¹, Shahbano² and M. N. Khan³
1Asian Biotech. Research Centre, Bhopal - 462 001 India.
²Bhoj Open University , Bhopal India.
³Saifia College of Science and Education, Bhopal - 462 001 India.
ABSTRACT: Lead is a abundant metal in nature, occuring in lead mineral. Lead intake is by diet, air and water each day in living beings.This Pb effect the heme synthesis of the bloodwhich causes hematological disruption of respiratory pigments such as cytochromes, aneaemia, kidney disfunctioning and brain damage.This can be cured by chelating agents
KEYWORDS: Lead(Pb), toxic, biochemical
Download this article as:| Copy the following to cite this article: Iqbal M, Shahbano S, Khan M. N. Effect of Environmental Lead on Human Health. Biosci Biotech Res Asia 2003;1(2). |
| Copy the following to cite this URL: Iqbal M, Shahbano S, Khan M. N. Effect of Environmental Lead on Human Health. Biosci Biotech Res Asia 2003;1(2). Available from: https://www.biotech-asia.org/?p=17834 |
Introduction
There are lots of chemicals in the environment. Many of these are toxic and the others are non-toxic. The toxic chemicals are discharge y industries into air, water and soil.They get into human food chain form the environment. As they enter in the biological system, they disturb the biochemicals processess, leading to fatal results. Chemical toxicology is the science that deals with the study of toxic chemicals and their mode of action.Many metals as environmental hazards are essential dietary trace elements required for normal growth and development of animal and human beings. These metals are Al, Sb, As, Ba, Bi, Be, Cd, Co, Cu, Ce, In, Pb, Hg, Mo, Ag, Te, Tl, Sn, Ti, W, U and Zn.
The well known toxic elements As, Pb and Cd are required in the trace quantities for the growth of animals. The so-called biologically intret Al causes different types of diseases
Toxic Chemicals in Air
As a matter of fact , thousand of chemicals pose the problems of health hazards so that it is necessary to excerise strict control on those which offer the most serious threats during manufacture and handling
Toxic Chemicals in Water:
A list of toxic trace elemants found in natural water and wsate water given in Table 1.
Some of these are essential at low levels, serving as nutrients for animals and plants life, but are toxic at higher levels.
The major souce of air borne Pb is the combustion of leaded petrol/gasoline.Pbis added in the form of tetra alkyl lead, primarily Pb (CH8)4 and Pb(C2H5)4, together with the scavengers 1,2-dichloroethane and 1,2-dichloroethane. In common with other particulate pollutants, Pb is removed from the atmosphere by wet and dry
Table 1 : Toxic trace elements in natural water and waste water
| Element | Sources | Effects and Significance |
| Lead | Industry, mining, plumbing, coal, | Toxic (anaemia, kidney disease, |
| gasoline | nervous disorders, wild-life destroyed |
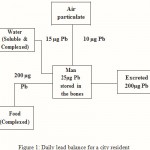 |
Figure 1: Daily lead balance for a city resident |
The major biochemical effect of Pb is its interference with heme synthesis, which leads to hematological damage. Pb inhibits several of the key enzymes involved in the overall process of heme synthesis whereby the metabolic intermediates accumulate. One such intermediate is delta-amino levulinic acid. An important phase of heme synthesis is the conversion of delta-aminolevulinic acid to prophobilinogen.
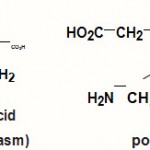 |
Scheme 1 |
deposition proces. As a result, street dusts and roadside soils becomes enrished with Pb, concentrations typically of the order 1000-4000mg kg-1 on busy streets.
It is noted that most of the Pb intake by a tpyical city dweller is from diet (about 200-300ug per day), air and water addinga futher 10-15 ug per day each .Of this total intake, 200 ug of Pb is excreted while 25 ug is stored in the bones each day.
Results and Discussion
The overall effect is the disruption of the synthesis of haemoglobin as well as other respiratory pigments, such as cytochromes, which require heme. Finally, Pb does not permit utilization of O2 and glucose for life-sustaining energy production. This interference can be detected as a head level in the blood of about 0.3ppm. The detection of (I) provides a sensitive test for Pb in the body at higher levels of Pb in the blood (>0.8 ppm) there will be symptoms of anaemia due to the deficiency of haemoglobin. Elevated Pb levels (>0.5-0.8 ppm) in the blood cause kidney dysfunction and finally brain damage.
Due to the chemical analogy of Pb2+ with Ca2+, bones actas repositories for Pb accumulated b the body. Subsequently, this Pb may be remobilized along with phosphates from the bones which exert a toxic effect when transported to soft tissues.
Lead poisoning can be cured by treatment with chelating agents which strongly bind Pb2+. Thus, calcium chelate in solution is fed to the victim of lead poisoning; Pb2+ displaces Ca2+ from the chelate and the resulting Pb2+ chelate is rapidly excreted in the urine.
Acknowledgement
Authors are thankful to Principal, Saifia College of Science and Education for providing library and laboratory facilities.
References
- Barry PSI. Concentrations of lead in the tissues of children. Br J Ind Med 38: 61–71 (1981)
- Btuman V, Maesaka JK, Haddad B, et al. The role of lead in gout nephropathy. N Engl J Med 304:520–3 (1981)
- Budd P, Montgomery J, Cox A, et al. The distribution of lead within ancient and modern human teeth: implications for long-term and historical exposure monitoring. Sci Total Environ Sep 18, 220(2–3):121–36 (1998)
- DeSilva PE. Determination of lead in plasma and studies on its relationship to lead in erythrocytes. Br J Ind Med 38, 209–17 (1981)
- Food and Drug Administration. Action levels for poisonous or deleterious substances in human food and animal feed. Washington:Department of Health and Human Services (1994)
- Flegal AR, Smith DR. Measurements of environmental lead contamination and human exposure. Rev Environ Contam Toxicol 143, 1–45 (1995)
- Gennart PH, Buchet JP, Roels H, et al. Fertility of male workers exposed to cadmium, lead or manganese. Am J Epidemiol 135, 1208–19 (1992)
- James HM, Milburn ME, Blair JA. Effects of meals and meal times on uptake of lead from the gastrointestinal tract of humans. Human Toxicol 4,401–7 (1985)
- Rabin R. Warnings unheeded: a history of child lead poisoning. Am J Public Health 79, 1668–74 (1989)

This work is licensed under a Creative Commons Attribution 4.0 International License.





