How to Cite | Publication History | PlumX Article Matrix
Pooja Malik* and Prashant Upadhyay
and Prashant Upadhyay
School of Pharmaceutical Sciences, Faculty of Pharmacy, IFTM University, Moradabad, Uttar Pradesh, India.
Corresponding Author E-mail: malikpooja094@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3084
ABSTRACT: This research aims to prepare microemulsion from Tea Tree and Rosemary essential oil to treat gram-positive and gram-negative bacterial infections. A phase titration has been used to make the proposed microemulsion, which contained 71% water, 23% of a 1:1 mixture of propylene glycol (surfactant), tween 80 (co-surfactant), and 6% of tea tree/rosemary essential oil. In Rosemary essential oil pale yellowish transparent microemulsion, droplet size, zeta potential, and polydispersity index (PDI) of 58.9, 883.6, and 0.678, respectively, showed a stable microemulsion. Tea tree oil loaded in a light, clear microemulsion with zeta potential, droplet size, and polydispersity index (PDI) values of 265.9, 746.8, and 0.987, respectively, indicated a stable microemulsion. The RMO/TTO micro emulsion's viscosity was 1.844/1.933 cps, and its pH ranged from 4.69 to 5.59. The formulation was determined to be a safe, reliable, and effective one in light and good in stability experiments. Escherichia coli, Staphylococcus aureus, and Candida albicans can all be treated using a microemulsion that contains 6% tea tree oil and rosemary oil by agar well diffusion method. Both the EOs-based microemulsion showed more zone of inhibition against Escherichia coli and Candida albicans while Staphylococcus aureus is the less susceptible zone of inhibition. The microemulsion formulation's minimum inhibitory concentration was effective against Staphylococcus aureus, Candida albicans, and E. coli on all physiological parameters. It can be concluded that TTO/RMO essential oil-loaded microemulsion is considered a promising substitute for the current gram-positive and gram-negative regimens.
KEYWORDS: Antibacterial; Antifungal Activity; Essential Oil; Microemulsion; Rosemary oil; Tea tree oil
Download this article as:| Copy the following to cite this article: Malik P, Upadhyay P. Formulation and Evaluation of Tea tree/ Rosemary Essential oil-based Microemulsion for Antimicrobial Activity . Biosci Biotech Res Asia 2023;20(1). |
| Copy the following to cite this URL: Malik P, Upadhyay P. Formulation and Evaluation of Tea tree/ Rosemary Essential oil-based Microemulsion for Antimicrobial Activity . Biosci Biotech Res Asia 2023;20(1). Available from: https://bit.ly/3XmWSiF |
Introduction
Essential oils produced from numerous plant species have been demonstrated to possess bactericidal activity against a variety of bacterial and fungal diseases. Even though they are frequently found in complex mixes, the conditions of their most significant mono and di-terpenoid components usually provide an adequate explanation for how they work. According to a review of monoterpenes’ antibacterial capabilities, they diffuse into cell membranes and harm their structure 1. Lipophilic monoterpenes will primarily separate into membrane structures from an aqueous phase 2. This results in membrane expansion, increased fluidity or disordered membrane constitution, and inhibition of membrane-embedded enzymes, as reported by Sikkema and colleagues 3. About 50% of the cyclic monoterpenes in tea tree essential oil (Melaleuca alternifolia) are oxygenated, whereas the remaining 50% are hydrocarbons. 4. It has been demonstrated to have broad-spectrum antibacterial activity, which is primarily attributed to terpinen-4-ol. This study shows that monoterpene membrane toxicity impacts microbial viability and cell membrane-related functions through an analysis of the mechanism of action of tea tree oil (TTO). The antibacterial and antifungal activity of monoterpenes may be due to, at least partially, a perturbation of the lipid fraction of bacterial plasma membrane permeability and leakage of intracellular materials. Furthermore, the monoterpenes cross the cell membranes, penetrating the interior of the cell and interacting with intracellular sites critical for antibacterial and antifungal activity 5.
There are more than 20 cultivars, or variants, of rosemary, which is scientifically known as Rosmarinus officinalis L. (rosemary), which grows naturally in the western Mediterranean basin. Since ancient times, R. officinalis has been utilized for several therapeutic, culinary, and decorative uses. For instance, R. officinalis is well recognized in the field of food science for its EOs (essential oils), which are used as food preservatives; nevertheless, because of its anti-inflammatory and antibacterial properties, it is also employed in medicine and pharmacology 6. Antibacterial and antifungal characteristics, anti-inflammatory activity, and antibacterial, antifungal, and oxidative agents are only a couple of pharmacological actions of R. officinalis that have been detailed and well documented in previous research 7, 8, 9.
Microemulsions are surfactant-stabilized mixes of two immiscible liquids that are clear, isotropic, and thermodynamically stable (and possibly other compounds). Both the range of many distinct microstructures that provide such an unambiguous macroscopic definition and the experimental methods for identifying them are described 10. The topical use of microemulsions on the skin has recently become popular. Due to the benefits including an increase in the pace at which essential oils penetrate the skin’s barrier and increasing the solubility, stability, and permeation, which are all closely related to the components of microemulsions, they can significantly improve their therapeutic properties 11,12. Among the essential oils’ active ingredients are flavonoids, phenylpropanoids, monoterpenes, and polyunsaturated omega-6 fatty acids. The antioxidant, antibacterial, antiviral, and other special intrinsic capabilities of these oils (rosemary and tea tree) enhance the overall superiority of the microemulsion system 13,14,15,16. Microemulsion compositions with tea tree and rosemary essential oils added have demonstrated antibacterial characteristics. EOs have been proven to have an antibacterial activity that does not require coming in touch with the EOs directly. Studies have indicated that EOs are more potent in antibacterial activity in the microemulsion-based system than in simply oil-based applications, and the microemulsion system phase seems to be primarily beneficial against bacterial and fungal infection 15,17. Salmonella enterica and listeria monocytogenes can both be inhibited by using tea tree and rosemary essential oils, according to prior research 18. Although essential oils have been used successfully in the past to diagnose a variety of illnesses and to promote health, they have become increasingly common in recent years for a wider range of applications, including medications, food additives, aromatherapy, and others 19,20
In this study, we evaluated the antimicrobial and antibacterial efficacy of two essential oils-loaded microemulsion formulations (Melaleuca alternifolia and Rosmarinus officinalis Linn) against clinical and food-borne bacterial pathogens, as well as a wide range of ecological bacteria and fungi. Investigations were done into these EOs’ microemulsion phase systems’ antibacterial properties. The EO doses necessary to consistently thwart the growth of diseases and ecological bacteria were identified through in vivo studies 21.
Materials and Methods
Materials
Commercially available items from Aroma Products Private Limited include tea tree essential oil (Melaleuca alternifolia) and rosemary oil (from leaves) (Rosmarinus officinalis) (New Delhi, India). Sodium nitroprusside, phosphoric acid, sulphanilamide, and naphthyl ethylene diamine dihydrochloride were primarily provided by Sigma-Aldrich Chemical Pvt. Limited, Bangalore. Sulab chemicals private limited, Vadodara, India, provided Tween 60 and Span 20 along with propylene glycol, isopropyl alcohol, xanthan gum, ascorbic acid, BHT, ethanol, and methanol. The primary analytical chemicals were utilized in this research investigation.
Methods
Preparation of microemulsion
Tween-80 and propylene glycol (Smix) were combined in a 1:1 ratio and swirled for an hour at ambient (room) temperature using a magnetic stirrer. The Smix and essential oils (Tea tree oil and rosemary oil) were then weighed into glass vials in the following ratios: 1:9, 2:8, 3:7, 4:6, 5; 5, 6:4, 7:3, 8:2, and 9:1. The clear glass vials were immediately sealed with stoppers made of rubber to inhibit the essential oils from volatilizing. Using an appropriate magnetic stirrer, water was gradually added to each greasy mixture until it reached a point where it was transparent. The pseudo-ternary phase diagram was created using the essential oils, Smix (surfactant and co-surfactant), and water, and the concentration of the essential oils were measured. This was done to determine the best outcomes 22.
Screening of Smix (surfactant and co-surfactant)
Several nonionic surfactants were titrated because they are highly compatible with both mutual cation and anion substances and do not ionize to a significant extent in the sample, including propylene glycol, IPA (isopropyl alcohol), and b-butanol. These nonionic surfactants include Tween -80, Tween -20, span-20, and co-surfactants, such as propylene glycol, and IPA. TTO/RMO was found to be more soluble in propylene glycol and Tween -80 when compared to span-20 and PEG-400. Propylene glycol and Tween-80 have been widely used because of their ability to stabilize microemulsion formulation so it was selected as co-surfactants and surfactants for the system’s essential oil-loaded microemulsion 23.
Structure of ternary phase diagram:
Table 1 (A): Pseudo ternary phase diagram for RMO
|
Oil(A) |
Surfactant(B) (Smix)(1:1) |
Water(C) |
Sum |
A |
B |
C |
Sum |
|
1 |
9 |
86 |
96 |
1.041667 |
9.375 |
89.58333 |
100 |
|
2 |
8 |
71 |
81 |
2.469136 |
9.876543 |
87.65432 |
100 |
|
3 |
7 |
55 |
65 |
4.615385 |
10.76923 |
84.61538 |
100 |
|
4 |
6 |
48 |
58 |
6.896552 |
10.34483 |
82.75862 |
100 |
|
5 |
5 |
34 |
44 |
11.36364 |
11.36364 |
77.27273 |
100 |
|
6 |
4 |
13 |
23 |
26.08696 |
17.3913 |
56.52174 |
100 |
|
7 |
3 |
3.4 |
13.4 |
52.23881 |
22.38806 |
25.37313 |
100 |
|
8 |
2 |
1.8 |
11.8 |
67.79661 |
16.94915 |
15.25424 |
100 |
|
9 |
1 |
0.5 |
10.5 |
85.71429 |
9.52381 |
4.761905 |
100 |
Table 1(B): Pseudo ternary phase diagram for TTO
|
Oil(A) |
Surfactant(B) (Smix 1:1) |
Water(C) |
Sum |
A |
B |
C |
Sum |
|
1 |
9 |
89 |
99 |
1.010101 |
9.090909 |
89.89899 |
100 |
|
2 |
8 |
74 |
84 |
2.380952 |
9.52381 |
88.09524 |
100 |
|
3 |
7 |
53 |
63 |
4.761905 |
11.11111 |
84.12698 |
100 |
|
4 |
6 |
47 |
57 |
7.017544 |
10.52632 |
82.45614 |
100 |
|
5 |
5 |
32 |
42 |
11.90476 |
11.90476 |
76.19048 |
100 |
|
6 |
4 |
12 |
22 |
27.27273 |
18.18182 |
54.54545 |
100 |
|
7 |
3 |
3.8 |
13.8 |
50.72464 |
21.73913 |
27.53623 |
100 |
|
8 |
2 |
1.2 |
11.2 |
71.42857 |
17.85714 |
10.71429 |
100 |
|
9 |
1 |
0.8 |
10.8 |
83.33333 |
9.259259 |
7.407407 |
100 |
 |
Figure-1(a): Pseudo ternary phase diagram for RMO with Smix (1:1). |
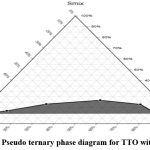 |
Figure-1(b): Pseudo ternary phase diagram for TTO with Smix (1:1). |
Formulation preparation by essential oil
Table 2: Formulation composition for EO-loaded microemulsion
|
Sample |
EO (%) |
Smix(%) |
Water (%) |
|
Rosemary oil microemulsion |
6.0 |
23.0 |
71.0 |
|
Tea tree oil microemulsion |
6.0 |
23.0 |
71.0 |
Preparation of stock solution
To perform antimicrobial activity against particular cultures, a stock solution of an essential oil-based microemulsion was prepared. A stock solution with a concentration of 100 ml/ml was made by dissolving 1 ml of a 6% TTO/RMO-based microemulsion in 10 ml of dimethyl sulfoxide (DMSO). The liquid from this solution was then separated by centrifugation, coated with paraffin wax, and kept at 4° C for later use.
Agar well diffusion method
To test for antibacterial activity, Muller-Hinton agar plates were prepared. On the appropriate media, 0.1 mL of freshly made, 18-hour-old broth culture was spread. Using a sterile cork borer, 6 mm-diameter wells were prepared in the middle of the plate after the culture had been spread out. With the use of sterile forceps, the wells were opened. Using a micropipette in each well, 100 mL of the initial stock solution was added. 1 mL was the final concentration in the well diffusion method. The prepared plates were kept at room temperature for 30 minutes and then incubated set at 37 °C for 24 hours to provide the TTO/RMO loaded microemulsion time to diffuse.
Results and Discussion
Characterization of Microemulsions
Optical microscopy
The optical microscopy of the formulation was optimized at different concentrations of Smix to determine the optical microscopy of the solution in a sterile and clear container with good lighting to prevent light scattering into the eyes and observed against a black and white reflection dark background 24. The formation of globules at 1:1 concentration of Smix for spherical globules with well-defined boundaries.
 |
Plate 1: Optical microscopy picture of ready microemulsion at various concentrations of surfactant and co-surfactant: |
Determination of pH
A pH meter with a glass electrode was used to measure pH. The value of hydrogen ion occupation in the sample solution is fundamentally represented by pH. The equation written down below defines it. This esteem was very close to the log of the reciprocal of the concentration of H+(hydrogen) ions in dilute solutions. The pH value for rosemary oil-loaded microemulsion was 4.69±0.03 and TTO oil-loaded microemulsion was 5.59±0.04.
Determination of viscosity
A viscometer called Brookfield viscometer was required in this procedure to determine the viscosity of a fluid. Viscosity is a measure of liquid resistance, which can be thought of as the inner friction caused by moving one layer of fluid over another. Also, determine the ratio of shearing stress to shear rate. The resulting viscosity was determined to be 1.844 0.010 for rosemary oil-loaded microemulsion and 1.993 0.019 for TTO oil-loaded microemulsion.
Determination of mechanical stress
The physical and chemical properties related to the stability of microemulsions containing essential oils were assessed using a mechanical stress study and phase separation.
The various microemulsion formulations (TTO and RMO ME) were centrifuged at 2000 rpm for varying time intervals (10,30, and 60 min) and the quantity of two-phase partition of the formulations was recorded 25. The Phase separation of TTO/ RMO loaded microemulsion formulation on 30min time interval was found to be 8.34±0.23 and 6.18±0.20 respectively.
Table 3: Physical characteristics of microemulsion loaded with Rosemary / Tea tree oil
|
Formulation |
pH |
Viscosity |
Density |
Phase separation (30min) |
Encapsulation efficiency |
|
|
Rosemary oil microemulsion |
4.69±0.03 |
1.844±0.010 |
1.018±0.003 |
6.18±0.20 |
82.6± 0.1 |
|
|
Tea tree oil microemulsion |
5.59±0.04 |
1.993±0.019 |
1.011±0.002 |
8.34±0.23 |
84.8± 0.2 |
|
Determination of particle size
Measurement of size distribution by the instrument particle size analyzer
The essential oil microemulsion formulation was subjected to the laser particle counting technique. At this stage, the sample was placed into the chamber used for regulating and delivering samples. After that, a suitable solvent dimethyl sulfoxide (DMSO) was pumped into chamber 26.
Measurement of zeta potential
All particles in the transmission share the same physical characteristic called the zeta potential. It is advantageous for the creation of emulsion and suspension formulations. For stable and continuous formulations, the zeta potential range is often recorded to be between -30 and +30 mV. For a stable microemulsion formulation, particles with a zeta potential greater than -30 or +30 mV are typically considered 27.
Determination droplet size distribution
Gujarat’s sophisticated instrumentation center (SICART), which is located in India, evaluated the zeta potential and particle size distribution (ZS-90, Worcestershire, UK version 7). They can be identified by evaluating the zeta potential average, PDI value, and size distribution assessment with the DLS instrument. TTO essential oil loaded microemulsion droplet size distribution on peaks 1(mean 265.9, width 66.8 nm) Peak 2, (mean 69.5, width 9.38 nm), and peak 3 (mean 1290 width 457.8 nm, respectively), and RMO loaded microemulsion droplet size distribution on peaks 1, (mean 810.9, width 178.9 nm) peak 2 (mean 58.9, width 6.08 nm) and peak 3 (mean 75.4, width 19.8nm respectively).
Table 4: The resultants Value of PDI, zeta potential, and droplet size distribution
|
Essential oil Loaded formulation |
Polydispersity Index (PDI) |
Zeta potential |
Distribution of droplet size |
||
|
Particle diameter 1(nm) |
Particle diameter 2(nm) |
Particle diameter 3(nm) |
|||
|
Rosemary oil microemulsion |
0.678 |
883.6 |
810.9(mean) 178.8(width) 94(% intensity) Droplet size (810.9± 178.9nm) |
58.9(mean) 6.08(width) 9.8(% intensity) Droplet size (58.9± 6.08nm) |
75.4(mean) 19.8(width) 22(% intensity) Droplet size (75.4± 19.8nm) |
|
Tea tree oil microemulsion |
0.987 |
746.8 |
265.9(mean) 66.8(width) 56(% intensity) Droplet size (265.9± 66.8nm) |
69.5(mean) 9.38(width) 23(% intensity) Droplet size (69.5± 9.38nm) |
1290(mean) 457.8(width) 28.5(% intensity) Droplet size (1290± 457.8nm) |
Table 5: Zeta potential analysis of microemulsion loaded with Rosemary / Tea tree oil.
|
Formulation |
Dispersant |
Zeta potential |
Stability |
|
Rosemary oil microemulsion |
Water |
-0.47.8 |
Good |
|
Tea tree oil microemulsion |
Water |
-0.48.6 |
Good |
Antimicrobial activity of EO-loaded microemulsion formulation:
The development of new antibacterial and antifungal formulations has recently received more attention. The evaluation of antibacterial activity and transmission techniques have thus received increased attention. The agar broth dilution method and disc diffusion method are widely used in in-vitro antimicrobial studies. The minimum inhibitory concentration is the very low concentration of antibacterial and antifungal agents (antimicrobial agent) that entirely inhibits the growth of microorganism in the micro-well diffusion method 28.
Antimicrobial activity of TTO-loaded microemulsion:
The effects of a 6.0 % EO-loaded microemulsion formulation on various parameters were measured for gram-negative (-) bacteria Escherichia coli and gram-positive (+) bacteria Staphylococcus aureus, as well as Candida albicans29. When treated cells are detached from the existence of TTO microemulsion and subcultured in a nutrient medium, cell viability counts reveal the extent to which they can survive and reproduce to form colonies. During the 60-minute experiment, E. coli and Candida albicans cultures were more susceptible to tea tree oil than S.aureus. Exposure to microemulsion significantly reduced respiratory oxygen consumption in cultures of all three organisms. The enzymes and cofactors that are unswervingly involved in the respirations ETC (Electron transport chain) span bactericidal cytoplasmic outer cell membrane as well as cytoplasm and plasma cell membrane. As a result, tea tree oil’s inhibitory effects are unswerving with the partitioning of its monoterpene chemical components into the outer cell membrane 30.
The propidium iodide (PI) is increased and outflow of 280nm fascinating resources in all three suggested microorganisms that the decline in viability and decrease the respiration rate was accompanied by improved microorganism cell membrane permeability 12,31. As a result, even negligible changes in the capacity of cell membrane structure can damage metabolism and show the way to cell death 32,34.
The antibacterial activity of a 6% concentration of tea tree oil-laded microemulsion was assessed against both gram-positive and gram-negative bacteria following the findings on cultured nutrient agar plates. E. coli (17mm) and Candida albicans (14mm) showed greater zones of inhibition when exposed to TTO-loaded microemulsion, while S. aureus (11mm) showed less susceptibility.
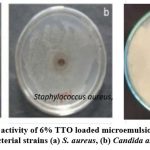 |
Plate 2(a): Antimicrobial activity of 6% TTO loaded microemulsion by agar well diffusion method on different bacterial strains (a) S. aureus, (b) Candida albicans, and (c) E. coli. |
Table 6(A): Antimicrobial activity of TTO-loaded microemulsion
|
Bacteria |
Physiological parameters of microorganisms |
||||
|
Bacteria Reduction in cell viability [%] |
Bacteria increase in Propidium iodide (PI) staining [%] |
Bacteria leakage 280 nm absorbing material [%] |
Bacteria Potassium ion effect [%] |
Bacteria decrease the respiration rate [%] |
|
|
Escheriahia Coli |
89.0 |
84.0 |
17.0 |
81.0 |
80.0 |
|
Staphylococcus Aureus |
27.0 |
12.0 |
14.0 |
18.0 |
26.0 |
|
Candida Albicans |
73.0 |
60.0 |
10.0 |
16.0 |
68.0 |
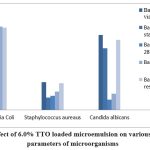 |
Figure 2(a): Effect of 6.0% TTO loaded microemulsion on various Physiological parameters of microorganisms |
Antimicrobial activity of RMO-loaded microemulsion
The antimicrobial activity of rosemary essential oil-loaded microemulsion has been determined in various assay types based on bacteria reduction in cell viability and inhibition of respiration in various gram-negative and gram-positive bacteria. This study found to be that both essential oil-loaded microemulsions inhibited microbial growth. Disc diffusion was used to test antibiotic susceptibility4. Both essential oils tested were found to be active against all clinical isolates of E. coli, Staphylococcus aureus, and Candida albicans 35. On all physiological parameters of the microorganism, the minimal inhibitory concentration for rosemary essential oil-loaded microemulsion 6 % concentration was effective against E. coli. At a 6% oil concentration, the formulation is also effective on Candida albicans and Staphylococcus aureus in all physiological parameters of gram-positive and gram-negative strains 36.
According to a cultivated nutrient agar plate, the antibacterial activity of a 6% tea tree oil-laded microemulsion was assessed against both gram-positive and gram-negative bacteria. E. coli (19mm) and Candida albicans (15mm) showed greater zones of inhibition in the TTO-loaded microemulsion, but S. aureus (12mm) showed decreased susceptibility to the RMO microemulsion.
 |
Plate 2(b): Antimicrobial activity of 6% TTO loaded microemulsion by agar well diffusion method on different bacterial strains (a) S. aureus, (b) Candida albicans, and (c) E. coli. |
Table 6 (B): Antimicrobial activity of RMO-loaded microemulsion
|
Bacteria |
Physiological parameters of microorganisms |
||||
|
Bacteria Reduction in cell viability [%] |
Bacteria ↑ in propidium iodide staining [%] |
Bacteria leakage at 280nm absorbing material [%] |
Bacteria Potassium ion effects [%] |
Bacteria Decrease the rate of respiration [%] |
|
|
Escheriahia Coli |
83.0 |
79.0 |
12.0 |
80.0 |
75.0 |
|
Staphylococcus aureus |
29.0 |
10.0 |
11.0 |
15.0 |
21.0 |
|
Candida albicans |
69.0 |
55.0 |
8.0 |
14.0 |
61.0 |
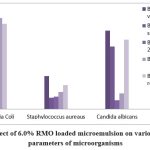 |
Figure 2(b): Effect of 6.0% RMO loaded microemulsion on various Physiological parameters of microorganisms. |
Conclusion
The ability of tea tree oil and rosemary oil-loaded microemulsion to inhibit respiration rate and increase outer cell membrane permeability in microorganism cells indicates that its lethal processes are principally the results shows that the inhibition rate of outer membrane-located metabolic pathways. The ability of a tea tree oil and rosemary oil-loaded microemulsion to decrease the rate of respiration and increase the permeability of the outer cell membrane in microorganism cells indicates that the primary causes of its lethal processes are inhibition of the metabolic processes that take place in the outer membrane and a loss of chemiosmotic control. The different susceptibilities of Staphylococcus aureus, Candida albicans, and E. coli can be attributed to variances in the amount of cell membrane damage caused by monoterpenes. This research study reveals that both kinds of essential oils are utilized in microemulsion systems and show their therapeutic benefits and potential for usage as medication delivery systems. The result indicated that using TTO/RMO had a beneficial effect in controlling the pathogenic microbial organisms and thus can be used in therapeutic formulation in near future.
Acknowledgment
The authors acknowledge the Vice-Chancellor, IFTM University, Moradabad, for providing continuous support & necessary facilities for this research work.
Conflict of interests
The authors declare that they have no conflict of interest.
Funding Sources
There was no particular grant for this assessment from any funding source in the public, commercial, or non-profit sectors.
References
- Cox S., Mann C Markham., J Gustafson., J Warmington, J Wyllie, S. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001; 6 (12), 87–91. https://doi.org/10.3390/60100087.
CrossRef - Bassett, I. B., Barnetson, R. S. C., Pannowitz, D. L. A Comparative Study of Tea-Tree Oil versus Benzoylperoxide in the Treatment of Acne. J. Aust. 1990, 153 (8), 455–458. https://doi.org/10.5694/j.1326-5377.1990.tb126150.x.
CrossRef - Liu, M., Gao, Q., Sun, C., Liu, B., Liu, X., Zhou, Q., Zheng, X., Xu, P., Liu, B. Effects of Dietary Tea Tree Oil on the Growth, Physiological and Non-Specific Immunity Response in the Giant Freshwater Prawn (Macrobrachium Rosenbergii) under High Ammonia Stress. Fish Shellfish Immunol. 2021, S1050464821004411.
CrossRef - Amenu, D. Antimicrobial Activity of Medicinal Plant Extracts and Their Synergistic Effect on Some Selected Pathogens. J. Ethnomedicine. 2019, 134 (7), 459-461.
- Dávila-Rodríguez, M., López-Malo, A., Palou, E., Ramírez-Corona, N., Jiménez-Munguía, M. T. Antimicrobial Activity of Nanoemulsions of Cinnamon, Rosemary, and Oregano Essential Oils on resh Celery. LWT 2019, 112, 108247.
CrossRef - Stojiljkovic, J. Antibacterial Activities of Rosemary Essential Oils and Their Components against Pathogenic Bacteria. Cytol. Pathol. 2018, 3 (4). https://doi.org/10.15406/acp.2018.03.00060.
CrossRef - Abd Rashed, A., Rathi, D.-N. G., Ahmad Nasir, N. A. H., Abd Rahman, A. Z. Antifungal Properties of Essential Oils and Their Compounds for Application in Skin Fungal Infections: Conventional and Nonconventional Approaches. Molecules 2021, 26 (4), 1093. https://doi.org/10.3390/molecules26041093.
CrossRef - Rašković, A., Milanović, I., Pavlović, N., Ćebović, T., Vukmirović, S., Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus Officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14 (1), 225. https://doi.org/10.1186/1472-6882-14-225.
CrossRef - Shapiro, S., Meier, A., Guggenheim, B. The Antimicrobial Activity of Essential Oils and Essential Oil Components towards Oral Bacteria. Oral Microbiol. Immunol. 1994, 9 (4), 202–208. https://doi.org/10.1111/j.1399-302X.1994.tb00059.x.
CrossRef - Tartaro, G., Mateos, H., Schirone, D., Angelico, R., Palazzo, G. Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials 2020, 10 (9), 1657. https://doi.org/10.3390/nano10091657.
CrossRef - Adamson, A. W. A Model for Micellar Emulsions. Colloid Interface Sci. 1969, 29 (2), 261–267. https://doi.org/10.1016/0021-9797(69)90195-7.
CrossRef - Thakur, D., Kaur, G., Puri, A., Nanda, R. Therapeutic Potential of Essential Oil-Based Microemulsions: Reviewing State-of-the-Art. Drug Deliv. 2021, 18 (9), 1218–1233. https://doi.org/10.2174/1567201818666210217161240.
CrossRef - Mohyaldinn, M., M. Hassan, A., A. Ayoub, M. Application of Emulsions and Microemulsions in Enhanced Oil Recovery and Well Stimulation. In Microemulsion – a Chemical Nanoreactor IntechOpen, 2019. https://doi.org/10.5772/intechopen.84538.
CrossRef - Jadhav, K., Shaikh, I., Ambade, K., Kadam, V. Applications of Microemulsion Based Drug Delivery System. Drug Deliv. 2006, 3 (3), 267–273. https://doi.org/10.2174/156720106777731118.
CrossRef - Francisconi, R. S., Huacho, P. M. M., Tonon, C. C., Bordini, E. A. F., Correia, M. F., Sardi, J. de C. O., Spolidorio, D. M. P. Antibiofilm Efficacy of Tea Tree Oil and of Its Main Component Terpinen-4-Ol against Candida Albicans. Oral Res. 2020, 34, e050. https://doi.org/10.1590/1807-3107bor-2020.vol34.0050.
CrossRef - Mehta, S. K., Kaur, G. Microemulsions: Thermodynamic and Dynamic Properties. In Thermodynamics; Tadashi, M., Ed.; InTech, 2011. https://doi.org/10.5772/12954.
CrossRef - Demo, M., Oliva, M. de las M., López, M. L., Zunino, M. P., Zygadlo, J. A. Antimicrobial Activity of Essential Oils Obtained from Aromatic Plants of Argentina. Biol. 2005, 43 (2), 129–134. https://doi.org/10.1080/13880200590919438.
CrossRef - Boonme, P. Applications of Microemulsions in Cosmetics: Applications of Microemulsions in Cosmetics. Cosmet. Dermatol. 2007, 6 (4), 223–228. https://doi.org/10.1111/j.1473-2165.2007.00337.x.
CrossRef - Safaei-Ghomi, J., Ahd, A. Antimicrobial and Antifungal Properties of the Essential Oil and Methanol Extracts of Eucalyptus Largiflorens and Eucalyptus Intertexta. Mag. 2010, 6 (23), 172. https://doi.org/10.4103/0973-1296.66930.
CrossRef - Gupta, S., Sanyal, S., Datta, S., Moulik, S. Preparation of Prospective Plant Oil Derived Micro-Emulsion Vehicles for Drug Delivery. Indian J. Biochem. Biophys. 2006, 43, 254–257.
- Carson, C. F., Hammer, K. A., Riley, T. V. Melaleuca Alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Microbiol. Rev. 2006, 19 (1), 50–62. https://doi.org/10.1128/CMR.19.1.50-62.2006.
CrossRef - Kawakami, K., Yoshikawa, T., Hayashi, T., Nishihara, Y., Masuda, K. Microemulsion Formulation for Enhanced Absorption of Poorly Soluble Drugs. Controlled Release 2002, 81 (1–2), 75–82. https://doi.org/10.1016/S0168-3659(02)00050-0.
CrossRef - Muzaffar, F.; Singh, U. K.; Chauhan, L. Review on microemulsion as futuristic drug delivery. 2009, 5 (3), 14.
- Yang, J., Xu, H., Wu, S., Ju, B., Zhu, D., Yan, Y., Wang, M., Hu, J. Preparation and Evaluation of Microemulsion-Based Transdermal Delivery of Cistanche Tubulosa Phenylethanoid Glycosides. Med. Rep. 2017, 15 (3), 1109–1116. https://doi.org/10.3892/mmr.2017.6147.
CrossRef - Callender, S. P., Mathews, J. A., Kobernyk, K., Wettig, S. D. Microemulsion Utility in Pharmaceuticals: Implications for Multi-Drug Delivery. J. Pharm. 2017, 526 (1–2), 425–442. https://doi.org/10.1016/j.ijpharm.2017.05.005.
CrossRef - Yadav, V., Jadhav, P., Kanase, K., Bodhe, A., Dombe, S. Preparation and evaluation of microemulsion containing antihypertensive drug. J. Appl. Pharm. 2018, 10 (5), 138. https://doi.org/10.22159/ijap.2018v10i5.27415.
CrossRef - Das, S., Lee, S. H., Chow, P. S.; Macbeath, C. Microemulsion Composed of Combination of Skin Beneficial Oils as Vehicle: Development of Resveratrol-Loaded Microemulsion Based Formulations for Skin Care Applications. Colloids Surf. B Biointerfaces 2020, 194, 111161. https://doi.org/10.1016/j.colsurfb.2020.111161.
CrossRef - Hammer, K. A. In Vitro Activity of Melaleuca Alternifolia (Tea Tree) Oil against Dermatophytes and Other Filamentous Fungi. Antimicrob. Chemother. 2002, 50 (2), 195–199. https://doi.org/10.1093/jac/dkf112.
CrossRef - D’auria, F. D., Laino, L., Strippoli, V., Tecca, M., Salvatore, G., Battinelli, L., Mazzanti, G. In Vitro Activity of Tea Tree Oil Against Candida Albicans Mycelial Conversion and Other Pathogenic Fungi. Chemother. 2001, 13 (4), 377–383. https://doi.org/10.1179/joc.2001.13.4.377.
CrossRef - Hammer, K. A., Carson, C. F., Riley, T. V. Susceptibility of Transient and Commensal Skin Flora to the Essential Oil of Melaleuca Alternifolia (Tea Tree Oil). J. Infect. Control 1996, 24 (3), 186–189. https://doi.org/10.1016/S0196-6553(96)90011-5.
CrossRef - Moreno, S., Scheyer, T., Romano, C. S., Vojnov, A. A. Antioxidant and Antimicrobial Activities of Rosemary Extracts Linked to Their Polyphenol Composition. Free Radic. Res. 2006, 40 (2), 223–231. https://doi.org/10.1080/10715760500473834.
CrossRef - Pazyar, N., Yaghoobi, R., Bagherani, N., Kazerouni, A. A Review of Applications of Tea Tree Oil in Dermatology. J. Dermatol. 2013, 52 (7), 784–790. https://doi.org/10.1111/j.1365-4632.2012.05654.x.
CrossRef - Farag, R. S., Daw, Z. Y., Hewedi, F. M., El-Baroty, G. S. A. Antimicrobial Activity of Some Egyptian Spice Essential Oils. Food Prot. 1989, 52 (9), 665–667. https://doi.org/10.4315/0362-028X-52.9.665.
CrossRef - Tomczykowa, M., Tomczyk, M., Jakoniuk, P., Tryniszewska, E. Antimicrobial and Antifungal Activities of the Extracts and Essential Oils of Bidens Tripartita. Folia Histochem. Cytobiol. 2008, 46 (3), 389–393. https://doi.org/10.2478/v10042-008-0082-8.
CrossRef - M, C., J, P., Cf, C., R, H., Tv, R. Tea Tree Oil as an Alternative Topical Decolonization Agent for Methicillin-Resistant Staphylococcus Aureus. Hosp. Infect. 2000, 46 (3). https://doi.org/10.1053/jhin.2000.0830.
CrossRef - Vuuren, S. F. V., Suliman, S., Viljoen, A. M. The Antimicrobial Activity of Four Commercial Essential Oils in Combination with Conventional Antimicrobials. Appl. Microbiol. 2009, 48 (4), 440–446. https://doi.org/10.1111/j.1472-765X.2008.02548.x.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





