Manuscript accepted on : 16-05-2023
Published online on: 20-06-2023
Plagiarism Check: Yes
Reviewed by: Dr. Francisco Solano
Second Review by: Dr. Nagham Mahmood Aljamali
Final Approval by: Dr. Eugene A. Silow
P. P. Sethumathi1 , V. V. Sathibabu Uddandrao1
, V. V. Sathibabu Uddandrao1 , P. Chandrasekaran1
, P. Chandrasekaran1 , S. Sengottuvelu2
, S. Sengottuvelu2 , P. Tamilmani3,1
, P. Tamilmani3,1 , P. Ponmurugan4
, P. Ponmurugan4 , S. Vadivukkarasi1
, S. Vadivukkarasi1 , M. Santhanakumar5
, M. Santhanakumar5 , M. Shabana Begum6
, M. Shabana Begum6 and G. Saravanan1*
and G. Saravanan1*
1Centre for Biological Sciences, Department of Biochemistry, K.S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode, Namakkal District, Tamilnadu, India.
2Department of Pharmacology, Nandha College of Pharmacy, Erode, Tamilnadu, India.
3Department of Biochemistry, PGP College of arts and science, Namakkal, Tamilnadu, India.
4Department of Botany, Bharathiar University, Coimbatore, Tamilnadu, India.
5Department of Pharmacology, Arulmigu Kalasalingam College of Pharmacy, Krishnankoil, Tamilnadu, India.
6Department of Biochemistry, Muthayammal College of Arts and Science, Rasipuram, Tamilnadu, India.
Corresponding Author E-mail: sarabioc@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3105
ABSTRACT: The current study is designed to evaluate the cardiorenal protective efficacy of the Biochanin-A (BCA) against high-fat-diet (HFD) and streptozotocin (STZ)-induced diabetes in rats. BCA (10mg/kg body weight) was administered to the diabetic rats for a period of 30 days and evaluated its effect on hyperglycemic markers, formation of lipid peroxidation, nitric oxide production and antioxidant enzymes such as superoxide dismutase, catalase and glutathione mediated enzymes. Further, we assessed the impact of BCA on nuclear factor erythroid 2-related factor-2 (Nrf-2) and heme oxygenase-1 (HO-1) along with antioxidant enzymes mRNA expressions by RT-PCR. BCA administration to diabetic rats resulted in attenuation of hyperglycemia and oxidative stress in both the kidney and heart. Further, BCA enhanced the endogenous antioxidant activities in the kidney and heart and up-regulated their corresponding mRNA expressions. In addition, BCA treatment produced notable up-regulation of Nrf-2 and HO-1 mRNA expressions in the cardiac and renal tissue of diabetic rats. In conclusion, the current study revealed that BCA could protect from diabetes-induced complications such as cardiomyopathy and nephropathy by activating the Nrf-2/HO-1 pathway and enhancing the endogenous antioxidant state in the kidney and heart.
KEYWORDS: Cardiorenal Damage; Diabetes mellitus; Nrf-2/HO-1 pathway; Nutraceuticals
Download this article as:| Copy the following to cite this article: Sethumathi P. P, Uddandrao V. V. S, Chandrasekaran P, Sengottuvelu S, Tamilmani P, Ponmurugan P, Vadivukkarasi S, Santhanakumar M, Begum M. S, Saravanan G. Biochanin-A Protects Rats from Diabetes-associated Cardiorenal Damage by Attenuating Oxidative Stress through Activation of Nrf-2/HO-1 Pathway. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Sethumathi P. P, Uddandrao V. V. S, Chandrasekaran P, Sengottuvelu S, Tamilmani P, Ponmurugan P, Vadivukkarasi S, Santhanakumar M, Begum M. S, Saravanan G. Biochanin-A Protects Rats from Diabetes-associated Cardiorenal Damage by Attenuating Oxidative Stress through Activation of Nrf-2/HO-1 Pathway. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/46dYUqH |
Introduction
Diabetes mellitus (DM) is a group of metabolic illnesses characterised by chronic hyperglycemia brought on by impaired insulin production, activity, or both. Constant hyperglycemia in DM is come with by damage, dysfunction, and malfunction of various organs and tissues, the progress of micro- (retinopathy, nephropathy, and neuropathy) (Uddandrao et al., 2023) and macro vascular (cardiovascular disorders) complications.1 DM is becoming more prevalent everywhere, with low- and middle-income nations describing the fastest growth. Type 2 diabetes (T2DM) leads to end-stage renal failure (ESRD) and significantly increases the threat of cardiovascular complications in the majority of developed countries.2 The primary mechanisms of altered glucose metabolism, oxidative damage to pancreatic beta-cells, and endothelial dysfunction are identified as the origins of elevated reactive oxygen species (ROS) formation in diabetes. Increased ROS production causes oxidative stress, which accelerates the deterioration of biologically important components including lipids, proteins, and DNA. By reducing the detrimental effects of ROS, the antioxidant defence system offers vital defence for the biological system.3
According to research results and data from experimental animal models of DM, nuclear factor erythroid 2-related factor-2 (Nrf-2) adjusts to altering oxidative and inflammatory stress by attempting to enhance both its function and expression levels. This adaptation aims to mitigate the early glucolipotoxicity effects.4 Similar findings were made by Uddandrao et al4, and they also put forth the theory that the up-regulation and activation of Nrf-2 during the premature phase of cardiac damage represents an effort by naturally occurring antioxidant mechanisms to stop the development of diabetic cardiomyopathy. The defensive systems, however, become overloaded with an excess of ROS, and the kidney damage advances to the advanced stages of ESRD.5 On the other hand, heme oxygenase-1 (HO-1), the inducible variety of HO, mostly exerts a cytoprotective impact in inflammatory situations. HO-1 can shield the cells from inflammatory damage by decreasing the production of inflammatory cytokines.6 As a result; targeting the Nrf-2/HO-1 pathway might be a possible therapeutic target to mitigate the oxidative stress caused by DM in several organs.
With no or less adverse effects, phytomedicines have long been established for their potential anti-diabetic, anti-hyperlipidemic, and antioxidant properties.7 The biochanin-A (BCA) is an O-methylated isoflavonoid, which is found in a wide range of plants including soy, peanuts, chickpeas, alfalfa sprouts, etc., has also been strongly connected to a number of biological and pharmacological effects.8,9 Additionally, BCA has the capacity to mitigate the abnormalities in the glucose metabolism of a number of vital organs (muscle, liver, and kidney) brought on by a high-fat-diet (HFD) and streptozotocin (STZ)-induced hyperglycemia, as demonstrated by our earlier work.10 The capability of BCA to protect against the cardiorenal damage induced by HFD-STZ, however, was not supported by any conclusive scientific evidence. Therefore, the rationale of this research is to examine if BCA may prevent cardiorenal damage from DM by reducing oxidative stress and its effects on the Nrf-2/HO-1 pathway.
Materials and methods
Chemicals
BCA was commercially purchased (Sigma Chemical Company, India) and other reagents used for this study were of analytical grade.
HFD Preparation
According to the procedures outlined by Uddandrao et al11 and Eraniappan et al12, HFD was prepared with the composition as depicted in table 1.
Table 1: Composition of normal and HFD diets (each ingredient in grams per kg diet).
|
Ingredients |
Normal diet |
HFD |
|
Corn Starch |
615 |
150 |
|
Sugar (Sucrose) |
0 |
150 |
|
Cellulose |
50 |
50 |
|
Corn oil |
80 |
0 |
|
Lard oil |
0 |
400 |
|
Casein |
200 |
195 |
|
Vitamin-mineral premix |
50 |
50 |
|
DL-Methionine |
3 |
3 |
|
Choline bitartrate |
2 |
2 |
Animals
We acquired 24 male Sprague-Dawley rats (body weight: 180-200g) from the Arulmigu Kalasalingam College of Pharmacy in Virudhunagar, India, and acclimatized them in standard lab settings (temperature: 22.2°C; moisture: 40-60%). The Arulmigu Kalasalingam College of Pharmacy’s Institute Animal Ethical Committee in Virudhunagar, Tamil Nadu, India (Approval number: AKCP/IAEC/83/20-21) approved the protocol of this study and permitted to do animal experiments.
Development of the T2DM rat model
The rats were given HFD for two weeks before receiving STZ (35 mg/kg i.p.) to produce the T2DM rat model. A standard diet was given to the rats used as controls. The rats included in the test had their blood glucose levels measured seven days after receiving an injection of STZ. The rats were selected for additional testing because it was thought that they would become diabetic if their blood glucose level rose beyond 200mg/dl after seven days of STZ administration. BCA therapy was started as soon as diabetes was confirmed and treatment continued for 30 days.
Experimental Design
The four groups into which the rats were segregated were as follows:
Group 1: Rats with normal diet and without STZ (control)
Group 2: Rats with HFD + STZ (diabetic)
Group 3: Diabetic + BCA (10mg/kg body weight).10
Group 4: Diabetic + gliclazide (5mg/kg body weight).10
All relevant drugs were orally administered for 30 days within a vehicle solution (normal saline) using an intragastric tube. Rats were anaesthetized intraperitoneally with pentobarbital sodium (40mg/kg/body weight) and blood was withdrawn using the retroorbital sinus puncture technique at the termination of the treatment phase. The heart and kidney were immediately removed and preserved at -80°C for subsequent utilization when the rats were sacrificed via cervical decapitation.
Assessment of glucose, insulin and glycated haemoglobin levels
The impact of BCA on hyperglycaemic markers namely, blood glucose, insulin and glycated haemoglobin was assessed by using the commercially available kits (Stan Bio Laboratory Kits, USA).
Estimation of renal and cardiac damage diagnostic biomarkers
A Roche automated biochemical analyzer was used to assess urinary N-acetyl-b-d-glucosaminidase (NAG), and urinary albumin to creatinine ratio (UACR), which is determined as urine albumin (mg) divided by urinary creatinine (g), was also measured. Following the manufacturer’s instructions, urine albumin, neutrophil gelatinase-associated lipocalin (NGAL), and transforming growth factor-β1 (TGF-β1) levels were tested using the appropriate ELISA kits. An automated biochemical analyzer was used to measure the amounts of plasma cardiac indicators such as lactate dehydrogenase (LDH), homocysteine, and creatine kinase-MB isoenzyme (CK-MB).
Renal and Cardiac oxidative stress markers
The concentration of NOx was measured using an enzymatic conversion method followed by reaction with a Griess reagent13, Malondialdehyde (MDA) level, the index of lipid peroxidation following the method of Buege and Aust14 and reduced glutathione (GSH) and oxidized glutathione (GSSG) was measured using colourimetric kits (G-Biosciences, USA, Cat. #786-075, 786-076) and following the manufacturer’s instructions.
Determination of renal and cardiac antioxidant status
The renal and cardiac antioxidant markers were determined by evaluating the level of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and Glutathione-S-transferase (GST) were described by Saravanan and Ponmurugan earlier.15
Measurement of Cardiac and Renal, Nrf-2 and HO-1
Nrf-2 and HO-1 levels in both cardiac renal tissues were assessed using ELISA kits as directed by the manufacturer (ElabscienceÒ, USA).
RT-PCR analysis
With the assistance of Trizol reagent (Invitrogen, Carlsbad, CA, USA), total RNA was extracted from the renal and cardiac tissue of control and experimental rats. This RNA was then reverse transcribed to produce cDNA using a DNA synthesis kit (RevertAid First Strand cDNA Synthesis Kit, Thermo ScientificTM, India). SOD, CAT, GPx, Nrf-2, and HO-1 were among the particular primers (Table 2) used in semi-quantitative PCR on 20ng of cDNA. The appropriate primers were used in 38 cycles of PCR amplification at the following cycling temperatures: 30 sec of denaturation at 94°C, 30 sec of annealing at 59°C, and 1 min of extension at 72°C. For standardization, the housekeeping gene β-actin was employed.
Table 2: Primers sequence
|
Primer |
Sequence |
|
SOD |
F: 5′-CATTCCATCATTGGCCGTACT-3′ R: 5′-CCACCTTTGCCCAAGTCATC-3′ |
|
CAT |
F: 5′-GTACAGGCCGGCTCTCACA-3′ R: 5′-ACCCGTGCTTTACAGGTTAGCT-3′ |
|
GPx |
F: 5′-GCGCTGGTCTCGTCCATT-3′ R: 5′-TGGTGAAACCGCCTTTCTTT-3′ |
|
Nrf-2 |
F 5′-GAGAGCCCAGTCTTCATTGC-3′ R 5′-TGCTCAATGTCCTGTTGCAT-3′ |
|
HO-1 |
F 5′-TTCTACCTGTTCGAGCATGTGG-3′ R 5′-TGTTAGCATGGAGCCAGCCT-3′ |
|
β-actin |
F 5′-CCTGCTTGCTGATCCACA-3′ R 5′CTGACCGAGCGTGGCTAC-3′ |
Statistical Analysis
SPSS (version 10.0) software was used for the statistical analysis. Using the mean and SD, the data were expressed. To evaluate the significance of intergroup differences, the one-way analysis of variance (ANOVA) and least significant difference (LSD) tests were applied. Significance level at p<0.05 was considered to indicate statistical significance.
Results
BCA attenuated hyperglycemia
The influence of BCA on hyperglycemic biomarkers in control and experimental diabetic rats is publicized in figure 1. In diabetic rats compared to control rats, there was a notable (P<0.05) rise in blood glucose and glycated hemoglobin and a concurrent decrease in insulin levels. However, the administration of BCA considerably (P<0.05) reverted glucose (Fig. 1A) and insulin levels (Fig. 1B) to a state that was close to normal and thereby avoiding the formation of glycated hemoglobin (Fig. 1C).
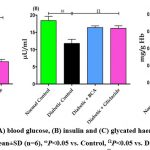 |
Figure 1: Effect of BCA on (A) blood glucose, (B) insulin and (C) glycated haemoglobin, The data were stated as mean±SD (n=6), ωP<0.05 vs. Control, ΩP<0.05 vs. Diabetic. |
BCA mitigated the DM-associated renal and cardiac damage
The impact of BCA on diagnostic indices of renal injury in control and experimentally diabetic rats is depicted in figure 2. The urine albumin excretion (Fig. 2A) and UACR (Fig. 2B), coupled with elevated levels of NAG (Fig. 2C), NGAL (Fig. 2D), and TGF-1 (Fig. 2E) were identified in the diabetic control rats, who demonstrated a substantial (P<0.05) tubular damage in the kidneys. However, as compared to untreated diabetic control rats, treatment with BCA produced notable (P<0.05) protection from renal damage, which was supported by a marked decrease in the levels of tubular injury markers.
Furthermore, CK-MB (Fig. 2F), LDH (Fig. 2G), and homocysteine (Fig. 2H), which are important markers of cardiac injury, all showed a momentous (P<0.05) increase in the diabetic control rats. Nevertheless, as compared to the diabetic control group of rats, the BCA therapy considerably (P<0.05) decreased the levels of CK-MB, LDH, and homocysteine.
BCA mitigated renal and cardiac oxidative stress
In diabetic control rat kidney and heart, supplementing with HFD and administering STZ caused an increase in MDA (Fig. 3A), NOx (Fig. 3B) and GSSG (Fig. 3C) and a concurrent reduction in the GSH content (Fig. 3D), which is evidence of oxidative stress in these organs. In addition, BCA treatment notably (P<0.05) augmented the GSH concentration in these organs, which in turn reduction in GSSG levels and reduced the formation of MDA and NOx in the kidney and heart and protected them from oxidative stress.
The antioxidant state in both the kidney and heart was enhanced by BCA treatment
When diabetic control rats were compared to normal control rats, there was a substantial (P<0.05) decline in the activity of antioxidant enzymes such as SOD (Fig. 4A), CAT (Fig. 4B), GPX (Fig. 4C), GR (Fig. 4D), and GST (Fig. 4E) in both renal and cardiac tissue. However, BCA administration improved the activity of these enzymes in the kidney and the heart.
Effect of BCA on cardiac and renal Nrf-2 and HO-1
In diabetic rats, the levels of Nrf-2 (Fig. 5A) and HO-1 (Fig. 5B) were substantially (P<0.05) lower than those in the normal control group in the cardiac and renal tissue. Additionally, Nrf-2 and HO-1 levels in the renal and cardiac tissues of diabetic rats with BCA treatment were noticeably (P<0.05) increased.
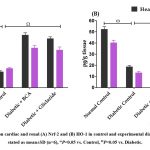 |
Figure 5: Effect of BCA on cardiac and renal (A) Nrf-2 and (B) HO-1 in control and experimental diabetic rats, The data were stated as mean±SD (n=6), ωP<0.05 vs. Control, ΩP<0.05 vs. Diabetic. |
BCA-activated Nrf-2/HO-1 pathway
The potential of BCA on the mRNA expressions of genes that affect antioxidant activity in both control and experimental diabetic rats is presented in figure 6. When compared to normal control rats, diabetic control rats showed a notable (P<0.05) down-regulation of the mRNA expressions of the SOD (Fig. 6A), CAT (Fig. 6B), GPx (Fig. 6C), Nrf-2 (Fig. 6D), and HO-1 (Fig. 6E) genes. As opposed to untreated diabetic rats, treatment of BCA effectively increased the mRNA expression of SOD, CAT, GPx, Nrf-2, and HO-1 in diabetic rats.
Discussion
The current investigation revealed more evident effects of the BCA on diabetic complications such as cardiomyopathy, nephropathy, and oxidative stress in diabetic rats that were generated with HFD and STZ in an animal model (rat) that mimics human DM. It may be applied in human trials when our theory has been validated and all parts of the impacts of our medication have been examined in animal modeling. The study’s main findings were beneficial in reducing diabetic complications in a rat model, and further research suggests promising therapeutic approaches. On these possible options, more study is required. As a result, we might do research that is supported by science and eventually broaden our research to include humans.
High blood sugar levels and decreased insulin production along with efforts to increase glycated hemoglobin cause T2DM. Our findings demonstrate the anti-diabetic potential of BCA by establishing how BCA lowers blood glucose and enhances insulin production. Intensive glucose-lowering strategies have been reported to minimize prognostic indicators of renal complications in people with T2DM, but there is no evidence that these strategies alter the severity of advanced renal issues.16 Accordingly, individuals with T2DM continue to be at a higher risk of mortality and morbidity from cardio renal causes despite improved glucose management and the use of single-agent renin-angiotensin-aldosterone system blockage.17 In the current investigation, we observed substantial cardio renal damage in diabetic rats produced by HFD and STZ, which was supported by enhanced tubular injury indicators and heart damage diagnostic markers, including CK-MB, LDH, and homocysteine. Conversely, BCA administration notably diminished the risk of cardio renal damage in diabetic rats. This suggests that, as we previously reported, BCA may protect the kidney and heart by reducing hyperglycemia and repairing the carbohydrate metabolic enzymes.10
Additionally, mounting data demonstrate that hyperglycemia induces the formation of ROS, which results in oxidative stress.18 The breakdown of delicate oxidant/antioxidant equilibrium may be one proposed molecular mechanism behind experimentally induced DM, which might result in cardiac and/or kidney disease through oxidative damage.5 The results of the present study demonstrated that giving rats HFD plus STZ created an excess of ROS generation, which in turn caused cardio renal oxidative injury. If enough antioxidant molecules are not present to stop the reaction of free radicals formed from ROS with lipids, lipid peroxides can occur which can cause significant damage.5 Since lipids make up a large portion of biological membranes, their oxidation can cause cell death and damage. It appears that the first step in the tissue becoming more vulnerable to oxidative damage is a large rise in the levels of MDA and NOx generation in diabetic rats. The enhanced lipid peroxidation in terms of MDA and NOx in both kidneys and hearts in the current study provides evidence that excessive ROS generation may be the cause of the observed cardio renal damage. Additionally, GSH is crucial for the regulation of a number of cellular activities as well as for defending cells from damage caused by free radicals.5 However, BCA treatment in diabetic rats considerably decreased the levels of MDA and NoX in cardio renal tissue, indicating that the antioxidant capabilities of BCA may be pharmacologically effective in protecting against cardio renal damage. An apparent defensive mechanism to prevent the excessive quenching of intracellular ROS necessary for insulin signaling appears to be the activation of GSH metabolism by the GST enzyme and recycling of GSH by GSSG-reductase. In diabetic rats treated with BCA or gliclazide, our findings clearly demonstrated that the regeneration of GSH from GSSG was greatly enhanced. Our findings, therefore, imply that BCA treatment of diabetic rats induces a GSH-dependent antioxidant adaptive response in cardiac and renal tissue.
The Nrf-2, transcription factor has been shown to be a fundamental transcription factor that controls the induction of phase 2 detoxifying and antioxidant genes that occur with HFD and STZ administration, and it has been acknowledged that this transcription factor possibly will play a part in the underlying biochemical and molecular stages during DM.19 We investigated the function of Nrf-2/HO-1 signaling activation in the cardio renal protective effect of BCA since it is essential for preventing DM-related renal and cardiac complications.20,21 Our results demonstrated that HFD and STZ administration caused down-regulation of Nrf-2 and HO-1 in the heart, which is consistent with previous studies.5,20,21 Additionally, we noticed that the activities of endogenous enzymatic antioxidants such SOD, CAT, GPx, GR, and GST were reduced in the kidney and the heart. An essential cytodefense redox mechanism for handling oxidative stress is the Nrf-2/HO-1 pathway.4 One specific hyperglycemia-induced event in the mitochondria, excessive superoxide generation by the electron-transport chain, has been linked to the activation of the Nrf-2-mediated pathways underpinning the development of diabetic complications.22 Additionally, research has indicated that the lack of Nrf-2 may make both type 1 and T2DM worse.23,24
Under normal circumstances, Nrf-2 is retained by Keap1, a protein that binds to Kelch-like ECHs and prevents Nrf-2 from entering the nucleus. Keap1 undergoes a conformational change after engaging with different inducers, releasing Nrf-2, which subsequently moves to the nucleus and attaches to antioxidant-related elements in the promoter regions of antioxidant and cytoprotective genes.25 In contrast to their indirect capability, which involves protecting against oxidative stress by promoting the production of phase II detoxifying and antioxidant enzymes, antioxidants have the potential to quench free radicals, ROS, and RNS by either giving hydrogen or electrons.5 On the other hand, HO-1 is an Nrf-2 controlled gene that plays a vital role in the deterrence of oxidative stress, inflammation, apoptosis and damage caused by ROS. The role of ROS scavenging activity in cellular homeostasis during cell development and maintenance is essential. Some of the enzymes involved in the removal of these free radical species from cells include SOD, GPx, and CAT. Degenerative disorders may develop if these enzymes are harmed by repeated instances of oxidative stress; Nrf-2 and HO-1 control these enzymes.26
This research demonstrates BCA’s potential to preserve cellular homeostasis and defend cells from oxidative damage. The expression of antioxidant enzymes including SOD, CAT, and GPx-1 was up-regulated in both the kidney and the heart after BCA treatment significantly elevating the mRNA levels of Nrf-2 and HO-1 and their corresponding levels in the tissue. In diabetic rats, severe oxidative stress consumed a significant quantity of Nrf-2, as seen by down-regulated mRNA expressions of Nrf-2 and its related antioxidant enzymes. However, supplementation with BCA increased the expression of Nrf-2 and HO-1 mRNA and stimulated Nrf-2 nuclear translocation, suggesting that BCA has protective effects on diabetic kidney and myocardial tissue that are mediated by the Nrf-2/HO-1 pathway. By controlling the expression of genes that are regulated by Nrf-2 to protect against oxidative damage, BCA may facilitate Nrf-2 translocation into the nucleus. According to the current research, Nrf-2 was increased by BCA and its depletion was stopped, enhancing the ability to restore redox equilibrium. These findings are consistent with the earlier research by Rani et al8, which showed that BCA reduced inflammation and oxidative stress, which in turn reduced the risk of cardiomyopathy linked with obesity.
Conclusion
Collectively, the results of this study show that BCA has cardio renal protective effects against HFD and STZ-induced DM in rats, primarily due to their capacity to improve antioxidant response by elevating the GSH level and the activities of enzymatic antioxidants in both the kidney and heart. Additionally, BCA demonstrated notable protective benefits against complications brought on by DM by reducing oxidative stress through regulation of the Nrf-2/HO-1 signaling pathway. Hence, the results of this study substantiate the cardio renal protective mechanisms of BCA, which might offer experimental support and new information for the development of alternative medicines for the treatment of DM-related complications.
Acknowledgements
The authors would like to express their sincere thanks to the management of K.S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode, Tamilnadu, India for providing the necessary facilities to conduct this work and their constant support. Further, we extend our sincere thanks to the management of Nandha College of Pharmacy, Erode, Tamilnadu and Arulmigu Kalasalingam College of Pharmacy, Krishnankoil, Tamilnadu for providing an animal house facility to carry out animal experiments.
Conflict of Interest
The authors declares that no conflict of interests to disclose.
Funding Sources
There are no funding Sources
References
- Uddandrao, V. V. S., Parim, B., Ramavat, R., Pothani, S., Vadivukkarasi, S., Ponmurugan, P., Chandrasekaran, P., & Ganapathy, S. (2023). Effect of S-allylcysteine against diabetic nephropathy via inhibition of MEK1/2-ERK1/2-RSK2 signalling pathway in streptozotocin-nicotinamide-induced diabetic rats. Archives of Physiology and Biochemistry, 129(1), 213–221. https://doi.org/10.1080/13813455.2020.1811731
CrossRef - Koye, D. N., Magliano, D. J., Nelson, R. G., & Pavkov, M. E. (2018). The Global Epidemiology of Diabetes and Kidney Disease. Advances in Chronic Kidney Disease, 25(2), 121–132. https://doi.org/10.1053/j.ackd.2017.10.011
CrossRef - Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F., & Nogueira-Machado, J. A. (2018). Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell death & Disease, 9(2), 119. https://doi.org/10.1038/s41419-017-0135-z
CrossRef - Uddandrao, V. V. S., Parim, B., Singaravel, S., Ponnusamy, P., Ponnusamy, C., Sasikumar, V., & Saravanan, G. (2022). Polyherbal Formulation Ameliorates Diabetic Cardiomyopathy Through Attenuation of Cardiac Inflammation and Oxidative Stress Via NF-κB/Nrf-2/HO-1 Pathway in Diabetic Rats. Journal of Cardiovascular Pharmacology, 79(1), e75–e86. https://doi.org/10.1097/FJC.0000000000001167
CrossRef - Sathibabu Uddandrao, V. V., Brahmanaidu, P., Ravindarnaik, R., Suresh, P., Vadivukkarasi, S., & Saravanan, G. (2019). Restorative potentiality of S-allylcysteine against diabetic nephropathy through attenuation of oxidative stress and inflammation in streptozotocin-nicotinamide-induced diabetic rats. European Journal of Nutrition, 58(6), 2425–2437. https://doi.org/10.1007/s00394-018-1795-x
CrossRef - Zhang, B., Xie, S., Su, Z., Song, S., Xu, H., Chen, G., Cao, W., Yin, S., Gao, Q., & Wang, H. (2016). Heme oxygenase-1 induction attenuates imiquimod-induced psoriasiform inflammation by negative regulation of Stat3 signaling. Scientific Reports, 6, 21132. https://doi.org/10.1038/srep21132
CrossRef - Unuofin, J. O., & Lebelo, S. L. (2020). Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxidative Medicine and Cellular Longevity, 2020, 1356893. https://doi.org/10.1155/2020/1356893
CrossRef - Rani, A. J. I., Uddandrao, V. V. S., Sangeethadevi, G., Saravanan, G., Chandrasekaran, P., Sengottuvelu, S., Tamilmani, P., Sethumathi, P. P., & Vadivukkarasi, S. (2021). Biochanin A attenuates obesity cardiomyopathy in rats by inhibiting oxidative stress and inflammation through the Nrf-2 pathway. Archives of Physiology and Biochemistry,1–16. https:// doi.org/10.1080/13813455.2021.1874017
- Sangeethadevi, G., Sathibabu Uddandrao, V. V., Jansy Isabella, R. A. R., Saravanan, G., Ponmurugan, P., Chandrasekaran, P., Sengottuvelu, S., & Vadivukkarasi, S. (2022). Attenuation of lipid metabolic abnormalities, proinflammatory cytokines, and matrix metalloproteinase expression by biochanin-A in isoproterenol-induced myocardial infarction in rats. Drug and Chemical Toxicology, 45(5), 1951–1962. https://doi.org/10.1080/01480545.2021.1894707
CrossRef - Pudhupalayam, S.P., Uddandrao, V.V.S., Chandrasekaran, P., Sengottuvelu, S., Tamilmani, P., Ponmurugan, P., Vadivukkarasi, S., Shabana Begum, M., & Saravanan, G. (2022). Biochanin A Attenuates Hyperglycemia in High-Fat Diet–Streptozotocin–Induced Diabetic Rats by Modulating the Activities of Carbohydrate-Metabolizing Enzymes in Vital Organs. Revista Brasileira de Farmacognosia, 32, 608–617 https://doi.org/10.1007/s43450-022-00280-8
CrossRef - Uddandrao, V. V. S., Rameshreddy, P., Brahmanaidu, P., Ponnusamy, P., Balakrishnan, S., Ramavat, R. N., Swapna, K., Pothani, S., Nemani, H., Meriga, B., Vadivukkarasi, S., Nivedha, P. R, & Ganapathy, S. (2020). Antiobesity efficacy of asiatic acid: down-regulation of adipogenic and inflammatory processes in high fat diet induced obese rats. Archives of Physiology and Biochemistry, 126(5), 453–462. https://doi.org/10.1080/13813455.2018.1555668
CrossRef - Eraniappan, S., Balasubramaniyan, P., Uddandrao, V. V. S., Roy, A., & Singaravel, S. (2023). Βeta-glucan suppresses high-fat-diet-induced obesity by attenuating dyslipidemia and modulating obesogenic marker expressions in rats. Fundamental & Clinical Pharmacology, https://doi.org/10.1111/fcp.12871
CrossRef - Pakdeechote, P., Bunbupha, S., Kukongviriyapan, U., Prachaney, P., Khrisanapant, W., & Kukongviriyapan, V. (2014). Asiatic acid alleviates hemodynamic and metabolic alterations via restoring eNOS/iNOS expression, oxidative stress, and inflammation in diet-induced metabolic syndrome rats. Nutrients, 6(1), 355–370. https://doi.org/10.3390/nu6010355
CrossRef - Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. doi:10.1016/s0076-6879(78)52032-6
CrossRef - Saravanan, G., Ponmurugan, P., Sathiyavathi, M., Vadivukkarasi, S., & Sengottuvelu, S. (2013). Cardioprotective activity of Amaranthus viridis Linn: effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infarcted rats. International Journal of Cardiology, 165(3), 494–498. https://doi.org/10.1016/j.ijcard.2011.09.005
CrossRef - Giorgino, F., Vora, J., Fenici, P., & Solini, A. (2020). Renoprotection with SGLT2 inhibitors in type 2 diabetes over a spectrum of cardiovascular and renal risk. Cardiovascular Diabetology, 19(1), 196. https://doi.org/10.1186/s12933-020-01163-9
CrossRef - Banerjee, D., Winocour, P., Chowdhury, T. A., De, P., Wahba, M., Montero, R., Fogarty, D., Frankel, A. H., Karalliedde, J., Mark, P. B., Patel, D. C., Pokrajac, A., Sharif, A., Zac-Varghese, S., Bain, S., Dasgupta, I., & Association of British Clinical Diabetologists and The Renal Association (2022). Management of hypertension and renin-angiotensin-aldosterone system blockade in adults with diabetic kidney disease: Association of British Clinical Diabetologists and the Renal Association UK guideline update 2021. BMC Nephrology, 23(1), 9. https://doi.org/10.1186/s12882-021-02587-5
CrossRef - Bhatti, J. S., Sehrawat, A., Mishra, J., Sidhu, I. S., Navik, U., Khullar, N., Kumar, S., Bhatti, G. K., & Reddy, P. H. (2022). Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free radical Biology & Medicine, 184, 114–134. https://doi.org/10.1016/j.freeradbiomed.2022.03.019
CrossRef - Al-Amarat, W., Abukhalil, M. H., Althunibat, O. Y., Alfwuaires, M. A., Alnamshan, M. M., Alqosaibi, A. I., Ahmeda, A. F., Kamel, E. M., Arab, H. H., & Mahmoud, A. M. (2021). Galangin Attenuates Liver Injury, Oxidative Stress and Inflammation, and Upregulates Nrf2/HO-1 Signaling in Streptozotocin-Induced Diabetic Rats. Processes, 9(9), 1562. https://doi.org/10.3390/pr9091562
CrossRef - Shen, X., Hu, B., Xu, G., Chen, F., Ma, R., Zhang, N., Liu, J., Ma, X., Zhu, J., Wu, Y., & Shen, R. (2017). Activation of Nrf2/HO-1 Pathway by Glycogen Synthase Kinase-3β Inhibition Attenuates Renal Ischemia/Reperfusion Injury in Diabetic Rats. Kidney & Blood Pressure Research, 42(2), 369–378. https://doi.org/10.1159/000477947
CrossRef - Alaofi A. L. (2020). Sinapic Acid Ameliorates the Progression of Streptozotocin (STZ)-Induced Diabetic Nephropathy in Rats via NRF2/HO-1 Mediated Pathways. Frontiers in Pharmacology, 11, 1119. https://doi.org/10.3389/fphar.2020.01119
CrossRef - Kaludercic, N., & Di Lisa, F. (2020). Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy. Frontiers in Cardiovascular Medicine, 7, 12. https://doi.org/10.3389/fcvm.2020.00012
CrossRef - Du, L., Wang, L., Wang, B., Wang, J., Hao, M., Chen, Y. B., Li, X. Z., Li, Y., Jiang, Y. F., Li, C. C., Yang, H., Gu, X. K., Yin, X. X., & Lu, Q. (2020). A novel compound AB38b attenuates oxidative stress and ECM protein accumulation in kidneys of diabetic mice through modulation of Keap1/Nrf2 signaling. Acta Pharmacologica Sinica, 41(3), 358–372. https://doi.org/10.1038/s41401-019-0297-6
CrossRef - Chen, X., Qian, J., Wang, L., Li, J., Zhao, Y., Han, J., Khan, Z., Chen, X., Wang, J., & Liang, G. (2018). Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine, 60(1), 83–94. https://doi.org/10.1007/s12020-018-1525-4
CrossRef - Baird, L., & Yamamoto, M. (2020). The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Molecular and Cellular Biology, 40(13), e00099-20. https://doi.org/10.1128/MCB.00099-20
CrossRef - Loboda, A., Damulewicz, M., Pyza, E., Jozkowicz, A., & Dulak, J. (2016). Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellular and molecular life sciences: CMLS, 73(17), 3221–3247. https://doi.org/10.1007/s00018-016-2223-0
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









