Manuscript accepted on : 17-06-2023
Published online on: 30-06-2023
Plagiarism Check: Yes
Reviewed by: Dr. Hind Shakir Ahmed
Second Review by: Dr. Avinash Cau
Final Approval by: Dr. Eugene A. Silow
Suman Jangir and Varalakshmi Kilingar Nadumane*
and Varalakshmi Kilingar Nadumane*
Department of Biotechnology, JAIN (Deemed-to-be University), Bengaluru, India.
Corresponding Author E-mail: kn.varalakshmi@jainuniversity.ac.in
DOI : http://dx.doi.org/10.13005/bbra/3126
ABSTRACT: Insects and their products have been linked to medical cures from age old now. Among all the other Insects, Ants of the order Hymenoptera possess a wide range of bioactive compounds that have shown to have potent anticancer properties. In a similar fashion, the present study investigates the in vitro antitumor effects of Bengaluru-based ant extracts. Different ant species were collected from various locations in Bengaluru and identified as Tetraponera rufonigra, Camponotus oblongus, Anoplolepsis gracilipes, Camponotus species. Further, A 3-(4, 5-dimethylthiazolyl2)-2, 5-diphenyltetrazolium bromide (MTT) assay was performed on hepatocellular carcinoma HepG2 after extracting the metabolites in 95% EtOH. The potential anticancer effect was again confirmed by Trypan blue cell staining assay using HepG2 (hepatocellular carcinoma) and MCF7 (human breast adenocarcinoma) cell line. Further, apoptotic induction was measured by Caspase-3 activity assay and different tests were performed to investigate the chemical composition of the extracts. All the crude extracts of ants have shown anticancer effects and increase in caspase-3 enzyme activity of Tetraponera rufonigra extract on hepatocellular carcinoma HepG2 while Anoplolepsis gracilipes on human breast cancer cell line MCF7 makes them good candidate for further purification and characterization. T. rufonigra extracts have shown the presence of all the tested chemicals like alkaloids, flavonoids, reducing sugars, phenols, steroids and amino acids.
KEYWORDS: Antitumor effects; Anoplolepsis gracilipes; Camponotus species; Camponotus oblongus; Insects; Tetraponera rufonigra
Download this article as:| Copy the following to cite this article: Jangir S, Nadumane V. Kilingar. Comparative Anticancer Efficacy Analysis of T. rufonigra, C. oblongus, A. gracilipes and Camponotus sp. of ants: An in Vitro Study. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Jangir S, Nadumane V. Kilingar. Comparative Anticancer Efficacy Analysis of T. rufonigra, C. oblongus, A. gracilipes and Camponotus sp. of ants: An in Vitro Study. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/3ptTO9c |
Introduction
It is well known fact that cancer is one of the most leading causes of death all over the world accounting for approximately 10 million deaths according to WHO reports. With the advanced chemotherapeutic approaches also, there is an extensive list of its side effects due to the non-specificity and development of resistance in anticancer drugs. Thus, the need of an alternative approach is required to combat this disease and that alternative approach is inclined towards the natural sources due to their omnipresence. Plants, microbes and marine organisms are the natural sources that contribute to more than 60% of anticancer drugs that are in clinical use today1. It is determined that apart from Plants, microbes and marine organisms, Insects also possess bioactive compounds that have great potential in antitumor activities as they have been extensively studied for their antimicrobial, antifungal, antithrombotic effects2.
In this Class Insecta, Ants belong to the Order Hymenoptera that is profusely present in the terrestrial environment with approximately 13,165 species discovered so far3. Ants use the chemicals present is them for defence and communications, these small species also have tiny glands in their body where they produce and stock an array of natural products. Thus, the whole-body of ants has been used in different forms for its health benefits from centuries now, that proves it to be a rich repository of bioactive compounds 4. The in vitro cytotoxic profile of four ant species’ solvent extracts against human breast cancer cell line MCF7 and hepatocellular carcinoma HepG2 were examined. Tetraponera rufonigra, an arboreal bicolour ant belonging to sub family pseudomyrmecinae is one of the most dangerous invasive species and is well known for its anaphylaxtic, pain and inflammation causing behaviour5. Camponotus genus have 83 species and subspecies diversity in India and amongst them 18 are found in Karnataka state6 but they are not studied for their cytotoxicity. Anoplolepsis gracilipes, the yellow crazy ant is an exotic ant introduced in India. It is considered one of the most dangerous invasive ant species due to its severe impact on biological diversity7.
Compared to the vast number of their existence all over the world and composition of their glandular and venom composition, the antitumor studies done is negligible. Taking this into consideration, the aim of the current study is to find the anticancer potential of above mentioned four ant species.
Materials and Methods
Sample collection, Identification and Authentication
Four different Ant species were collected from different locations in Bengaluru actively; the method used was hand-picking and keeping them in separate glass jar for each species. All the samples were kept in -20 º C until the extraction procedure and some ants were kept in 70% EtOH for species identification8. The Identification and authentication of ants were done by Dr. Himender Bharti (Lead Investigator, Ant Systematics and Molecular Biology Lab, Department of Zoology and Environmental Sciences, Punjabi University, Patiala).
Metabolite extraction
Each ant species samples were defrosted, washed with distilled water, air-dried, weighed (Dry weight) and macerated separately in 95% Ethanol and kept for three days in solvent with occasional shaking. Further the extracts were centrifuged, and the supernatant was collected and dried in hot air oven at 40º C overnight. The dried compounds were kept in 4°C until used for further experiments9.
Cell lines and Culture
The National Centre for Cell Sciences (NCCS), Pune, India provided the HepG2 and MCF7 cancer cell lines. These cell lines were subcultured in DMEM (HiMedia, India) supplemented with 10% Fetal Bovine Serum in T-25 flasks, using Trypsin (HiMedia, India) and were incubated at 37°C with 5% CO2 (Thermo Scientific USA). Both the cell lines were maintained in these conditions throughout the course of the study.
Cytotoxicity assay
The HepG2 cells were seeded in 96-well microtiter plate at a density of 1×104 cells/mL and incubated for 24 hours. The cancer cells were treated with the ant extracts at different concentrations of 0.025, 0.05, 0.1 and 0.4 mg/mL along with controls and incubated for 24-, 48- and 72- hours of time period. Percentage Cell viability was measured using MTT assay 10 according to the standard protocol.
Trypan blue cell staining assay
The MCF7 and HepG2 cells were seeded at a density of 1×105 cells/mL in 12 well plates. After 24 hours of incubation, each sample was administered separately to the cells at a concentration of 0.05 mg/mL. Further, cancer cells were harvested by trypsinization and resuspended in 1mL of phosphate buffered saline (PBS) after 48 hours of treatment period. Equal volume of cell suspension and 0.4 % of trypan blue solution were mixed in sterile vial and incubated for two-three minutes. The stained and unstained cells were counted using a haemocytometer under an inverted microscope (Labomed, Germany)11. The total cell concentration (per mL) was determined as per the standard protocol. The percentage cell viability was calculated using the formula:
Cell Viability (%) = No. of live cells (per mL)/ Total no. of cells (Live + Dead) (per mL) x 100
Caspase-3 activity assay
The MCF7 and HepG2 cell lines were cultured and treated at a concentration of 0.05 mg/mL of each sample in separate flasks, untreated flasks for both the cell lines were considered as control. After 48 hours of incubation, the apoptotic induction was measured by Caspase-3 activity assay kit (Elabscience, Catalog no. E-CK-A311). The absorbance was measured with Elisa Plate Reader at 405 nm at zero-time interval and after overnight extension of reaction time. The percentage increase in the Caspase activity was calculated using the OD values between the Control and Treated samples.
Chemical screening
Various biochemical tests were done to determine the contents present in the ant extracts according to standard protocol[12]. Tests for Alkaloids (Picric acid test), Reducing sugars (Benedict’s test), Flavonoids (Conc. H2SO4 test), Phenols (FeCl3 test), Steroids (Liebermann-Burchard test), Amino acids (Ninhydrin test) were performed.
Statistical analysis
All the results were calculated as mean ± standard deviation. The statistical significance was determined using one way ANOVA via GraphPad Prism® 9.0 software. Dunnett’s multiple comparison test was used to compare Control group and Experimental group means. A significance level of p < 0.05 and p < 0.01 was used to establish the significant difference between control data and the treated data.
Results and Discussion
Ants are one of the most underrated natural resources for anticancer drugs and have now become a topic of interest. Previous studies on the compounds that have been isolated from ants have resulted in major findings such as cancer signaling pathway inhibition and tumor growth inhibition in vivo. For instance, as per previous report Solenopsin A, an alkaloid isolated from red imported fire ant, blocks the PI3K (Phosphoinositide-3-kinase) signaling pathway in cells upstream of PI3K, which may underlie its effects of angiogenesis inhibition[13]. Samsum ant venom was reported as to have significant dose- dependent antineoplastic activity against human breast adenocarcinoma MCF7, hepatocellular carcinoma HepG2 and human colorectal adenocarcinoma LoVo cancer cells and the ability to induce apoptosis in vivo in rats14. Therefore, we have tried collecting some different types of species that might contain some chemicals inhibiting the cancer cells.
Sample collection, Identification and Authentication of the species
Four different ant species were collected from different locations as mentioned in Table1 and identified as well as authenticated by Dr. Himender Bharti as Tetraponera rufonigra (Jerdon, 1851) Camponotus oblongus (Smith, 1858), Anoplolepsis gracilipes (Smith, 1857) and Camponotus species (Mayr, 1861) (Figure 1).
Table 1: Four different ant species collected from Bengaluru, their species identification and location of collection.
|
S. No. |
Scientific Name |
Location |
|
1. |
Tetraponera rufonigra (Jerdon, 1851) |
12.997698° Lat. 77.590581° Long.
|
|
2. |
Camponotus oblongus (Smith,1858) |
12.939986° Lat. 77.692067° Long
|
|
3. |
Anoplolepsis gracilipes (Smith,1857) |
12.951137° Lat. 77.584938° Long.
|
|
4. |
Camponotus species (Mayr, 1861) |
12.977778° Lat. 77.596723° Long. |
 |
Figure 1: (a) Tetraponera rufonigra (Jerdon, 1851) (b) Camponotus oblongus (Smith, 1858) (c) Anoplolepsis gracilipes (Smith, 1857) (d) Camponotus species (Mayr, 1861).
|
Cytotoxicity of the crude extracts
The ongoing discussion of Insects being analyzed for their various roles in human favour is escalating these days, especially with their antibacterial and anticancer potentials. However, all these species are more focussed on their venom composition for these benefits but only taking venom has its own side effects like it can cause more damage to normal cells along with cancer cells15. Apart from the venom, the whole body of ants also contains several chemical compounds that would be useful for inhibiting cancer cell growth and might not damage normal cells as well. Hence, we have focused on the whole-body extracts of these ant species and checked the percentage of cytotoxicity these extracts possess towards the HepG2 cancer cell line.
The percentage cell viability of HepG2 cells at 0.05 mg/mL concentration of T. rufonigra and C. oblongus extracts after 72 hours of treatment was 73.51 ± 0.06 % and 60.10 ± 0.01% respectively. A. gracilipes has shown 78.54 ± 0.05% viability at 0.05 mg/mL concentration after 72 hours treatment while Camponotus sp. has demonstrated 64.97 ± 0.32% viability at 0.1 mg/mL after 48 hours of treatment (Figure 2).
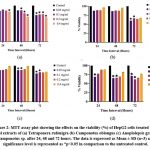 |
Figure 2: MTT assay plot showing the effects on the viability (%) of HepG2 cells treated with ethanol extracts of (a) Tetraponera rufonigra (b) Camponotus oblongus.
|
Trypan Blue cell staining
For the confirmation of cytotoxicity towards cancer cells, we again checked the anticancer samples against MCF7 and HepG2 cell lines. The treatment of each ants’ extract was given separately for 48 hours at 0.05 mg/mL. After counting the dead and viable cells, calculated percentage cell viability for T. rufonigra, C. oblongus, A. gracilipes and C. species are shown in Table 2. T. rufonigra showing the minimum viability of 52.94 % against MCF7 cell lines and not so significant cytotoxicity towards HepG2 cell lines indicates that this species might contain some chemical compounds that is inhibiting the specific cancer type while Camponotus sp. showing 50% inhibition against MCF7 and 46.67 % against HepG2 cells clearly explains its potential for the cancer inhibitory chemical compounds present in them.
Table 2: Viability (%) of ant extract treated MCF7 and HepG2 cancer cells determined by Trypan blue cell count.
|
Cells |
Treatment |
No. of Viable cells (1 × 104 cells/mL) |
No. of Dead cells (1 × 104 cells/mL) |
Cell Viability (%) |
|
MCF7 |
Control T. rufonigra C. oblongus A. gracilipes Camponotus sp. |
38 ± 2 18 ± 2 24 ± 1 19 ± 1 16 ± 2 |
2 ± 1 16 ± 1 10 ± 2 11 ± 2 16 ± 2 |
95 52.94 70.58 63.33 50 |
|
HepG2 |
Control T. rufonigra C. oblongus A. gracilipes Camponotus sp. |
31 ± 1 25 ± 3 25 ± 1 23 ± 2 16 ± 2 |
01 ± 1 08 ± 2 06 ± 1 11 ± 1 14 ± 3 |
96.87 75.76 80.64 67.64 53.33 |
Caspase-3 activity assay
Caspase-3 enzyme activity in the treated MCF7 and HepG2 cells was determined to confirm the apoptotic induction in comparison to the untreated control cells. For MCF 7 cell line, C. oblongus, A. gracilipes and Camponotus sp. extracts have shown 0.15-, 2.65- and 0.55- fold increase respectively, while there were no significant changes observed in T. rufonigra. For HepG2 cell line, T. rufonigra and A. gracilipes extracts have shown 0.43- and 0.27- fold increase, when compared to the control. The maximum apoptotic induction ability (as displayed by caspase activity enhancement) by A. gracilipes on MCF-7 and by T. rufonigra on HepG2 cell line, indicating cell line specificity of the compounds present in these ant extracts.
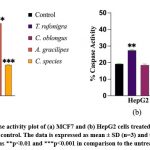 |
Figure 3: The % Caspase activity plot of (a) MCF7 and (b) HepG2 cells treated with ant extracts (0.05 mg/mL) and untreated control.
|
Chemical screening
The extracts of T. rufonigra, C. oblongus, A. gracilipes and C. species were subjected to different tests to investigate the chemical composition of the compounds present in the samples as shown in Table 3. Tetraponerines are the alkaloids isolated from Tetraponera binghami has exhibited impressive cytotoxicity against MCF 7 cell lines and their analogues have given cytotoxicity against colorectal adenocarcinoma HT29 cells[16]. Likewise, we have worked on the same genus Tetraponera but different species rufonigra that have shown the presence of all the tested compounds i.e., alkaloids, flavonoids, reducing sugars, phenols, steroids and amino acids. C. oblongus and A. gracilipes have only shown the presence of flavonoids, reducing sugars and amino acids. In Camponotus sp. we could detect the presence of phenols, reducing sugars and amino acids. The chemical compounds like Alkaloids in general is involved in modulation of key signaling pathways in cancer cell proliferation, metastasis, induction of cell cycle arrest, etc.17. Flavonoids induce excessive autophagy in cancer cells18 alongwith anticarcinogenic properties like apoptotic induction19, cell cycle arrest20, etc. Phenols inhibit angiogenesis21. Overexpression of estrogen hormone is one of the causes of breast cancer22, thus the presence of steroids in T. rufonigra could also act as an anticancer agent due to its antihormonal properties. Therefore, the anticancer effect shown by all these extracts could be due to the presence of all these chemicals.
This is the first report to study the anticancer effect of whole body ethanol extracts of T. rufonigra, C. oblongus, A. gracilipes and Camponotus sp. which have shown promising cytotoxicity towards MCF7 and HepG2 cancer cell lines. There are 61 genera and 257 species of ants located in Karnataka state[6] itself to explore in this regard. Out of these, we collected and screened only 3 genera and 4 species of ants in the current study. From this study, it is worth stating that T. rufonigra, and Camponotus sp. can be taken for further purification, characterization studies followed by other in vitro assays to verify and validate anticancer application potential of these species of ants.
Table 3: Chemical contents of T. rufonigra, C. oblongus, A. gracilipes and Camponotus sp. extracts. Remarks: Plus sign (+) means detected, Minus sign (-) means not detected
|
Tests |
T. rufonigra |
C. oblongus |
A. gracilipes |
Camponotus sp. |
|
Alkaloids |
+ |
– |
– |
– |
|
Reducing sugars |
+ |
+ |
+ |
+ |
|
Flavonoids |
+ |
+ |
+ |
– |
|
Phenols |
+ |
– |
– |
+ |
|
Steroids |
+ |
– |
– |
– |
|
Amino acids |
+ |
+ |
+ |
+ |
Conclusion
In conclusion, the crude extracts of four different ants have shown cytotoxicity against HepG2 cancer cell lines via MTT assay that is further confirmed by Trypan blue cell staining assay and enhanced Caspase activities on both MCF7 and HepG2 cell lines. Hence, these ant species can be taken up for future studies on their purification, characterization and anticancer activities.
Acknowledgements
Both the authors are indebted to JAIN (Deemed-to-be University) for the material and infrastructural facilities. We are thankful to CSIR for the financial support. We are grateful to Dr. Himender Bharti Lead Investigator, Ant Systematics and Molecular Biology Lab, Department of Zoology and Environmental Sciences, Punjabi University, Patiala for the ant species identification and authentication.
Conflict of Interest
The authors find no conflicts of interest related to this research work.
Funding Sources
There is no funding source related to this work.
References
- Rayan A., Raiyn J., Falah M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS One. 2017;12(11):e0187925. Published 2017 Nov 9. doi:10.1371/journal.pone.0187925
CrossRef - Xie J., Zhang D., Liu C., Wang L. A periodic review of chemical and pharmacological profiles of Tubiechong as insect Chinese medicine. RSC Advances. 2021;11:33952–33968. doi: 10.1039/D1RA05425B
CrossRef - Bolton B. An online catalogue of the ants of the world. http://antcat.org [accessed on 7 October 2015].
- Attygalle A.B. and Morgan D.E. Chemicals from the Glands of Ants. RSC London. 1984;13(3):245–78. doi: 10.1039/CS9841300245
CrossRef - Naephrai S., Khacha-Ananda S., Pitchakarn P., Jaikang C. Composition and Acute Inflammatory Response from Tetraponera rufonigraVenom on RAW 264.7 Macrophage Cells. Toxins (Basel). 2021;13(4):257. Published 2021 Apr 3. doi:10.3390/toxins13040257
CrossRef - Bharti H., Guénard B., Bharti M., Economo E.P. An updated checklist of the ants of India with their specific distributions in Indian states (Hymenoptera, Formicidae). Zookeys. 2016;(551):1-83. Published 2016 Jan 11. doi:10.3897/zookeys.551.6767
CrossRef - Lee C.Y., Scotty Yang C.C. Biology, Ecology, and Management of the Invasive Longlegged Ant, Anoplolepis gracilipes. Ann Rev Ent. 2022; 67: 43-63. doi: 10.1146/annurev-ento-033121-102332
CrossRef - Schauff M.E. Collecting and Preserving Insects and Mites: Techniques and Tools. Systematic Entomology Laboratory, USDA; National Museum of Natural History, [Beltsville, Md.], Washington, D.C., 2001
- Yeerong K., Sriyab S., Somwongin S., Punyoyai C., Chantawannakul P., Anuchapreeda S., Prommaban A., Chaiyana W. Skin irritation and potential antioxidant, anti-collagenase, and anti-elastase activities of edible insect extracts. Sci Rep. 2021;11(1):22954. doi: 1038/s41598-021-02382-0
CrossRef - Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55-63. doi:10.1016/0022-1759(83)90303-4
CrossRef - Freshney I.R. (4th): Culture of animal cells- a manual of basic techniques. 4th edition A. John Wiley & sons, Inc, New York; 2000.
- Shaikh J.R. and Patil M.K. Qualitative tests for preliminary phytochemical screening: An overview. Int J Chem Stud. 2020;8(2):603-608. doi: 22271/chemi.2020.v8.i2i.8834
CrossRef - Arbiser J.L., Kau T., Konar M., Narra K., Ramachandran R., Summers S.A., Vlahos C.J., Ye K., Perry B.N., Matter W., Fischl A., Cook J., Silver P.A., Bain J., Cohen P., Whitmire D., Furness S., Govindarajan B., Bowen J.P. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. 2007;109(2):560-5
CrossRef - Al-Tamimi J., Semlali A., Hassan I., Ebaid H., Alhazza I.M., Mehdi S.H., Al-Khaliffa M., Alanazi M.S. Samsum Ant Venom Exerts Anticancer Activity Through Immunomodulation In Vitro and In Vivo. Cancer Biother Radiopharm. 2018;33(2):65-73. doi:10.1089/cbr.2017.2400
CrossRef - Saidemberg D.M., da Silva-Filho L.C., Tognoli L.M., Tormena C.F., Palma M.S. Polybioside, a neuroactive compound from the venom of the social wasp Polybia paulista. J Nat Prod. 2010;73(4):527-531. doi:10.1021/np900424t
CrossRef - Rouchaud A., Braekman J.C. 2009. “Synthesis of new analogs of the tetraponerines.” Euro J Org Chem. 2009;2009(16): 2666-74.
CrossRef - Efferth T., Oesch F. Repurposing of plant alkaloids for cancer therapy: Pharmacology and toxicology. Semin Cancer Biol. 2021;68:143-163. doi:10.1016/j.semcancer.2019.12.010
CrossRef - Pang X., Zhang X., Jiang Y., Su Q., Li Q., Li Z. Autophagy: Mechanisms and Therapeutic Potential of Flavonoids in Cancer. Biomolecules. 2021; 11(2), 135. doi: 10.3390/biom11020135
CrossRef - Monasterio A., Urdaci M.C., Pinchuk I.V., López-Moratalla N., Martínez-Irujo J.J. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50(1):90-100. doi:10.1207/s15327914nc5001_12
CrossRef - Haddad A.Q., Venkateswaran V., Viswanathan L., Teahan S.J., Fleshner N.E., Klotz L.H. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006;9(1):68-76. doi:10.1038/sj.pcan.4500845
CrossRef - Wahle K.W., Brown I., Rotondo D., Heys S.D. Plant phenolics in the prevention and treatment of cancer. Adv exp med biol. 2010; 698, 36–51. https://doi.org/10.1007/978-1-4419-7347-4_4
CrossRef - Chen G.G., Zeng Q., Tse G.M. Estrogen and its receptors in cancer. Med Res Rev. 2008;28(6):954-974. doi:10.1002/med.20131
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





