How to Cite | Publication History | PlumX Article Matrix
Karthikeyan Kandhasamy and Kumpati Premkumar*
Cancer Genetics and Nanomedicine laboratory, Department of Biomedical Science, Bharathidasan University, Tiruchirappalli – 620 024, Tamil Nadu, India.
Corresponding Author E-mail: prems@bdu.ac.in
DOI : http://dx.doi.org/10.13005/bbra/3104
ABSTRACT: Recent years have seen a dramatic uptick in both research into and practical application of nanoparticles (NPs). Many biomedical applications have found success with the use of nanoparticles due to their wide spectrum of significant biological effects, including antibacterial and antioxidant properties. Nanoparticles that aren't harmful are gaining traction as a promising new class of antioxidants. Cerium oxide is a lanthanide rare-earth element. Cerium oxide nanoparticles (CNPs) exhibit a large surface area and good catalytic activity, the result of the dual oxidation state of CNPs, Ce3+ and Ce4+, has good antibacterial and antioxidant activity. CNPs were characterised by using analytical techniques such as the UV-Visible spectrophotometer, scanning electron microscopy, X-ray diffraction, zeta potential, Fourier transform infrared spectroscopy, and dynamic light scattering (DLS). CNPs exhibited a strong zone of inhibition against S. aureus (15mm) and E. coli (14mm). In vitro antioxidant activity of CNPs was investigated using the DPPH and ABTS techniques, with 50% of their radical scavenging potential being observed at concentrations of 47.61μg/mL and 49.26μg/mL respectively. Thus, our study reports that CNPS could be used as a prominent and efficient antioxidant and antibacterial agent. However, further studies are needed to understand the possible mechanisms of toxicity assessment.
KEYWORDS: Antioxidant; ABTS; Cerium oxide nanoparticles (CNPs); cell wall damage; DPPH
Download this article as:| Copy the following to cite this article: Kandhasamy K, Premkumar K. Fabrication of Cerium Oxide Nanoparticles with Improved Antibacterial Potential and Antioxidant Activity. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Kandhasamy K, Premkumar K. Fabrication of Cerium Oxide Nanoparticles with Improved Antibacterial Potential and Antioxidant Activity. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/41mKukq |
Introduction
Nanotechnology is one of the most exciting research frontiers in current materials science because of their unique physiochemical properties1. Synthesis of metallic nanoparticles with a size range of 1nm to 100 nm or less has a peculiar property, for example, size, shape, and high distribution, with enhanced applications such as the biomedical field2. In cancer therapy, nanoparticles worked with minimal side effects and improved pharmacokinetics3.
The metal oxide nanoparticles are used in highly potential biological applications due to their controlled size, shapes, chemical constituents, and valence state. Among the metal oxide nanoparticles, TiO2, FeO2 and CeO2 are extremely reactive oxides that acutely interconnect with the metabolic networks of cells4. Cerium oxide (CeO2) is a rare earth metal in the lanthanide family. Among the researchers, cerium oxide nanoparticles are becoming the most significant material due to their two oxidation states, Ce3+and Ce4+ as well as their greater oxygen mobility and loading capacity5,6. The cubic fluorite structure of cerium oxide nanoparticles (CNPs) is active in biomedical applications such as anticancer, antioxidant, antibacterial, bone implant material, device fabrication, and drug carrier6,7 due to their topography, size, and biocompatibility8. Cerium oxide nanoparticles are active and play a significant role in toxicity against yeast, bacteria, and fungi9 by interacting with the microbe’s cell wall through electrostatics 10, 11. The paired oxidation states Ce3+ and Ce4+ of cerium oxide nanoparticles are responsible for the enhanced antibacterial potential. Sufficient dispersibility makes them exist as a catalytic location for the attachment and long-chain phospholipid hydrolysis found on the surface of the bacterial membranes12. The Ce atom’s ability to transition between the 3+ and 4+ states of oxidation consists of cerium oxide nanoparticles that exhibit autocatalytic activity, which results in a strong radical-scavenging potential whenever this state occurs in a biological system at pH 7.4 13, 14.
In this present study, we demonstrate CNPs as good hybrid nanomaterials for bacterial growth inhibition Staphylococcus aureus and Escherichia coli also have good antioxidant potential for radical scavenging that was investigated by using the DPPH and ABTS assays. Further cerium oxide nanoparticles have been characterised using different techniques such as the UV-Visible spectrophotometer, morphology identification using SEM, functional groups of nanoparticles identification by FT-IR, DLS to determine hydrodynamic diameter, Zeta potential analyses to identify the surface charge of nanoparticles, and XRD for confirmation of the crystalline nature of nanoparticles.
Materials and Methods
Cerium oxide nanoparticle Synthesis.
Cerium oxide nanoparticles were synthesised according to the work of Pinna et al., 202014 with some modifications. In 10 mL of 2-propanol, 3.5g of Ce(NO3)3.6H2O was dissolved, and then 1M of 0.25mL HCl was added and stirred until complete dissolution. In a separate vial, 1g of urea was dissolved in 10 mL of 2-propanol that contained 1M of 0.25mL HCl and stirred for 5 minutes. The solution of urea was added dropwise to the Ce(NO3)3 solution under stirring conditions, and NH4OH 7mL was added to that mixture when the addition was complete. The samples were then microwaved four times at 600 W for 10 seconds before being washed with water and centrifuged at 10,000 rpm. After that, the discarded supernatant (5 mL of suspension) was allocated with a light yellow milky pellet (500mg of CeO2).
Characterization
UV-visible spectroscopy measurements were performed at room temperature using a spectrophotometer UV-2450 (Shimadzu) and observed in the 200–800 nm range for cerium oxide nanoparticles (CNP). The topographical architecture of CNPs was studied using scanning electron microscopy (SEM) (JSM-6480 LV). The hydrodynamic size of CNPs is measured by DLS, and the surface charge of CNPs is determined by the zeta potential of (ζ) using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK). Fourier transform infrared spectroscopy (FT-IR) analysis was performed at a range of 4000-400cm-1 (Perkin Elmer, USA). X-ray diffractometer (XRD) analysis confirmed the crystalline morphology of the CNPs (SmartLab, Rigaku Corporation, Japan).
Investigation of antibacterial activity
Diffusion method in agar well
The antibacterial potential of CNPs was assessed using the agar well diffusion technique against S. aureus and E. coli. Bacterial culture was spread using cotton swabs on nutrient agar (HiMedia, India) plates. Wells were performed using gel puncture (the diameter of the wells was 6 mm) on nutrient agar plates. In each well added with different doses of CNPs (25-100μg/mL), streptomycin (HiMedia, India) was used as a positive control (10μg/mL) and 30μL of autoclaved, double distilled water acted as a negative control. After that, the nutrient agar plates were incubated for 24 hours at 37°C. After the incubation period, the zone of inhibition diameter was measured in mm15.
Analysis of bacterial Growth curve
Staphylococcus aureus and Escherichia coli were treated with different concentrations of CNPs 25, 50, 75, 100μg/mL which were compared with the untreated bacteria as a control to assess the bacterial growth curve. Briefly, a 96-well microliter plate was filled with 250 μL of LB and 20μL of bacterial suspension (107 CFU/mL) and incubated at 37 °C for 24 hours. Every 3 hours for 24 hours, bacterial growth rate was measured at 580 nm using a Synergy HT Multimode Reader (Biotek, Winooski, USA). The experiments were performed in triplicates16.
Evaluation of antioxidant activity in in vitro
Radical scavenging activity by DPPH method
The scavenging activity of CNPs against 2, 2-diphenyl-1-picrylhydrazile (DPPH) (HiMedia, India) was calculated according to the17. In a 96 well plate, was added at increasing doses (20, 40, 60, 80 and 100μg/mL), and the control was ascorbic acid (Vitamin C). 1mM of DPPH 100μL solution was added to each well, and the samples were incubated in the dark for 30 minutes at room temperature. The solution colour was changed from violet to yellow colour indicate that reactive oxygen species have been scavenged, and it has been measured at 517 nm using the Synergy HT Multimode Reader (Biotek, Winooski, USA). Finally, the percentage of scavenging ability was calculated using the following equation:
Whereas, Ac – OD value of blank, As – OD value of CNPs treated.
Radical scavenging activity by ABTS method
The scavenging activity of CNPs at various concentrations (20 – 100μg/mL) against radicals was evaluated using the ABTS+ (2,2′-azinobis( 3-ethylbenzothiazoline-6-sulfonic acid) (HiMedia, India) assay, adapting a procedure from a previous study. In the ABTS assay, ascorbic acid was used as the standard antioxidant, and the ability of CNPs to scavenge the ABTS radical (ABTS.ABTS+) was compared. The ABTS solution was prepared by simply mixing 7mM of aqueous ABTS solution with 2.45mM of potassium persulfate in a dark medium at room temperature for 12 hours. After diluting the same standard solution with ethanol, the ABTS+ reaction mixture was added to each well, and the reaction was measured at 734nm after 10 minutes in the dark using the Synergy HT Multimode Reader (Biotek, Winooski, USA)15.
Result and Discussion
Analytical characterization of CNPs
UV-Visible spectrophotometer of CNPs
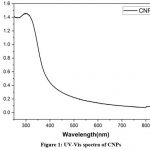 |
Figure 1: UV-Vis spectra of CNPsClick here to view figure |
The UV-Vis spectrophotometer investigations have been helpful for structural integrity, changes, and keeping track of the formation of nanoparticles determinination18. The absorption spectra of CNPs were observed at 300nm absorbance, as shown in (Fig.1). The stability of the CNPs was evaluated based on the time intervals from 0 to 60 days as shown in (Fig.2), when the incubation time was increased, a hypochromic shift was observed, which indicates the CNPs are partially stable 19. Strong absorption peaks at 300 nm were visible in the absorption spectra, which is the particular characteristic mentioned in earlier studies20. The interaction of Ce ions with cancer cells and microbes to exhibit anticancer and antibacterial activity, respectively, has been previously reported for CNPs21.
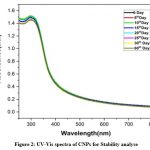 |
Figure 2: UV-Vis spectra of CNPs for Stability analyse.Click here to view Figure |
SEM Analysis
We examined the topography of the spherical CNPs using a scanning electron microscope, as shown in (Fig.3). The CNPs’ spherical appearance and restricted particle range imply that particle distributions are highly constant and that spherical agglomerates have formed. As mentioned in a previous article, synthesis duration, temperature, solvent, and calcination temperature22 are all critical reaction parameters that may explain why this kind of reaction emerges. According to phenomenological evidence, CNPs have a spherical shape with smooth surfaces.
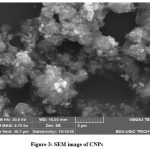 |
Figure 3: SEM image of CNPs.Click here to view Figure |
Particle size distribution and Zeta potential study of CNPs
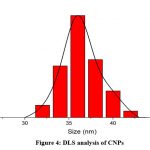 |
Figure 4: DLS analysis of CNPsClick here to view Figure |
Dynamic light scattering (DLS) analysis was used to investigate the hydrodynamic size distribution of CNPs. The average size of dispersion CNPs was 35 ±0.4 nm, as shown in (Fig. 4). CNP zeta potential investigation revealed +24.3 mV as shown in (Fig. 5).
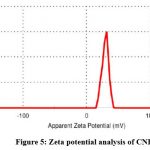 |
Figure 5: Zeta potential analysis of CNPs |
Fourier transforms infrared spectroscopy (FT-IR)
The Fourier transform infrared spectroscopy (FT-IR) approach has been shown to be helpful in determining the functional groupings of CNPs, as shown in (Fig.6).This particle absorption at a specific regain is observed as minor and major peaks for respective particles. The significant absorption at 3435cm−1 in the high-frequency region of the spectrum is due to physically absorbed water, an O-H stretching vibration, or a surface hydroxyl group. The bending vibration of linked structures shows a small shoulder peak at 2073cm−1 responsible for (H-O-H). The 1633cm−1 peaks are associated with O-C-O symmetric stretching, but the 666 cm−1 peaks are directly associated with the frequency of CeO stretching8, 23.
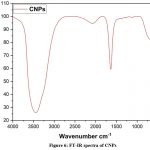 |
Figure 6: FT-IR spectra of CNPsClick here to view Figure |
XRD analysis of CNPs
CNPs are precipitated, as evidenced by the X-ray diffraction (XRD) pattern in (Fig.7). The XRD pattern of the nanoparticles reveals that they are made of cubic fluorite, CeO2. For diffraction peaks with values around 28.41°, 32.87°, 47.54°, 56.38°, and 60.18°, the planes (111), (200), (220), (311), and (222) are chosen. This set of peak deflections agrees with the powder X-ray diffraction standard JCPDS No. 34-0394. The XRD pattern showed no peaks related to impurities or other phases, suggesting that the CNPs generated are made of a pure crystal of CeO221.
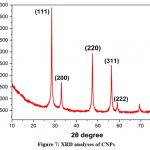 |
Figure 7: XRD analyses of CNPs.Click here to view Figure |
Antimicrobial activity Study
CNPs have been tested for antibacterial potential against bacteria such as S. aureus and E. coli. According to the (Fig. 8) revealed a higher inhibition zone was observed. These findings show that the communication between CNPs and the bacteria’s cell wall has efficiently promoted the toxicity of the bacteria and caused cell death. Among the four dosages of CNPs, 100µg/mL depicts a higher inhibition zone for E. coli (14 mm) and S. aureus (15 mm). The nanoparticles attached to the bacterial cell wall through ionic interactions between negatively charged organisms and positively charged nanoparticles. The binding of CNPs to peptidoglycan consists of gram positive bacteria, which are bound with teichoic acid; this may be a possible interaction with CNPs in antibacterial activity. More importantly, the possible mechanism of antibacterial activity of CNPs may interact with the bacterial cell membrane and bind with the mesosome, which is involved in cellular breathe, ability to cell divide, replicate their DNA, and, ultimately leading to cell death24. The observed antibacterial potential of CNPs at 100 µg/mL concentration exhibits significant efficacy against S. aureus and E. coli, due to the strong electrostatic forces the nanoparticles attach to the cell membrane to inhibit the growth of bacteria. In an early study, it was reported that CeO2/GO nanocomposites are potentially active against wound-associated infectious pathogens such as S. typhi, S. aureus, E. coli, and P. aeruginosa and present an absence of visible condition23. The antibacterial activity of Ag-Au loaded CeO2 nanoparticles against E. coli and S. aureus has potentially improved compared to other nanocomposite materials due to their individual metals containing ions25- 27. The possible mechanism of antibacterial activity has been electrostatic communication between bacterial cell walls and Ag-Au-loaded CeO2 nanocomposites that influence inhibition of microbial growth and induce microbial death, as reported in past studies1, 6, 28-30. Another mechanism of antibacterial activity reported in previously small nanoparticles has been to easily penetrate inside the cell wall of bacteria and cause cell death5,8. In an early study21, CNPs potentially damaged the cell wall of E. coli at a concentration of 0.06mg/mL when exposed to X-ray radiation.
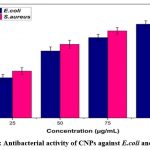 |
Figure 8: Antibacterial activity of CNPs against E.coli and S.aureusClick here to view Figure |
Effect of CNPs on bacterial growth
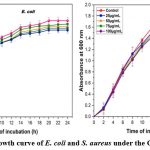 |
Figure 9: Growth curve of E. coli and S. aureus under the CNPs treatmentClick here to view Figure |
The effect of CNPs on E.coli and S.aureus growth was demonstrated. The lag, log, stationary, and death phases of E. coli and S. aureus growth curves were clearly represented in (Fig. 9). While the effect of different doses of CNPs from 25µg/mL to 100µg/mL has been observed, the constriction of the log phase was visible, demonstrating that CNPs have a dose-dependent manner to exploit against E. coli and S. aureus. The findings could imply that the binding of CNPs to the bacterial cell membrane surface, due to their dual oxidation state of Ce3+ and Ce4+, provided a catalytic site for the bonding and hydrolysis of long-chain phospholipids found on bacteria’s cell walls, resulting in cell wall damage and biomass reduction31.
Radical Scavenging activity by DPPH method
The antioxidant activity of CNPs was determined using the (DPPH)-2, 2-diphenyl-1-picrylhydrazyl method. When DPPH combines with an antioxidant, it produces a stable free radical that can be converted into a non-radical state. The DPPH radical was initially purple, but an antioxidant converted it to yellow, the colour of the non-radical version. As compared to the industry standard (Vitamin-C), CNPs’ free radical scavenging (DPPH) activity revealed significant antioxidant power, with an IC50 percentage of 47.61μg/mL as shown in (Fig.10). A previous study found that the levan coated CNPs have higher antioxidant capacity because polysaccharides contain multiple hydroxyl groups that can react with free radicals, and reduced radical chain reactions are related to antioxidant32,33. The probable mechanism of the radical scavenging potential of cerium oxide nanoparticles, it has a dual oxidation state that converts together based on pH, generating ROS in acidic pH and scavenging the ROS in normal pH, as reported in early study34,35. In DPPH assay 50μg/mL of CNPs has highly effective with 87.6% of scavenging potential compared with control α-tocopherol 76.3 % and BHA 52.9% has reported in earlier36. Free radical scavenging activity of nanoceria with a dose dependent manner, which is exposing the concentration of 50µg/500µl for 48.58% of radical inhibition in an early study, is reported at32. Early studies found that 9mg/mL of CeO2NPs increased DPPH inhibition by up to 67%, while polysaccharide-coated CeO2NPs were 85% effective at getting rid of DPPH21.
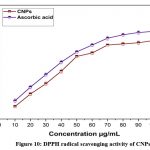 |
Figure 10: DPPH radical scavenging activity of CNPsClick here to view Figure |
Both solvothermal CeO2 nanoparticles and hydrothermal CeO2 nanoparticles were able to get rid of up to 55% and 30% of DPPH, respectively37. The IC50 value for the antioxidant activity of CeO2 NPs is 4.38 mg/ml. CeO2 nanoparticles displayed higher antioxidant activity (IC50 = 8-10 mg/mL) than ZnO nanoparticles, according to the literature38-40. According to past reports, the CNPs have been potentially active against radicals in the concentration range of 0.05g/L upto 0.06g/L when the activity has been low, below the concentration of 0.05g/L41.
ABTS Radical Scavenging activity
The ABTS method was used to assess the antioxidant activity of CNPs, and the reduction of free radicals caused by the CNPs was seen at 734 nm. CNPs have scavenged free radicals in a dose-dependent manner, with the best scavenging ability being noted in (Fig.11). The obtained result has depicted that CNPs have a dose-dependent inhibitory effect on the generation of ABTS radicals with an IC50 percentage of 46.26μg/mL. It is reasonable to believe that antioxidant capabilities increase directly with nanoparticle concentration15. ABTS•+ scavenging activity of Ce2O3NPs is significantly high with 87.2% compared with standard α-tocopherol 74.9 % and BHA 50.1% reported in early study 36. In previous report, it was shown that the CNPs have potentially active and scavenged free radicals and inhibit the production of ABTS+ radicals in a dose dependent manner compared with Trolox15. The scavenging potential of Mentha royleana-mediated CNPs is 46.7%, compared to 49.7% for control ascorbic acid at an IC50 value of 5.39 g/ml, whereas the IC50 value for Mentha royleana was found at a concentration of 5.57g/ml, as reported in an earlier study. The ABTS+ radical’s species was reduced in proportion to the amount of greenly synthesised CeONPs. Other evidence suggests that CeONPs scavenge ROS from normal cells preferentially, protecting them from reactive oxygen species42-44.
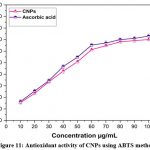 |
Figure 11: Antioxidant activity of CNPs using ABTS methodClick here to view Figure |
Conclusion
The current study demonstrated cerium oxide nanoparticles (CNPs) have been a considerable help in combating microbial pathogens with significantly formed zones of inhibition against E.coli and S.areus and reduced biomass with investigation of the bacterial growth curve, which shows considerably reduced growth of E.coli and S,areus in a dose dependent manner up to the incubation period of 24 hours. The ionic interaction between CNPs and bacterial cell membranes is crucial to their antibacterial activity. This is because the CNPs dual oxidation state of Ce3+ and Ce4+ surface provides a catalytic site for the attachment and hydrolysis of long-chain phospholipids found on the cell wall of bacteria, damaging the cell wall and decreasing the biomass. The obtained results from the present study unequivocally proved CNPs have good antibacterial properties. DPPH and ABTS are the methods that were used to investigate the in vitro antioxidant activity of CNPs. In the DPPH assay, the CNPs have an IC50 concentration of 47.61μg/mL, which yields a significant result when compared with the control. The result that was obtained from this study, which was the ABTS radical scavenging assay of CNPs, demonstrated that there was an inhibition of ABTS radical production in a dose-dependent manner, in addition to a significant IC50 concentration of 46.26μg/mL. This finding was made possible by the fact that the ABTS radical scavenging assay of CNPs was conducted. According to the results that were obtained, CNPs have beneficial antioxidant properties, with the paired oxidation states Ce3+ and Ce4+ that contain CNPs having the most significant influence. The SEM, XRD, DLS, Zeta potential, FTIR and UV-Visible spectrophotometer characterizations studies are supported to the antibacterial and antioxidant activity properties of CNPs. Thus, the present study demonstrated that cerium oxide nanoparticles could be used as effective antimicrobial and antioxidant agents. In our subsequent research, we focused on the toxicity of CNPs that had been functionalized with phytochemicals in both in vitro and in vivo studies to better understand the potential mechanisms involved.
Acknowledgments
The authors are grateful to Bharathidasan University for having received Department of Science and Technology Science and Engineering Research Board Empowerment and Equity (DST-SERB-EEQ), Government of India, project fellow Ref. No. 03056/P4/2019 Dated 28.06.2019.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
This work was supported by Department of Science and Technology Science and Engineering Research Board Empowerment and Equity (DST-SERB-EEQ), Government of India, Ref. No. 03056/P4/2019 Dated 28.06.2019. Dr. K. Premkumar has received research support from Bharathidasan University, Trichy.
Reference
- Nithya P, Sundrarajan M Ionic liquid functionalized biogenic synthesis of AgAu bimetal doped CeO2 nanoparticles from Justicia adhatoda for pharmaceutical applications: Antibacterial and anti-cancer activities. Journal of Photochemistry and Photobiology B: Biolog. 2020; 202:111706. https://doi.org/10.1016/j.jphotobiol.2019.111706
CrossRef - Liang Y, Guo N, Li L, Li R, Ji G, Gan S Fabrication of porous 3D flower-like Ag/ZnO heterostructure composites with enhanced photocatalytic performance. Applied Surface Science. 2015;332:32-9. https://doi.org/10.1016/j.apsusc.2015.01.116
CrossRef - Farokhzad OC, Langer R Impact of nanotechnology on drug delivery. ACS nano. 2009;3(1):16-20. https://doi.org/10.1021/nn900002m
CrossRef - Ghibelli L, Mathur S. Biological interactions of oxide nanoparticles: The good and the evil. Mrs Bulletin. 2014;39(11):949-54. DOI: https://doi.org/10.1557/mrs.2014.250
CrossRef - Magdalane CM, Kaviyarasu K, Raja A, Arularasu MV, Mola GT, Isaev AB, Al-Dhabi NA, Arasu MV, Jeyaraj B, Kennedy J, Maaza M. Photocatalytic decomposition effect of erbium doped cerium oxide nanostructures driven by visible light irradiation: investigation of cytotoxicity, antibacterial growth inhibition using catalyst. Journal of Photochemistry and Photobiology B: Biology.2018;185:275-82. https://doi.org/10.1016/j.jphotobiol.2018.06.011
CrossRef - Magdalane CM, Kaviyarasu K, Vijaya JJ, Siddhardha B, Jeyaraj B Facile synthesis of heterostructured cerium oxide/yttrium oxide nanocomposite in UV light induced photocatalytic degradation and catalytic reduction: synergistic effect of antimicrobial studies. Journal of Photochemistry and Photobiology B: Biology.2017; 173:23-34. https://doi.org/10.1016/j.jphotobiol.2017.05.024
CrossRef - Dhall A, Self W Cerium oxide nanoparticles: a brief review of their synthesis methods and biomedical applications. Antioxidants. 2018;7(8):97. https://doi.org/10.3390/antiox7080097
CrossRef - Khadar YS, Balamurugan A, Devarajan VP, Subramanian R, Kumar SD. Synthesis, characterization and antibacterial activity of cobalt doped cerium oxide (CeO2: Co) nanoparticles by using hydrothermal method. Journal of Materials Research and Technology. 2019;8(1):267-74. https://doi.org/10.1016/j.jmrt.2017.12.005
CrossRef - Masui T, Fujiwara K, Machida KI, Adachi GY, Sakata T, Mori H. Characterization of cerium (IV) oxide ultrafine particles prepared using reversed micelles. Chemistry of Materials. 1997;9(10):2197-204. https://doi.org/10.1021/cm970359v
CrossRef - Abbas F, Jan T, Iqbal J, Ahmad I, Naqvi MS, Malik M. Facile synthesis of ferromagnetic Ni doped CeO2 nanoparticles with enhanced anticancer activity. Applied Surface Science. 2015;357:931-6. https://doi.org/10.1016/j.apsusc.2015.08.229
CrossRef - Azad AM, Matthews T, Swary J. Processing and characterization of electrospun Y2O3-stabilized ZrO2 (YSZ) and Gd2O3-doped CeO2 (GDC) nanofibers. Materials Science and Engineering: B. 2005;123(3):252-8. https://doi.org/10.1016/j.mseb.2005.08.070
CrossRef - Khulbe K, Karmakar K, Ghosh S, Chandra K, Chakravortty D, Mugesh G Nanoceria-based phospholipase-mimetic cell membrane disruptive antibiofilm agents. ACS Applied Bio Materials. 2020; 3(7):4316-28. https://doi.org/10.1021/acsabm.0c00363
CrossRef - Pinna A, Figus C, Lasio B, Piccinini M, Malfatti L, Innocenzi P. Release of ceria nanoparticles grafted on hybrid organic–inorganic films for biomedical application. ACS Applied Materials & Interfaces. 2012;4(8):3916-22. https://doi.org/10.1021/am300732v
CrossRef - Pinna A, Cali E, Kerherve G, Galleri G, Maggini M, Innocenzi P, Malfatti L. Fulleropyrrolidine-functionalized ceria nanoparticles as a tethered dual nanosystem with improved antioxidant properties. Nanoscale Advances. 2020;2(6):2387-96. DOI: 10.1039/D0NA00048E
CrossRef - Pop OL, Mesaros A, Vodnar DC, Suharoschi R, Tăbăran F, Magerușan L, Tódor IS, Diaconeasa Z, Balint A, Ciontea L, Socaciu C Cerium oxide nanoparticles and their efficient antibacterial application in vitro against gram-positive and gram-negative pathogens. Nanomaterials. 2020;10(8):1614. https://doi.org/10.3390/nano10081614
CrossRef - Deepika MS, Thangam R, Vijayakumar TS, Sasirekha R, Vimala RT, Sivasubramanian S, Arun S, Babu MD, Thirumurugan R. Antibacterial synergy between rutin and florfenicol enhances therapeutic spectrum against drug resistant Aeromonas hydrophila. Microbial pathogenesis. 2019;135:103612. https://doi.org/10.1016/j.micpath.2019.103612
CrossRef - Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci Technol. 1995; 28(1), 25-30. https://doi.org/10.1016/S0023-6438(95)80008-5
CrossRef - Deepika MS, Thangam R, Sheena TS, Sasirekha R, Sivasubramanian S, Babu MD, Jeganathan K, Thirumurugan R A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomedicine & Pharmacotherapy. 2019 ;109:1181-95. https://doi.org/10.1016/j.biopha.2018.10.178
CrossRef - Badi’ah HI, Seedeh F, Supriyanto G, Zaidan AH Synthesis of silver nanoparticles and the development in analysis method. In IOP Conf. Ser.: Earth Environ. Sci., 2019; (Vol. 217, No. 1, p. 012005). IOP Publishing. https://doi:10.1088/1755-1315/217/1/012005
CrossRef - Parimi D, Sundararajan V, Sadak O, Gunasekaran S, Mohideen SS, Sundaramurthy A (2019) Synthesis of positively and negatively charged CeO2 nanoparticles: investigation of the role of surface charge on growth and development of drosophila melanogaster. ACS omega. 4(1):104-13. https://doi.org/10.1021/acsomega.8b02747
CrossRef - Nurhasanah I, Safitri W, Arifin Z, Subagio A, Windarti T. Antioxidant activity and dose enhancement factor of CeO2 nanoparticles synthesized by precipitation method. InIOP Conference Series: Materials Science and Engineering 2018; (Vol. 432, No. 1, p. 012031). IOP Publishing. https://doi.org/doi:10.1088/1757-899X/432/1/012031
CrossRef - Habib IY, Kumara NT, Lim CM, Mahadi AH Dynamic light scattering and zeta potential studies of ceria nanoparticles. InSolid State Phenomena. 2018; (Vol. 278, pp. 112-120). Trans Tech Publications Ltd. https://doi.org/10.4028/www.scientific.net/SSP.278.112.
CrossRef - Sharma G, Prema D, Venkataprasanna KS, Prakash J, Sahabuddin S, Venkatasubbu GD. Photo induced antibacterial activity of CeO2/GO against wound pathogens. Arabian Journal of Chemistry. 2020;13(11):7680-94. https://doi.org/10.1016/j.arabjc.2020.09.004.
CrossRef - Gopinath K, Karthika V, Sundaravadivelan C, Gowri S, Arumugam A Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. Journal of Nanostructure in Chemistry. 2015; 5(3):295-303. https://doi.org/ 10.1007/s40097-015-0161-2.
CrossRef - Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano letters. 2006;6(8):1794-807. https://doi.org/10.1021/nl061025k
CrossRef - Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS nano. 2008;2(10):2121-34. https://doi.org/10.1021/nn800511k
CrossRef - Burello E, Worth AP. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology. 2011;5(2):228-35. https://doi.org/10.3109/17435390.2010.502980
CrossRef - Agarwal H, Menon S, Kumar SV, Rajeshkumar S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chemico-biological interactions. 2018;286:60-70. https://doi.org/10.1016/j.cbi.2018.03.008
CrossRef - Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proceedings of the National Academy of Sciences. 2007;104(7):2050-5. https://doi.org/10.1073/pnas.0608582104
CrossRef - Azzam EM, Zaki MF. Surface and antibacterial activity of synthesized nonionic surfactant assembled on metal nanoparticles. Egyptian Journal of Petroleum. 2016;25(2):153-9. https://doi.org/10.1016/j.ejpe.2015.04.005
CrossRef - Chatterjee S, Bandyopadhyay A, Sarkar K Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application. Journal of Nanobiotechnology. 2011;9(1):1-7. doi:10.1186/1477-3155-9-34
CrossRef - Pitchumani Krishnaveni M, Annadurai G Biosynthesis of nanoceria from bacillus subtilis: characterization and antioxidant potential. Res J Life Sci. 2019;5(3):644. https://doi.org/10.26479/2019.0503.52
CrossRef - Pozharitskaya ON, Obluchinskaya ED, Shikov AN Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Marine drugs. 2020; 18(5):275. https://doi.org/10.3390/md18050275.
CrossRef - Asati A, Santra S, Kaittanis C, Perez JM. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS nano. 2010;4(9):5321. https://doi.org/10.1021/nn100816s.
CrossRef - Nourmohammadi E, Khoshdel-Sarkarizi H, Nedaeinia R, Sadeghnia HR, Hasanzadeh L, Darroudi M, Kazemi oskuee R. Evaluation of anticancer effects of cerium oxide nanoparticles on mouse fibrosarcoma cell line. Journal of cellular physiology. 2019;234(4):4987-96. https://doi.org/10.1002/jcp.27303.
CrossRef - Nadaroglu H, Onem H, Alayli Gungor A. Green synthesis of Ce2O3 NPs and determination of its antioxidant activity. IET nanobiotechnology. 2017;11(4):411-9. https://doi.org/10.1049/iet-nbt.2016.0138.
CrossRef - Soren S, Jena SR, Samanta L, Parhi P. Antioxidant potential and toxicity study of the cerium oxide nanoparticles synthesized by microwave-mediated synthesis. Applied biochemistry and biotechnology. 2015;177:148-61. DOI 10.1007/s12010-015-1734-8
CrossRef - Nethravathi PC, Shruthi GS, Suresh D, Nagabhushana H, Sharma SC. Garcinia xanthochymus mediated green synthesis of ZnO nanoparticles: photoluminescence, photocatalytic and antioxidant activity studies. Ceramics International. 2015;41(7):8680-7. https://doi.org/10.1016/j.ceramint.2015.03.084.
CrossRef - Suresh D, Shobharani RM, Nethravathi PC, Kumar MP, Nagabhushana H, Sharma SC. Artocarpus gomezianus aided green synthesis of ZnO nanoparticles: Luminescence, photocatalytic and antioxidant properties. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015;141:128-34. https://doi.org/10.1016/j.saa.2015.01.048.
CrossRef - Madan HR, Sharma SC, Suresh D, Vidya YS, Nagabhushana H, Rajanaik H, Anantharaju KS, Prashantha SC, Maiya PS. Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2016;152:404-16. https://doi.org/10.1016/j.saa.2015.07.067.
CrossRef - Al-Mashhadani AH. Study of in vitro and in vivo free radical scavenging activity for radioprotection of cerium oxide nanoparticles. Iraqi Journal of Physics. 2017;15(35):40-7. https://doi.org/10.30723/ijp.v15i35.52.
CrossRef - Aseyd Nezhad S, Es‐haghi A, Tabrizi MH. Green synthesis of cerium oxide nanoparticle using Origanum majorana L. leaf extract, its characterization and biological activities. Applied Organometallic Chemistry. 2020 Feb;34(2):e5314. https:// doi. org/ 10. 1002/ aoc. 5314.
CrossRef - Homayouni-Tabrizi M, Asoodeh A, Mashreghi M, Rezazade Bazaz M, Kazemi Oskuee R, Darroudi M. Attachment of a frog skin-derived peptide to functionalized cerium oxide nanoparticles. International Journal of Peptide Research and Therapeutics. 2016 Dec;22:505-10. https:// doi. org/ 10. 1007/ s10989- 016- 9531-y.
CrossRef - Khan M, Raja NI, Asad MJ, Mashwani ZU. Antioxidant and hypoglycemic potential of phytogenic cerium oxide nanoparticles. Scientific Reports. 2023 Mar 18;13(1):4514. https://doi.org/10.1038/s41598-023-31498-8.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






