How to Cite | Publication History | PlumX Article Matrix
Microbe-Based Synthesis of Gold Nanoparticles and its Catalytic Applications
Rakshi Anuja Dinesh , Srishti Raja
, Srishti Raja , Nisha Kishanlal
, Nisha Kishanlal , Valli Nachiyar C*
, Valli Nachiyar C* and Swetha Sunkar
and Swetha Sunkar
Sathyabama Institute of Science and Technology (Deemed to be University), Jeppiaar Nagar, Rajiv Gandhi Salai, Chennai, Tamilnadu, India.
Corresponding Author E-mail:vnachiyar@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3106
ABSTRACT: The application of microbes to synthesize metallic NPs is due to their increased capability to survive at maximum concentrations of metallic ions. The gold nanoparticles are used as the catalytic agent in the degradation of organic dyes, bioremediation, and antibacterial and antimicrobial effects. Despite the fact that the production of metal gold nanoparticles is relatively new, the relationships amongst microorganisms and metals have been thoroughly documented. In the subject of bioremediation, the capacity of bacteria to accumulate metals has also been acknowledged. Recently, the diversity of microorganisms has been used as factories for fabricating AuNPs both intracellularly and extracellularly. Microbial cells, upon treatment with gold salts, synthesize gold nanostructures, which are further isolated and purified using varied methodologies to acquire AuNPs. Control over the size and shape of AuNPs can be achieved by manoeuvring the main growth parameters.
KEYWORDS: Antimicrobial; Bioremediation; Catalysis; Fabricate; Gold Nanoparticles; Microbes; Nanostructures
Download this article as:| Copy the following to cite this article: Dinesh R. A, Raja S, Kishanlal N, Nachiyar C. V, Sunkar S. Microbe-Based Synthesis of Gold Nanoparticles and its Catalytic Applications. Biosci Biotech Res Asia 2023;20(2). |
| Copy the following to cite this URL: Dinesh R. A, Raja S, Kishanlal N, Nachiyar C. V, Sunkar S. Microbe-Based Synthesis of Gold Nanoparticles and its Catalytic Applications. Biosci Biotech Res Asia 2023;20(2). Available from: https://bit.ly/3LcPfby |
Introduction
By combining biologic, mechanical, and chemical elements, nanotechnology creates nano-sized particles with specialised capabilities22, 37, 43, 64. Noble metallic nanostructures like silver, gold, platinum, palladium, and others, as well as artificial, non-metallic oxides such as zinc oxide and titanium oxide, have been widely used for this purpose owing to their distinct electrical, mechanical, optical, chemical, and magnetic characteristics1,31,36,52. Nanoparticles are used in a variety of fields, including drug discovery, optoelectronics, display devices, catalysis, the manufacture of biological sensors, the diagnosis or monitoring of diseases like cancer cells, and the detection of environmental toxic metals or reagents. These special properties include their larger surface area per unit volume, size, shape such as spherical or rod, and others10, 17, 60. There has been a boom in the promotion of sustainable and ecologically friendly methods that do not employ dangerous ingredients for the manufacture of nanostructures. Due to the employment of reductants that are extremely unstable or toxic to humans or the environment and are also very expensive for upscale manufacture, physical or chemical approaches for generating metal nanoparticles are neither efficient nor sustainable. Microorganisms such as fungi, bacteria, algae, viruses, and plants are used in biosynthesis as reducing agents. Algae is referred to as the “bio-nano factories” since it is ecologically advantageous, affordable, macroscopic, and has a high metal absorption capacity20, 21. Toxic compounds created during nanoparticle manufacturing can be rapidly destroyed by enzymes found in microorganisms or plants. The binding of Au (III) ion to the cell surface occurs during the reduction of Au (III) ion to Au atom, and other reduced Au also binds and aggregates to produce gold nanoparticles. The localised surface plasmon resonance (LSPR), the shift in individual specialized levels of energy, the novel distinguishing qualities with the quantum size effects, and other electrical properties are just a few of the unusual characteristics of gold (Au) that are produced in nanosized particles. Numerous jobs associated with many fields of application, including medicine, diagnostics, therapeutic or tumor treatment, such as anti-angiogenesis, anti-arthritic, antimalarial medications, are carried out by gold nanoparticles of various shapes produced by diverse microorganisms. On electrochemically active biofilms (EABs), nanocomposites of Ag-graphene, Au-graphene, or Au-SnO2 are generated, which help in the bio-reduction of gold nanoparticles without the use of capping or surfactants. These nanocomposites are used in a range of applications, including detectors, photoelectrodes, optical devices, photocatalytic degradation, photoelectric, ultra – capacitors, due to their superior photoelectrochemical and photocatalytic characteristics32, 33, 34.
Synthesis of gold nanoparticles
Types of synthesis
Metal capture
A straightforward approach for producing gold nanoparticles using an ion coater and a viscous liquid capture medium has been described below. The nanomaterials’ size grew as the discharge current increased during stuttering with the thermal treatment. SPR peaks were visible in the spectroscopic analysis of the gold colloidal solutions, proving that the ion applicator was successful in producing gold nanoparticles. Ion coaters are relatively basic deposition apparatuses, as opposed to typical sputtering systems, which are made up of sophisticated components, allowing this nanoparticle production approach to be inexpensive in both cost and energy consumption. Additionally, direct sputtering deposition of homogeneous gold nanoparticles onto glycerin is a simple, rapid, and inexpensive approach that does not require additions, reducing, or steadying agents. This is a non-toxic, ecologically acceptable technology that may be utilized to create nanoparticles for biological and medicinal purposes38.
Enzymatic reduction
At early times, UV-Vis spectroscopy, transmission electron microscopy (TEM), and dynamic light scattering are used to explore the production of gold nanoparticles (Au NPs) from HauCl4 employing EDTA as a reducing agent (DLS). The size of the resulting Au NPs is determined by the responsiveness of ionized Au(III) species and the charge distribution of EDTA molecules with pH. Furthermore, by changing the pH of the reaction media, it is possible to adjust the size of Au NPs between 25 and 100 nm. Time-resolved Ultra violet and TEM examination of the nucleation and development of Au NPs revealed the presence of nanotubes that gradually grow in size as the connected network is divided into tiny segments prior to the creation of the final spherical particles. Several 1 Dimension- and 2D14 Nuclear Magnetic Resonance (NMR) spectra were recorded to distinguish process end products and phases emerging from the reduction of Au(III) by EDTA. Our findings reveal that EDTA-mediated reduction of Au(III) à Au(0) NPs is accompanied by decarbonylation of EDTA and the generation of formaldehyde, which is liable on pH, inhibits with the reduction and acts to develop synthesis. Aside from the enormous elasticity that EDTA can provide for additional chemical conjugation to expand the surface functionality and thus in order to widen the scope of the application, a lot of synthetic approaches involving the development of nanostructures and core-shell metal nanoparticles employ hazardous formaldehyde in excess amounts as a reductant. Utilizing EDTA as a reduction agent in the preliminary step for replacing the use of formaldehyde in the synthesis can be a practical first step given the involvement in biological systems of such nanomaterials and the crucial role of formaldehyde as a reducing agent in the fabrication process19.
Capping
Capping agents are often used in colloidal dispersions to precisely control the growth, aggregation, and physicochemical nanostructures54. The capping agent is an amphiphilic molecule with a polar group and a non-polar hydrocarbon tail. It confers functionality and improve compatibility with other phases due to their amphiphilic nature. As seen in Fig 1, the polar head interfaces with the metal atom of the nanosystem, whereas the non-polar tail engages with the surroundings23. Surfactants, tiny ligands, polymers, dendrimers, cyclodextrins, and polysaccharides have all been utilized as capping agents in the creation of nanoparticles. All of these have been effectively used as capping agents capable of inducing minor modifications in nanoparticles, disclosing substantial medicinal and environmental cleaning effects 56. The numerous forms of capping agents are depicted in Fig 2.
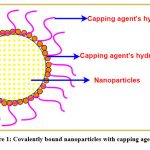 |
Figure 1: Covalently bound nanoparticles with capping agents28. |
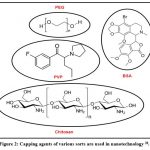 |
Figure 2: Capping agents of various sorts are used in nanotechnology 28. |
Three distinct capping systems were used to create gold nanoparticles (NPS): a tetraalkylammonium salt, an alkanethiol, and a thiol-derivatized neoglycoconjugate. Gold nanoparticles supported on a porous TiO2 substrate have also been studied. The electrical performance of various capped systems in terms of electron/hole density has been determined using X-ray absorption spectroscopy (XAS). Size and surface impacts, and the significance of the micrographs, have all been thoroughly investigated using EXAFS (extended X-ray absorption fine structure) data. Due to Au-S charge transfer, very tiny gold NPs functionalized with thiol-derived molecules exhibit a rise in d-hole density at the gold site. For high S: Au atomic ratios and core-shell microstructures where an atomically abrupt Au-S boundary is unlikely to exist, this effect overcomes size effects which contribute to a modest increase in d-electron density. It has also been demonstrated that thiol functionalization of extremely tiny gold NPs introduces a significant distortion when compared to the fcc order. In contrast, d-electron thickness at the gold site may increase more than in bare gold clusters as a result of electron transfer from reduced support oxides to gold NPs.
Strategies for the Production of Nanoparticles Using Microbes
It is yet unknown exactly how biological agents can be used to make nanoparticles. This is due to the diverse ways that different biological agents interact with metal ions to form nanoparticles. A large number of microorganisms produce inorganic substances either intracellularly or extracellularly. However, the process for the creation of nanoparticles differs amongst biological entities. The intracellular technique requires a unique ion transport system within the microbial cell. The microbial cell wall plays a crucial role in the intracellular creation of nanoparticles. The procedure is an electrostatic interaction between the positively charged ions of the metal ions and the negatively charged ions of the cell wall. The ions are changed into nanoparticles by the enzymes present in the cell wall, which are then distributed throughout the cell wall Fig 3. A sequential approach for intracellular manufacture of nanoparticles utilizing Verticillium sp. 47 explains that the mechanism of nanoparticle creation includes trapping, bioreduction, and capping. When a fungal cell surface comes into touch with metal ions, electrostatic interactions occur and the ions are trapped. Enzymes found in the cell wall convert metal ions to metal nanoparticles. The earliest phase in the bacterium Lactobacillus sp. is the nucleation of metal ion clusters 51. As a result, the bacterial cell and metal clusters interact electrostatically, resulting in the formation of nanoclusters. The microscopic nanoclusters are then transported through the bacterial cell wall. Metal ion reduction occurs on the surface of mycelia together with the cytoplasmic membrane in actinomycetes, leading to the creation of nanoparticles 2.
Thermomonospora sp., an alkalothermophilic actinomycete that decreases gold ions, produces extracellular gold nanoparticles. Plectonema boryanum, a photoautotrophic cyanobacterium, was previously characterised for its extracellular manufacture of silver nanoparticles 40. The synthesis was carried out in the dark for up to 28 days at temperatures of 25, 60, and 1000°C.
Only at 1000°C did silver entirely precipitate from solutions after 28 days. The precipitate was created by adding AgNO3 to the microbial cells both inside and outside. On the cell surface, silver nanoparticles were generated at 600°C. At 1000°C, silver nanoparticles covered the cyanobacterial cells. The range of the nanomaterials inside the cell was between 1 and 40 nm. The silver nanoparticles that precipitated outside of the bacterial cells were between 1 and 200 nm in size.
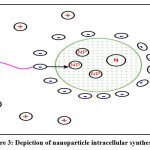 |
Figure 3: Depiction of nanoparticle intracellular synthesis25. |
Microbe-mediated biosynthesis of gold Nanoparticles
Due to their ease of cultivation, rapid rate of development, and ability to thrive under temperature, atmospheric pH, and pressure parameters, microorganisms like fungi, algae, yeast and bacteria are frequently preferred for NP synthesis. Different biological agents interact with various metal solutions to produce NPs in various ways. Metal ions are first trapped on the cell surface, and then, with the influence of enzymes produced by the bacteria, they are reduced to NPs.4
Bacteria
In addition to being efficient catalysts for material synthesis, metal-reducing bacteria have been proven to be ecologically beneficial bioremediation agents. In general, it has been discovered that the genus Shewanella produces a variety of metal oxides by microbial respiration mechanisms. Crystal formation in bioremediation processes is usually facilitated by electron transfer from reduced organic to oxidised inorganic chemicals via microbial dissimilatory anaerobic respiration. It is well known that the genus Shewanella can assist in the reduction of inorganic metals acting as electron acceptors and the oxidation of organic acids acting as electron donors.
The extracts obtained from Enterobacter cloacae MF01 were used to biosynthesize gold-containing nanostructures in both aerobic and anaerobic environments4 .
Actinobacteria
Due to their distinctive and powerful visible-range plasmon resonance and their use in biological sciences, gold nanoparticles (Au NPs) are regarded as a very important subject of study. A safe, hygienic, non-toxic, and eco-friendly technique for creating nanoparticles is required46. It is generally recognized that Streptomyces sp. has the potential to create a wide range of secondary metabolites, including antibiotics, immunosuppressive agents, and a variety of other physiologically active substances. Streptomyces-related organisms are commonly used for industrial manufacturing processes as well as the enzyme and pharmaceutical sectors3. Streptomyces hygroscopicus59, Streptomycetes viridogens (HM10), as well as Streptomyces avidinii have all been implicated in the production of Au NPs. Biosynthesized zinc oxide nanoparticles using Aeromonas hydrophila were reported to have antifungal activity against Candida spp.—Aspergillus flavus, Aspergillus niger, and Candida albicans. Aspergillus tubingensis and Bionectria ochroleuca demonstrated excellent extracellular capacity to synthesize silver nanoparticles58 and Aspergillus flavus and Bionectria Allicin-loaded polybutyl cyanoacrylate nanoparticles were tested for their effectiveness on Microphyton gypsum and Microsporum canis in vitro 41. The goal of the current work is to evaluate the anti-dermatophytic activity of synthetic Au NPs with the help of Streptomyces sp. VITDDK3 against M. gypseum and Trichophyton rubrum.
Actinobacter spp.
Due to their remarkable optoelectronic capabilities, there has been a lot of interest in improving the production processes for anisotropic metal nanoparticles. Various shapes, such as bars, blocks, tetrapods, and crystals, can be created by combining different processes. When studied with gold chloride and in the presence of Bovine Serum Albumin (BSA), Bharde et al. proved that the bacterium Actinobacter spp. produces anisotropic AuNPs physiologically. Additionally, it has been observed that the synthesis of AuNPs takes place simultaneously with the bacterium’s stimulation of protease in the presence of BSA. BSA speeds up the production of AuNP while also allowing for some shape control. The response rate and particle morphology are strongly influenced by basic test parameters, such as incubation temperature and oxygen availability. According to various test results, chemical proteases can work to both reduce and reshape the coordinating agent 9.
Bacillus marisflavi
An essential component of the investigation of nanotechnology is the creation of a green method for synthesising nanomaterials. The extracellular synthesis of AuNPs using Bacillus marisflavi YCIS MN 5 is described in a paper by Nadaf and Kanase as being more environmentally friendly. By adding gold chloride to a cell-free extract (CFE) of B.marisflavi, they were produced at room temperature with an internal temperature of 90.6 h. The combined nanoparticles were found to be crystalline spheres with an average length of around 14 nm. Because the CFE served as both a lowering and a stabilising agent, no additional stabilising or capping agents were needed. The ability of these AuNPs to reduce methylene blue and Congo purple dyes has been examined. It was discovered that the discount response exhibits pseudo-first-order kinetics, with response charges of 0.2484 and 0.2192 min-1 for methylene blue and Congo purple, respectively. It was discovered in this way that the produced AuNPs have excellent synergistic motion in the breakdown of and methylene blue and Congo purple. These results support the use of manufactured gold nanoparticles mediated by B. marisflavi as prospective nanocatalysts for the breakdown of methylene blue and Congo purple dyes50.
Pseudomonas veronii AS41G
Biogenic nanoparticle synthesis had already drawn a lot of attention as a green technique since the advent of nanotechnology. Baker and Satish suggested synthesising AuNPs extracellularly utilising the cell-free supernatant of Pseudomonas veronii AS41G, a new endophyte isolated from Annona squamosa L. The use of UV-Visible spectrophotometry to confirm the production of gold nanoparticles. Different functional groups responsible for stabilising AuNPs and lowering metal salts were predicted by FTIR analysis. The XRD pattern demonstrated the crystallinity of the nanoparticles. The morphological characteristics of nanoparticles of various sizes were revealed by TEM investigation. In contrast to conventional techniques, Baker and Satish suggest this simple process for the production of AuNPs, emphasising the innovative role of the bacterium Pseudomonas veronii AS41G7.
Plectonema boryanum
In this study, Lengke et al. synthesised AuNPs using the filamentous cyanobacterium Plectonema boryanum UTEX 485. The results suggested that the cubic, 100 nm-sized manufactured nanoparticles39.
Fungi
Due of the stiff opposition of fungal mycelial mesh to increased agitation and flow in bioreactors, fungal NP synthesis is chosen over other microbial synthesis techniques. It was found that the component of the fungal cell wall system most crucial to heavy metal complexation and the production of NPs is chitin. Fungi are more productive than bacteria in the production of NPs because of their large number of bioactive metabolites, robust aggregation, and increased efficiency. Many filamentous fungi have already been reported to be capable of Au NP production. This research explored multiple techniques to biosynthesize Au NPs. Fungi, as opposed to bacteria, can make more nanoparticles because they release more proteins, which results in higher overall production of nanoparticles45.
Candida albicans
To decrease metal ions and ultimately transform nanocrystals into their insoluble state, dimorphic fungus release an unusual amount of both intracellular and extracellular redox proteins. Due to stability, oxidation resistance, biocompatibility as well as the various microbial synthesis strategies that have been widely used up to this point for achieving improved yield and stability, gold nanoparticles have an advantage. Candida albicans was cultivated in normal gravity and induced microgravity, and the filtrate from that culture was used to create gold nanoparticles in a novel, environmentally friendly approach. Strong plasmon resonance was confirmed at 540–550 nm by gold nanoparticles. XRD , FTIR, TEM, and UV-Vis spectroscopy were used to characterise the differences in the produced nanoparticles under the two circumstances62.
Trichoderma viride
Several Trichoderma species are found in soil and are known to be effective biocontrol agents and stimulators of plant development 13,24. The species are also now being used in nanotechnology, notably in the creation of metal nanoparticles (NPs). More recently, reports of their resistance to various nanocompounds have been made; however, little is known about how these chemicals affect Trichoderma relationships and how they contribute to the manufacture of metal NPs through the tolerance these organisms develop to them 8,42. Trichoderma, on the other hand, is suggested as potential bioremediation for contaminated environments because of its high tolerance to metals65.
Rhizopus oryzae
A simple, one-pot green chemical method is presented for producing innovative gold nano-bio-conjugates (AuNBC) by reducing chloroauric acid (HauCl4) along with a protein extract from Rhizopus oryzae. By adjusting the HauCl4-protein extract ratio, AuNBCs have been created in a range of diameters from 5 to 65 nm. Measurements of spectroscopy, electron microscopy, electrophoretic mobility, and light scattering have been used to describe the conjugates. The aqueous AuNBC suspensions were discovered to be extremely stable over a wide pH, ionic strength, and temperature range. The effects of ionic strength and pH revealed that stabilisation results from electrostatic repulsion brought on by the conjugate proteins’ negative charge. Less than the fungal proteins’ denaturation temperature, the AuNBCs remained stable. By examining the reduction of p-nitrophenol with borohydride, the catalytic activity of the synthesised AuNBCs was measured16.
Yeast
Through the use of an aqueous extract of Baker’s yeast, gold nanoparticles with a diameter of 13.0 0.9 nm were produced under visible light (Saccharomyces cerevisiae). It has been established for the first time that the components of the aqueous yeast extract are , glucose, indole-3-acetic acid, trimethylsilyl derivatives of butan-2,3-diol and undecanoic acid. For gold nanoparticles, this extract functions as a reducing and capping agent6.
Yarrowia lipolytica
It is believed that Yarrowia lipolytica produces gold nanoparticles that are connected with cells. A component that accounted for the formation of nanoparticles was found to be the pigment melanin, which is connected to yeast. Gold nanoparticle formation was mediated by cell-extracted melanin as well as that which was brought about by treating cells with 3,4-dihydroxy-L-phenylalanine (L-DOPA). Several commonly used approaches had been employed to characterize these nanoparticles. The resulting nanoparticles were powerful antibiofilm agents4.
Magnusiomyces ingens LH-F1
The utilisation of cell-free extracts of the yeast Magnusiomyces ingens LH-F1 allowed for the development of an environmentally friendly and organic method for the manufacture of gold nanoparticles (AuNPs). A prominent absorption band at about 540 nm, which corresponds to the surface plasmon resonance of AuNPs, was confirmed by UV-Vis spectra. According to images taken using a transmission electron microscope, AuNPs have pseudo- and almost spherical forms. A few proteins with amino- and carboxyl groups in cell-free extracts were absorbed in the surfaces of nanoparticles, according to analysis of sodium dodecyl sulfate-polyacrylamide gel electrophoresis Fourier transform infrared spectroscopy. These proteins can function as reducing and capping agents for the synthesis of AuNPs55.
Sponge
Acanthella elongate
In order to create gold (Au) nanoparticles from gold precursors, an extract from the marine sponge Acanthella elongata of the early phylum Porifera was used. The reduction of gold ions to nano-sized Au particles was specifically caused by water-soluble organics present in marine sponge extract. The sponge extract was mixed with a 10-3 M HauCl4 aqueous solution at 45°C, where it was changed to a pinkish ruby-red colour. After 4 hours of continuous stirring, the bioreduction was confirmed. A peak of around 526 nm was confirmed by the UV-Visible spectrum of the aqueous media containing gold nanoparticles. With dimensions ranging from 7 to 20 nm, high-resolution transmission electron micrographs (HR-TEM) verified the monodispersed and spherical morphology27. The gold precursor was reduced while attempting an extraction from the marine sponge using water, resulting in spherical monodispersed gold nanoparticles 20.
Actinomycetes
Actinomycetes are recognised as one of the best subgroups of microbial species and are of commercial importance due to their saprophytic behaviour and production of several bioactive extracellular enzymes and secondary metabolites. Streptomyces species are thought to be the leading candidate for the biosynthesis, and only a small number of actinomycete taxa, including , Nocardia, Streptomyces, Thermomonospora and Rhodococcus and Thermomonospora, have been proposed57.
Aerobic gram-positive bacteria known as actinomycetals are often stationary and orderly. Actinomycetes are frequently simple to cultivate in conventional settings. The majority of actinomycetes in soil are still saprophytic, and there are very few harmful species. Actinomycetes that are isolated from soil create a variety of unique antibiotics, some of which have significant medical use, such as streptomycin and neomycin35.
Algae
Sargassum cymosum, an algae extract, was used in the green synthesis process to create gold nanoparticles (Au-NPs). Different extracts, quantities of tetrachloroauric acid, temperatures, pH levels, and stirring rates were used to complete the synthesis. After a few minutes, the reaction medium’s colour began to change, and subsequent dynamic light scattering (DLS) and UV-Vis spectrophotometry confirmed the formation of gold nanoparticles (Au-NPs), with an average size that varied based on the experimental conditions between 5 and 22 nm14.
Stoechospermum marginatum
The study’s important alginophyte was Stoechospermum marginatum (Phaeophyta, Dityotales). A novel spatane diterpene, 17,18-epoxy, 5(R), 16-dihydroxyspat 13(14)-ene, was discovered by studying the biochemical composition of these seaweed constituents, including vitamins, proteins, fatty acids, trace elements, amino acids and minerals in S. marginatum, a brown alga5, 67.
Sargassum wightii Greville
Using Sargassum wightii, the investigation of the extracellular biosynthesis for gold nanoparticles and carried out the rapid production of gold nanoparticles. The plasmon absorbance of AuNPs was confirmed by a peak at 527 nm in the UV-Vis spectra of the aqueous media containing gold ions. The production of gold nanoparticles in the 8–12 nm size range was confirmed by transmission electron microscopy (TEM). The gold nanoparticles’ X-ray diffraction (XRD) spectra showed Bragg reflections that were in line with their size63.
Merits and Demerits of microbe mediated gold nanoparticle synthesis
Table 1: Merits and Demerits of Microbe-Mediated Gold Nanoparticle synthesis
| METHOD | MERITS | DEMERITS |
| Top-down synthesis | Extremely regulated particle shape and size dispersion | · Extreme environment
· Cutting-edge equipment · Inflated prices |
| Bottom-up synthesis | · Cost-effective
· Exact control over the distribution and form of the particle sizes |
Environmental degradation and lethality are increased by leftover poisons and potentially dangerous capping ligands. |
| Bacteria | Agents that are economical, safe for the environment, and biologically effective for stabilising AuNPs | · Massive nanoparticles that have a wide particle size distribution
· Organic components must be present for pure nanoparticles to be produced. |
| Fungi | · Smaller than bacterially produced nanoparticles
· Environmentally safe · Cost-effective · For the stability of AuNPs, capping agents and extracellular redox reactions are present in high concentrations. · Simple scale-up |
· Wide range of particle sizes
· Poor reproducibility · Impossibe to produce purified nanoparticles devoid of organic components |
Catalytic Applications
In recent centuries, the advances and approaches in nano sciences and nanotechnologies has been increasing by researchers and they have given a broad aspect of these studies and among them Nano catalysis stands as one of the most prominent aspects. Catalysis by Nanoparticles have become central concept as the oil consumption increasedin early 1960s. Catalysis now has a new chance to take the lead in society because to the rarification of energy sources and the requirement for a more rational use of the energy that is currently available. Heterogeneous and homogeneous catalyst are the two basic classifications used to categorise catalysis. The transformation of raw materials into chemicals has been greatly aided by heterogeneous catalyst, which operates at the solid-gas interface, It is technically the most frequently used because it offers greater advantages in product yield through high turnover and long lifespan through low deactivation. whereas homogenous catalyst, which often uses transition metal elements that is surrounded by ligands and dissolved in organic solvents, involves in improved control of kinetics, mechanism, and consequently of high selectivity was only developed in last quarter of 20th centuries. These types of catalysts exhibit extremely high selectivity and are crucial to the pharmaceutical sector. Nanocrystals (such as chitin, Nanofiber, polymers) of size between one to a few nanometers give highest catalytic efficiency, and their support is more crucial for the synergistic activation (combined effect of two drugs is greater than the sum of individual drug activity) of substrates, as best demonstrated by Haruta’ s well-known method for catalysis of CO oxidation by O2 by oxide – supported gold nanoparticles (NP) at 200 K. The discovery that the catalytic selectivity and turnover correlate with the size and shape of nanoparticles has led to the promotion of bottom-up strategy over top-down one61, rendering this traditional frontier obsolete. Across the globe, numerous scientists are involved in common objective for new upcoming techniques for synthesis of nanomaterials, and nanocatalyst has a chance to produce catalysts with controlled size and shape. Photocatalytic degradation of organic dyes by nanoparticles of has attracted lot of research interest in the past decade. However, this may limit its applications by slower reaction rate, requirement of strict irradiation conditions and high photocatalyst concentration of. This observation, in combination with characterisation methods, enables us to conduct investigations at the molecular or atomic level of catalyst particles under ideal circumstances and leads to revolutionary advancements in the branch of catalysis science known as “nanocatalysis.”
Degradation of Dyes
Organic (azo) dyes (which is considered to be one of the major groups of effluents widely used in textile, plastic, pharmaceutical sector and many other industries) 49 waste water discharged after improper treatment has direct impact harming environment and living creatures because of the discharge of unwanted product in air and water bodies, while the hazardous effects of organic dyes in waste water have been a major concern due to the significant pollution issues they have caused. Although various methods were performed, the majority of them had one or more drawbacks such as high cost, release of secondary pollutants (such as ozone, peroxyacyl nitrates (PANs), nitrogen dioxide), low efficiency, complexity etc. These dye industries deplete large amount of high content colour pollutant generated by Azo basic, acidic, cationic, etc these azo dyes are carcinogenic, highly poisonous and mutagenic in nature and also are resistant to destruction by conventional methods. The presence of these hazardous and complicated chemicals in environment has given rise to bioaccumulation, where heavy metals get accumulated inside human and animal bodies which gives rise to various disorders such as dysentery, skin irritation, neuro-muscular and central nervous system disorder and liver and kidney damage44. Due to their ability to block sunlight, the buildup of these dyes in water bodies results in eutrophication, decreases the capacity for reoxygenation, and severely harms aquatic creatures49. Additionally, they must be susceptible to microbial attack resistance. They cannot degrade, thus wastewater treatment systems do not usually remove them from . The advantages of catalytic degradation by nanoparticles over traditional methods including adsorption, ultrafiltration, chemical, and electrochemical approaches are fast oxidation, no creation of polycyclic products, and oxidation of contaminants. There are other several methods introduced for removing the dyes from the industrial effluents by synthesizing nanoparticle including physical (milling, vapour phase synthesis, mechanical grinding), chemical (pyrolysis, electrolysis, sol gel process) and green synthesis of nanoparticles commonly known as biological approaches. The biological method (involvement of microorganisms) is seen to be the most advantageous of all because it is quick, simple, environmentally safe, and carried out in a comfortable environment. In the recent years, utilizing metallic nanoparticles (gold, silver, titanium, platinum etc) for the degradation of hazardous dyes has drawn wide attention, since metal nanoparticles have high surface area, it increases catalytic activity and can be easily separated and recycled. It is possible to create nanoparticles for the catalytic degradation of organic or inorganic dyes using a variety of physical, chemical, and biological processes. For instance, the biological approaches require raw materials such as plants, bacteria, fungi, and algae, whereas the physical route requires large energy inputs to synthesise silver nanoparticles. Nanoparticles used for the catalytic degradation of textile or organic dyes can be synthesized by various processes such as physical, chemical and biological process. On the other side, chemical approaches are more suited and adaptable in nature to achieve well-controlled nanoparticles in terms of shape, structure, and stability. Nanoparticles are thought to be the best method for improving water quality and environmental safety since they reduce potentially dangerous effects.
4-nitophenol
For environmental remediation, it is crucial to develop effective nanoparticle catalysts that can break down organic contaminants or transform them into chemicals that are safe and even valuable. In humans and animals, 4-nitrophenol (4-NP) is a major pollutant that can cause blood problems, eye, skin, and kidney damage, as well as poisoning of the central nervous system53. In the chemical industry, 4-NP has been widely employed as a raw material for the production of insecticides, synthetic colours, medicines, for treating leather, and in a number of military applications. For the reduction reaction of 4-NP, a number of silver, titanium, and gold nanoparticle-based catalysts have been identified.
Zr and Ag co-doped with TiO2 nanoparticles that was carried out at room temperature (30°C), using sodium borohydride (NaBH4) acting as reducing is one of the approch for reduction of 4-NP53 , converting 4-Nitophenol to 4-aminophenol. TiO2 has been used to increase the photocatalytic activity using techniques including noble metal doping. As it can be seen, transition element doping of TiO2 results in crystal defects that can alter the material’s photocatalytic capabilities. TiO2 is regarded as one of the best supports for metal nanoparticles because of its chemical and thermal stability, non-toxicity, and affordability.
AuNPs, which demonstrate exceptional catalytic characteristics for dyes degradation and 4-NP reduction with noticeably greater catalytic efficiencies than many previously described heterogeneous catalysts, can also be used for 4-NP degradation.
P-nitroaniline
The non-biodegradable pollutant P-nitroaniline (PNA)71 is typically produced as an intermediary in the manufacturing of drugs, azo dyes, and corrosion inhibitors. However, PNA is toxic once it contaminates the water and is regarded as a high-risk chemical for human health and aquatic microorganisms even at comparatively low concentrations due to the presence of a nitro group in the aromatic ring, which enhances stability and results in resistance to biological degradation. Iron oxide magnetic nanoparticles (MNPs) are a potential method for degrading resistant organic pollutants, such p-nitroaniline (PNA), in wastewater because they can efficiently activate persulfate and produce free sulphate radicals (SO-4). MNPs were synthesized using the liquid-phase co-precipitation process, and their shape and structure were examined using X-ray diffraction (XRD) and transmission electron microscopy (TEM). Sulfate radicals (SO-4) can destroy the target contaminant PNA depending on several factors, including persulfate, Fe3O4, PNA concentrations, pH, and temperature. The findings demonstrate that PNA decomposition follows pseudo-first-order kinetics (behaving like first-order reaction). According to research, the mineralization rate reached 67% after 300 minutes of reaction time, while the PNA removal efficiency was roughly 100% within 270 minutes.
Oxidation of Hydroxylamine
In particular, hydroxylamine is utilised as a reducing agent because it is a colourless, odourless nitrogenous base (NH3O) that reacts similarly to ammonia but is less basic. It is also a very important intermediate in nitrification, having a direct connection with the production of nitrous oxide in biological wastewater treatment processes.
The nanoparticles characterization can be done using X-ray diffraction analysis, energy dispersive X-ray, high resolution transmission electron microscopy, selected area electron diffraction and UV–Vis spectroscopy. The higher dispersity and smaller size of the nanoparticles may account for the observation that the reduction/degradation rate increased with increasing amount of the photocatalyst.
Reduction of methylene blue
A cationic dye and heterocyclic aromatic chemical (tricyclic phenothiazine), methylene blue is used as a staining agent and in medical. Reduction of methylene blue by Nanocatalyst were used more widely making it cost efficient, eco-friendly and more advantageous. ZnO nanoparticles’ photocatalytic activity was studied utilising the Heber Multi Lamp photoreactor(HML), a type of photoreactor, to photodegrade methylene blue in aqueous solution. According to studies, 1–15% of the dye used in the textile industry is lost during the dyeing process and discharged into the wastewater, endangering the environment and harming human health. The reducing chemical NaBH4 is used to reduce Methylene Blue (MB), which establishes the size-dependent catalytic activity of the produced nanoparticles. Data storage media, holographic production, and data recordings are few examples of the many uses for the reversible reduction of MB to Leuco Methylene Blue (LMB) 53, a less hazardous and biodegradable substance.
Copper oxide (CuO) nanoparticles (NPs), which have attracted significant interest recently due to their unique properties such as a high surface to volume ratio, increased activity, compared to those of the bulk materials, and easy synthesis of CuO NPs by chemical precipitation process, which was considered as an excellent method to synthesise CuO NPs in mild conditions, were used in another approach.
Reduction of Rhodamine B
Rhodamine B (RhB), a type of organic dye that contains nitrogen, is dangerous for both people and animals and is frequently employed in industrial colourants used as fluorescent dye in the textile industry69. Therefore, it is vitally important to detect RhB in the environment and catalyse its destruction or reduction using a sustainable biological mechanism. The traditional methods for removing RhB include emulsification, photocatalysis, membrane separation or ultra filtration, and adsorption. However, their widespread use is constrained by their expensive and complicated operations. Ag/Au NPs have been widely used in catalysis, electronics and antibacterial agents because of the outstanding properties of physics and chemistry. Au Nps can be extracted from many environmental resources (soil, microorganisms, etc) and its catalytic application gave a successful role in reduction of organic dye. The dye degradation can be generally visualized in UV-Vis spectroscopy at 554nm. NaBH4 is a strong reducing agent used in this process and the reduction process is traced by colour change by formation of leuco RhB from pale pink to colourless.
Reduction of Methyl orange
Methyl orange dye (MO) which is one of the very common water-soluble azo dyes, frequently used in the laboratory, coloring agents in leather, food, textile, and pharmaceutical industries. Methyl orange is mutagenic and carcinogenic, and it is not easily biodegradable. Copper nanoparticles (Cu NPs) are synthesised using a variety of techniques, including thermal decomposition, plasma, pulsed wire explosion, sol gel, vacuum vapour deposition, electrochemical, radiolysis, and many more18 . The majority of these techniques are challenging and have disadvantages including the use of hazardous organic solvents, high costs, toxic byproducts, extreme reaction conditions, difficulty isolating NPs, longer times, etc. Due to their potent optical, catalytic, electrical, mechanical, antifungal, and antibacterial capabilities, copper nanoparticles (CuNPs) have recieved a great deal of attention. The target dye with Cu Nps and the reducing agent (NaBH4) , aquartz cuvette as a reaction vessel and was kept in UV-Vis spectrometer, to observe a catalytic reduction, finding the range of wavelength by obtaining a peak, the higher the peak the more intense the colour is and resulting in higher concentration. Under ideal circumstances, the dye solutions’ colour would vanish, indicating complete degradation.
Bioremediation
There is an increasing need for new technologies that might accelerate the decontamination of hazardous areas and lower their costs. Recently, more focus has been placed on the usage of nanomaterials, with iron nanoparticles being one of the most useful and inventive methods for cleaning up contaminated sites. Though numerous studies on nanomaterials have been conducted11 little is known about how these materials behave in soil pores, adsorb to mineral particles, and interact with soil microbes.
One common method for treating contaminants in soil, sediment, and groundwater is bioremediation, which employs living things like bacteria and fungi. However, due to the low concentration and restricted dispersion of dissolved oxygen (DO)68 in these locations, the effectiveness of remediation, particularly for microbes that need oxygen to survive, has diminished. One of the oxygen-releasing chemicals, calcium peroxide (CaO2) nanoparticles have been used to increase the effectiveness of cleaning up polluted areas. These nanoparticles react with water in presence of ferrous ions as a catalyst that is used to oxidize contaminants or waste water, this reaction is called Fenton reaction. The supplied oxygen in this reaction rapidly oxidizes pollutants and feeds aerobic microorganisms.
Triclosan, also known as TCS or 2,4,40-trichloro-20-hydroxydiphenyl ether, is a synthetic antimicrobial agent that is frequently used in personal care products, household goods like textiles and plastics,48 as well as for disinfection. TCS and its transformation product, methyl-TCS, a toxic compound, have been found in a variety of environmental matrices, including wastewaters, freshwater, seawater, agricultural soils, and biotic samples like fish and human breast milk, as a result of its widespread applications in products containing TCS and incomplete degradation of TCS in sewage treatment. Microbial palladium nanoparticles from Bacillus megaterium Y-4, a rod-like, gram-positive, aerobic spore-forming bacteria found in a variety of environments, perform well in TCS degradation12. Under aerobic conditions, the B. megaterium Y-4-produced microbial Pd-NPs were employed to detoxify TCS in the presence of formic acid. Formic acid, which has a variety of beneficial qualities, can be employed as a hydrogen source for TCS breakdown when microbial Pd-NPs are present. The cytotoxicity of the contaminated system was gradually reduced due to this technology of increasing TCS breakdown. Due to the need for few chemical reagents, low energy requirements, and low cost, the breakdown of TCS utilising microbial Pd-NPs is thus particularly promising.
Antibacterial effect on Fusarium nucleatum
A gram-negative, obligate anaerobe bacteria known as Fusarium nucleatum (F. nucleatum)70 poses a risk to human health. It can lead to a number of severe diseases, including colorectal cancer, which is fatal to humans, as well as oral problems like periodontitis, gingivitis, and peri-implantitis. Recent developments in nanomaterials that display antimicrobial activity, including nanoparticles that treat infections once they have occurred and inhibit bacterial adhesion66. With a focus on the creation of carrier nanosystems with features that target bacteria and biofilm, researchers have developed nanoparticles with inherent antibacterial activity and nanoparticles serving as nanovehicles to combat infection. AgNPs use a variety of techniques to exhibit their antibacterial activity. The most prevalent antimicrobial action modalities of AgNPs have been identified as adhesion15 to microbial cells followed by penetration inside the cells as a result of ROS and free radical production and regulation of microbial signal transduction pathways.
The method of Surfactin-loaded carrageenan30 oligosaccharides linked cellulose nanofibers (CO-CNF) nanoparticles was developed recently and can inhibit the growth of Fusobacterium nucleatum by showing a reduction in biofilm formation and metabolic activity of the bacteria, which was confirmed by crystal violet and MTT assay, respectively. In terms of immunological response, anti-tumor activity, and stem cell proliferation, carrageenan’s potential medical applications are being researched.
Discussion
The booming technologies introduced in nanotechnology have given a broad aspect of these studies and their applications in modern problems. Nanoparticle and their catalytic role in treatment of wastewater has given a new step towards a better world. Innovative application of contemporary techniques for synthesis of nanomaterials for catalytic reactions in conjunction with a thorough comprehension of basic molecular surface chemistry and improvements in characterization techniques has resulted in significant advancement in some of the most important and chanllenging issues in the field. Numerous organisations throughout the world have been interested in the development of novel approaches for the synthesis of well-defined and well-controlled nanomaterials for catalysis, and current attempts to optimise nanoparticle chemistry are particularly encouraging. Nanoparticles synthesized from microbes and their catalytic role in bioremediation plays an important role in solving major environmental problems. As nanoparticles are used as catalytic process it is neccesary to control their morphology and stability increasing the demand to easy-to-apply methodologies that drive towards sustainable and environment-friendly chemistry. Industrial wastewater can be efficiently treated utilising a variety of metal nanoparticles, including Ag, Fe, Zn, Sn, and Au. Fungal NP synthesis is preferred over other microbial synthesis methods because of the strong resistance of fungal mycelial mesh to higher agitation and flow in bioreactors. Transmission electron microscopy (TEM) was used to establish the synthesis of nanoparticles in Sargassum wightii.
Conclusion
Nanocatalyst played an important role in degradation of organic harmful dyes discharged into the waste water after improper treatment has led to many health problems for humans and aquatic species. Newer environmentally friendly treatments and approaches have been developed to eliminate highly toxic azo dyes When creating nanoparticles from biological sources including plants, algae, and microbes, a number of variables (such as pH, temperature, concentration, etc.) must be optimised in order to produce nanoparticles that are very stable and efficient. The size of the nanoparticles is crucial for the catalytic process; the smaller the size of the nanoparticles, the better the efficiency. Metal nanoparticles produced biologically are used in a variety of industries, including pharmaceuticals, bioengineering, energy, nanotechnology, defence and security, agriculture, wastewater treatment, and optical engineering. It is thought that nanomaterials have a greater chance of totally replacing the traditional approaches for dye degradation.
Acknowledgement
Rakshi AD, Srishti Raja and Nisha conceived and designed the manuscript. Valli Nachiyar C and Swetha Sunkar oversaw and edited the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- Abdel-Mohsen AM, Hrdina R, Burgert L, Krylová G, Abdel-Rahman RM, Krejčová A, Steinhart M, Beneš L (2012) Green synthesis of hyaluronan fibers with silver nanoparticles. Carbohydr Polym 89:411–422. doi: 10.1016/j.carbpol.2012.03.022
CrossRef - Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, Srinivas V, Sastry M (2003) Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete,Rhodococcus species. Nanotechnology 14:824. doi: 10.1088/0957-4484/14/7/323
CrossRef - Alani F, Moo-Young M, Anderson W (2012) Biosynthesis of silver nanoparticles by a new strain of Streptomyces sp. compared with Aspergillus fumigatus. World J Microbiol Biotechnol 28:1081–1086. doi: 10.1007/s11274-011-0906-0
CrossRef - Apte M, Girme G, Nair R, Bankar A, Ravi Kumar A, Zinjarde S (2013) Melanin mediated synthesis of gold nanoparticles by Yarrowia lipolytica. Mater Lett 95:149–152. doi: 10.1016/j.matlet.2012.12.087 CrossRef
- Arockiya Aarthi Rajathi F, Parthiban C, Ganesh Kumar V, Anantharaman P (2012) Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim Acta – Part A Mol Biomol Spectrosc 99:166–173. doi: 10.1016/j.saa.2012.08.081 CrossRef
- Attia YA, Farag YE, Mohamed YMA, Hussien AT, Youssef T (2016) Photo-extracellular synthesis of gold nanoparticles using Baker’s yeast and their anticancer evaluation against Ehrlich ascites carcinoma cells. New J Chem 40:9395–9402. doi: 10.1039/c6nj01920j
CrossRef - Baker S, Satish S (2015) Biosynthesis of gold nanoparticles by Pseudomonas veronii AS41G inhabiting Annona squamosa L. Spectrochim Acta – Part A Mol Biomol Spectrosc 150:691–695. doi: 10.1016/j.saa.2015.05.080
CrossRef - Banik S, Pérez-de-luque A (2017) In vitro effects of copper nanoparticles on plant pathogens, beneficial microbes and crop plants. Spanish J Agric Res 15. doi: 10.5424/sjar/2017152-10305 CrossRef
- Bharde A, Kulkarni A, Rao M, Prabhune A, Sastry M (2007) Bacterial enzyme mediated biosynthesis of gold nanoparticles. J Nanosci Nanotechnol 7:4369–4377. doi: 10.1166/jnn.2007.891
CrossRef - Cai F, Li J, Sun J, Ji Y (2011) Biosynthesis of gold nanoparticles by biosorption using Magnetospirillum gryphiswaldense MSR-1. Chem Eng J 175:70–75. doi: 10.1016/j.cej.2011.09.041 CrossRef
- Cecchin I, Reddy KR, Thomé A, Tessaro EF, Schnaid F (2017) Nanobioremediation: Integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int Biodeterior Biodegrad 119:419–428. doi: 10.1016/j.ibiod.2016.09.027
CrossRef - Chen Y, Chen Y, Jia J, Yan B (2022) Triclosan detoxification through dechlorination and oxidation via microbial Pd-NPs under aerobic conditions. Chemosphere 286:131836. doi: 10.1016/j.chemosphere.2021.131836
CrossRef - Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in arabidopsis. Plant Physiol 149:1579–1592. doi: 10.1104/pp.108.130369
CrossRef - Costa LH, Hemmer J V., Wanderlind EH, Gerlach OMS, Santos ALH, Tamanaha MS, Bella-Cruz A, Corrêa R, Bazani HAG, Radetski CM, Almerindo GI (2020) Green Synthesis of Gold Nanoparticles Obtained from Algae Sargassum cymosum: Optimization, Characterization and Stability. Bionanoscience 10:1049–1062. doi: 10.1007/s12668-020-00776-4
CrossRef - Dakal TC, Kumar A, Majumdar RS, Yadav V (2016) Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol 7:1–17. doi: 10.3389/fmicb.2016.01831
CrossRef - Das SK, Dickinson C, Lafir F, Brougham DF, Marsili E (2012) Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem 14:1322–1334. doi: 10.1039/c2gc16676c
CrossRef - Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8
CrossRef - Devi HS, Singh TD (2014) Synthesis of Copper Oxide Nanoparticles by a Novel Method and its Application in the Degradation of Methyl Orange. Adv Electron Electr Eng 4:83–88.
- Dozol H, Mériguet G, Ancian B, Cabuil V, Xu H, Wang D, Abou-Hassan A (2013) On the synthesis of Au nanoparticles using EDTA as a reducing agent. J Phys Chem C 117:20958–20966. doi: 10.1021/jp4067789
CrossRef - Du L, Jiang H, Liu X, Wang E (2007) Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem commun 9:1165–1170. doi: 10.1016/j.elecom.2007.01.007
CrossRef - Dumur F, Guerlin A, Dumas E, Bertin D, Gigmes D, Mayer CR (2011) Controlled spontaneous generation of gold nanoparticles assisted by dual reducing and capping agents. Gold Bull 44:119–137. doi: 10.1007/s13404-011-0018-5
CrossRef - Gnanajobitha G, Paulkumar K, Vanaja M, Rajeshkumar S, Malarkodi C, Annadurai G, Kannan C (2013) Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J Nanostructure Chem 3. doi: 10.1186/2193-8865-3-67
CrossRef - Gulati S, Sachdeva M, Bhasin KK (2018) Capping agents in nanoparticle synthesis: Surfactant and solvent system. AIP Conf Proc 1953. doi: 10.1063/1.5032549
CrossRef - Hermosa R, Viterbo A, Chet I, Monte E (2012) Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158:17–25. doi: 10.1099/mic.0.052274-0
CrossRef - Hulkoti NI, Taranath TC (2014) Biosynthesis of nanoparticles using microbes-A review. Colloids Surfaces B Biointerfaces 121:474–483. doi: 10.1016/j.colsurfb.2014.05.027
CrossRef - Ilunga AK, Meijboom R (2016) Catalytic oxidation of methylene blue by dendrimer encapsulated silver and gold nanoparticles. J Mol Catal A Chem 411:48–60. doi: 10.1016/j.molcata.2015.10.009 CrossRef
- Inbakandan D, Venkatesan R, Ajmal Khan S (2010) Biosynthesis of gold nanoparticles utilizing marine sponge Acanthella elongata (Dendy, 1905). Colloids Surfaces B Biointerfaces 81:634–639. doi: 10.1016/j.colsurfb.2010.08.016
CrossRef - Javed R, Zia M, Naz S, Aisida SO, Ain N ul, Ao Q (2020) Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J Nanobiotechnology 18:1–15. doi: 10.1186/s12951-020-00704-4
CrossRef - Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, Gaurav K, Karthik L, Rao KVB (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta – Part A Mol Biomol Spectrosc 90:78–84. doi: 10.1016/j.saa.2012.01.006
CrossRef - Johnson A, Kong F, Miao S, Thomas S, Ansar S, Kong ZL (2021) In-vitro antibacterial and anti-inflammatory effects of surfactin-loaded nanoparticles for periodontitis treatment. Nanomaterials 11:1–23. doi: 10.3390/nano11020356
CrossRef - Joy Prabu H, Johnson I (2015) Plant-mediated biosynthesis and characterization of silver nanoparticles by leaf extracts of Tragia involucrata, Cymbopogon citronella, Solanum verbascifolium and Tylophora ovata. Karbala Int J Mod Sci 1:237–246. doi: 10.1016/j.kijoms.2015.12.003
CrossRef - Khan ME, Khan MM, Cho MH (2015) Green synthesis, photocatalytic and photoelectrochemical performance of an Au-Graphene nanocomposite. RSC Adv 5:26897–26904. doi: 10.1039/c5ra01864a CrossRef
- Khan ME, Khan MM, Cho MH (2015) Biogenic synthesis of a Ag-graphene nanocomposite with efficient photocatalytic degradation, electrical conductivity and photoelectrochemical performance. New J Chem 39:8121–8129. doi: 10.1039/c5nj01320h
CrossRef - Khan MM, Ansari SA, Lee JH, Lee J, Cho MH (2014) Mixed culture electrochemically active biofilms and their microscopic and spectroelectrochemical studies. ACS Sustain Chem Eng 2:423–432. doi: 10.1021/sc400330r
CrossRef - Korzybski T, Kowszyk-Gindifer Z, Kurylowicz W (2013) Antibiotics: origin, nature and properties. Elsevier
- Krishnaraj C, Muthukumaran P, Ramachandran R, Balakumaran MD, Kalaichelvan PT (2014) Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Reports 4:42–49. doi: 10.1016/j.btre.2014.08.002
CrossRef - Kumar DA, Palanichamy V, Roopan SM (2014) Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta – Part A Mol Biomol Spectrosc 127:168–171. doi: 10.1016/j.saa.2014.02.058
CrossRef - Lee SH, Jung HK, Kim TC, Kim CH, Shin CH, Yoon TS, Hong AR, Jang HS, Kim DH (2018) Facile method for the synthesis of gold nanoparticles using an ion coater. Appl Surf Sci 434:1001–1006. doi: 10.1016/j.apsusc.2017.11.008
CrossRef - Lengke MF, Fleet ME, Southam G (2006) Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold(I)-Thiosulfate and gold(III)-chloride complexes. Langmuir 22:2780–2787. doi: 10.1021/la052652c
CrossRef - Lengke MF, Fleet ME, Southam G (2007) Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir 23:2694–2699. doi: 10.1021/la0613124 CrossRef
- Luo DQ, Guo JH, Wang FJ, Jin ZX, Cheng XL, Zhu JC, Peng CQ, Zhang C (2009) Anti-fungal efficacy of polybutylcyanoacrylate nanoparticles of allicin and comparison with pure allicin. J Biomater Sci Polym Ed 20:21–31. doi: 10.1163/156856208X393473
CrossRef - Luo Z, Zhu M, Guo M, Lian Z, Tong W, Wang J, Zhang B, Wei W (2017) Ultrasonic-Assisted Dispersion of ZnO Nanoparticles and Its Inhibition Activity to Trichoderma viride . J Nanosci Nanotechnol 18:2352–2360. doi: 10.1166/jnn.2018.14397
CrossRef - Mariselvam R, Ranjitsingh AJA, Usha Raja Nanthini A, Kalirajan K, Padmalatha C, Mosae Selvakumar P (2014) Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim Acta – Part A Mol Biomol Spectrosc 129:537–541. doi: 10.1016/j.saa.2014.03.066
CrossRef - Mehta M, Sharma M, Pathania K, Jena PK, Bhushan I (2021) Degradation of synthetic dyes using nanoparticles: a mini-review. Environ Sci Pollut Res 28:49434–49446. doi: 10.1007/s11356-021-15470-5
CrossRef - Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: Technological concepts and future applications. J Nanoparticle Res 10:507–517. doi: 10.1007/s11051-007-9275-x
CrossRef - Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar P V., Alam M, Kumar R, Sastry M (2001) Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett 1:515–519. doi: 10.1021/nl0155274
CrossRef - Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Ajayakumar P V., Alam M, Sastry M, Kumar R (2001) Bioreduction of AuCl4− Ions by the Fungus, Verticillium sp. and Surface Trapping of the Gold Nanoparticles Formed D.M. and S.S. thank the Council of Scientific and Industrial Research (CSIR), Government of India, for financial assistance. Angew Chemie Int Ed 40:3585. doi: 10.1002/1521-3773(20011001)40:19<3585::aid-anie3585>3.0.co;2-k
CrossRef - Murugesan K, Bokare V, Jeon JR, Kim EJ, Kim JH, Chang YS (2011) Effect of Fe-Pd bimetallic nanoparticles on Sphingomonas sp. PH-07 and a nano-bio hybrid process for triclosan degradation. Bioresour Technol 102:6019–6025. doi: 10.1016/j.biortech.2011.02.099
CrossRef - Muthukrishnan S BS, Muthukumar M SM, Rao MV SKT (2015) Catalytic Degradation of Organic Dyes using Synthesized Silver Nanoparticles: A Green Approach. J Bioremediation Biodegrad 06. doi: 10.4172/2155-6199.1000312
CrossRef - Nadaf NY, Kanase SS (2019) Biosynthesis of gold nanoparticles by Bacillus marisflavi and its potential in catalytic dye degradation. Arab J Chem 12:4806–4814. doi: 10.1016/j.arabjc.2016.09.020 CrossRef
- Nair B, Pradeep T (2002) Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactobacillus Strains. Cryst Growth Des 2:293–298. doi: 10.1021/cg0255164
CrossRef - Nalawade P, Mukherjee P, Kapoor S (2014) Biosynthesis, characterization and antibacterial studies of silver nanoparticles using pods extract of Acacia auriculiformis. Spectrochim Acta – Part A Mol Biomol Spectrosc 129:121–124. doi: 10.1016/j.saa.2014.03.032
CrossRef - Naraginti S, Stephen FB, Radhakrishnan A, Sivakumar A (2015) Zirconium and silver co-doped TiO2 nanoparticles as visible light catalyst for reduction of 4-nitrophenol, degradation of methyl orange and methylene blue. Spectrochim Acta – Part A Mol Biomol Spectrosc 135:814–819. doi: 10.1016/j.saa.2014.07.070
CrossRef - Niu Z, Li Y (2014) Removal and utilization of capping agents in nanocatalysis. Chem Mater 26:72–83. doi: 10.1021/cm4022479
CrossRef - Qu Y, You S, Zhang X, Pei X, Shen W, Li Z, Li S, Zhang Z (2018) Biosynthesis of gold nanoparticles using cell-free extracts of Magnusiomyces ingens LH-F1 for nitrophenols reduction. Bioprocess Biosyst Eng 41:359–367. doi: 10.1007/s00449-017-1869-9
CrossRef - Radini IA, Hasan N, Malik MA, Khan Z (2018) Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J Photochem Photobiol B Biol 183:154–163. doi: 10.1016/j.jphotobiol.2018.04.014 CrossRef
- Ranjitha VR, Rai VR (2017) Actinomycetes mediated synthesis of gold nanoparticles from the culture supernatant of Streptomyces griseoruber with special reference to catalytic activity. 3 Biotech 7:1–7. doi: 10.1007/s13205-017-0930-3
CrossRef - Rodrigues AG, Ping LY, Marcato PD, Alves OL, Silva MCP, Ruiz RC, Melo IS, Tasic L, De Souza AO (2013) Biogenic antimicrobial silver nanoparticles produced by fungi. Appl Microbiol Biotechnol 97:775–782. doi: 10.1007/s00253-012-4209-7
CrossRef - Sadhasivam S, Shanmugam P, Yun KS (2010) Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surfaces B Biointerfaces 81:358–362. doi: 10.1016/j.colsurfb.2010.07.036
CrossRef - Sarkar J, Ray S, Chattopadhyay D, Laskar A, Acharya K (2012) Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata. Bioprocess Biosyst Eng 35:637–643. doi: 10.1007/s00449-011-0646-4
CrossRef - Schmid G (2004) Nanoparticles: From Theary to Application
- Sheet S, Lee TMY (2016) Biosynthesis and characterization grown Candida albicans mediated gold nanoparticles with enhanced potentiality towards antimicrobial and antifungal activity under controlled microgravity environment. 25:2016
- Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K (2007) A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surfaces B Biointerfaces 57:97–101. doi: 10.1016/j.colsurfb.2007.01.010
CrossRef - Suresh AK, Pelletier DA, Wang W, Broich ML, Moon JW, Gu B, Allison DP, Joy DC, Phelps TJ, Doktycz MJ (2011) Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater 7:2148–2152. doi: 10.1016/j.actbio.2011.01.023 CrossRef
- Tripathi P, Singh PC, Mishra A, Chauhan PS, Dwivedi S, Bais RT, Tripathi RD (2013) Trichoderma: A potential bioremediator for environmental clean up. Clean Technol Environ Policy 15:541–550. doi: 10.1007/s10098-012-0553-7
CrossRef - Truong VK, Truong NP, Rice SA (2021) Antibacterial activity of nanoparticles. Nanomaterials 11:15–17. doi: 10.3390/nano11061391
CrossRef - Venkateswarlu Y, Farooq Biabani MA (1995) A spatane diterpene from the brown alga Stoechospermum marginatum. Phytochemistry 40:331–333. doi: 10.1016/0031-9422(95)00231-U CrossRef
- Yeh CS, Wang R, Chang WC, Shih Y hsin (2018) Synthesis and characterization of stabilized oxygen-releasing CaO2 nanoparticles for bioremediation. J Environ Manage 212:17–22. doi: 10.1016/j.jenvman.2018.01.068
CrossRef - Zhang X, Zheng H, Jin S, Jiang Y, Wang Y, Liu Y (2021) Fe3Pt-Ag nanoparticles: A novel generic approach towards detection and reduction for Rhodamine B. J Solid State Chem 293:121802. doi: 10.1016/j.jssc.2020.121802
CrossRef - Zhao J, Jin S, Delgado AHS, Chen Z, Matinlinna JP, Tsoi JKH (2021) Self-assembled PHMB titanium coating enables anti-fusobacterium nucleatum strategy. Coatings 11:1–11. doi: 10.3390/coatings11101190
CrossRef - Zhao YS, Sun C, Sun JQ, Zhou R (2015) Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep Purif Technol 142:182–188. doi: 10.1016/j.seppur.2014.12.035
CrossRef - Tao, F. F., Schneider, W. F., & Kamat, P. V. (2015). Heterogeneous catalysis at nanoscale for energy applications. John Wiley & Sons.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





