How to Cite | Publication History | PlumX Article Matrix
Divya Christy.L and Jayshree Nellore *
and Jayshree Nellore *
Department of Biotechnology, School of Bio and Chemical Engineering, Sathyabama Institute of Science and Technology (Deemed to be University), Jeppiaar nagar, Rajiv Gandhi Salai, Chennai India
Corresponding Author E-mail: jayshree.biotech2021@gamil.com
DOI : http://dx.doi.org/10.13005/bbra/3131
ABSTRACT: This article discusses the embryonic development of zebrafish, which are essential aquatic models for investigating neurotoxicity caused by environmental toxins. Zebrafish are one of the few fish species that can survive in highly toxic environments, making them an interesting model for assessing pollutants' performance and determining their toxicity. Zebrafish's limited life expectancy, ease of maintenance and growth, transparent embryos, and homology of genetic and biological features make them an interesting tool for studying the effects of common substances like insecticides, nanoparticles, and food supplements on human health. The study focuses on the regulation of morphology and behavior, stress caused by oxidative damage, transcription, neurogenesis, and neuron progression. Further research is needed at cellular and signaling levels to understand the detrimental processes of pollutants in relation to epigenetic toxicity, negative interference of the BB barrier, and manipulation of the brain-gut-microbiota axis. Environmental pollution is a significant issue that poses a severe threat to public health. Biotechnological principles are now being widely used to monitor environmental pollutants due to their versatile applications. In this regard, zebrafish has established as a prominent vertebrate prototype organism, which offers a unique platform for toxicity screening and efficacy testing of various chemicals. Studies have shown that exposure to toxicants during embryonic development can cause developmental, cardiovascular, and neurodevelopmental toxicity, as well as hepatic disorders in zebrafish. Therefore, zebrafish has been extensively used to study the neurotoxicity of environmental pollutants, including pesticides, nanoparticles, food additives, and other pollutants. Additionally, the review discusses the use of zebrafish-derived embryonic stem cells (ZESCs) for environmental pollutants monitoring. Stem cells are highly sensitive to the toxicity of environmental chemicals during embryogenesis, which can lead to adverse effects on embryonic and fetal development. Thus, ZESCs cultivation and utilization in toxicological assays have become a valuable tool for evaluating the toxicity of potential environmental toxicants. The application of green science principles to evaluate the toxicity of environmental pollutants using zebrafish and ZESCs has enormous potential. This approach offers a sustainable and environmentally friendly way to monitor pollutants, and it can contribute to the development of effective mitigation strategies. Overall, the use of zebrafish and ZESCs in biotechnological pollutants monitoring could have far-reaching effects for public health and environmental sustainability.
KEYWORDS: Environmental Toxicants; In Vitro; Stem Cells; Toxicological Assay; Zebrafish, Zsecs
Download this article as:| Copy the following to cite this article: Christy L. D, Nellore J. A Comprehensive Review on Zebrafish and Zebrafish Embryonic Stem Cells (Zescs) as the Versatile Biotechnological Green Tool for Detecting Environmental Pollutants. Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Christy L. D, Nellore J. A Comprehensive Review on Zebrafish and Zebrafish Embryonic Stem Cells (Zescs) as the Versatile Biotechnological Green Tool for Detecting Environmental Pollutants. Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/43uLNig |
Introduction
This article provides a summary of the embryonic development in relation to the production of developmental, cardiovascular, and neurodevelopmental toxicity in zebrafish, in addition to hepatic difficulties and the consequent modifications of certain genes. As a direct consequence of the continual development of increasingly sensitive detection technology, an ever-increasing number of pollutants that have been found to be present in the environment have been uncovered. Because of the significant risk that they provide to both vertebrates and invertebrates, in addition to the ecosystem as a whole, neurotoxic pollutants in particular have attracted a lot of people’s consideration in latest years. Because of their superior molecular and physiological properties, zebrafish (Danio rerio) have transpired as an essential aquatic prototypical for the investigation of the neurotoxicity caused by environmental toxins. This is due to the fact that zebrafish are one of the few fish species that can survive in highly toxic environments. This stands in stark contrast to the circumstances surrounding the existence of other model species. Because of its many desirable traits—including its limited life expectancy, easiness of maintenance and growth, transparent embryos, and noteworthy homology of genetic and biological features the zebrafish provides an interesting foundation to employ it as a versatile tool for assessment the performance of pollutants and determining their toxicity. These features all contribute to the fact that it is possible to use zebrafish as a model for numerous research facilities have made use of zebrafish in order to examine the effects of common substances found in the milieu, such as insecticides, nanoparticles, food supplements, and other kinds of contaminants. These research have been conceded out in order to learn more about how these substances can affect human health. In addition, this article discusses the use of varied stem cells and the function that they play in efficient The present investigation on the toxicity of pollutants from the environment to the nervous system of zebrafish is primarily centered on the regulation of morphology and behavior, stress caused by oxidative damage, transcription, the production of neurons and release, and the progression of neurons. Further investigation is needed at the cellular and signaling levels to elucidate the detrimental processes employed by pollutants in relation to epigenetic toxicity, negative interference of the BB barrier, and manipulation of the brain-gut-microbiota axis. The present article offers a comprehensive overview of the existing literature on the deleterious impact of environmental toxicants, such as heavy metals such as lead, cobalt, arsenic and organic contaminants, on the neurological systems of zebrafish. Additionally, it presents a succinct summary of the principal study’s outcomes, along with some evaluative remarks on the findings. In addition, the article includes a discussion on the findings. This study covers not only a review of the difficulties, but also of the hot regions in the existing research, as well as the potential of the subjects that could be further researched.
Zebrafish
When it comes to the study of neurodegenerative disorders and the pathophysiological processes that are passed down from one generation to the next, the zebrafish is an organism that serves as a model that is not only effective but also has a great deal of promise. In addition, zebrafish are excellent for high-throughput behavioural and pharmacological testing, and their behaviour often resembles that of human species as well as clinical behaviour that is connected with neurodegeneration. Zebrafish may be found in freshwater and saltwater environments. Yet, despite the many advantages that have been discussed in this article, the potential of zebrafish has not yet been fully realised. This is the case despite the many benefits that have been mentioned. As a consequence of this, there is a pressing need for further investigation into novel neurodegenerative disease biomarkers, mechanisms, and treatments using this powerful model organism. 1 We presented a brief overview of the current research standing and potential of the toxicological consequences and the processes associated with environmental substances on the nervous system of zebrafish, involving oxidative damage, epigenetic modification, toxic effects, the barrier between the brain and bloodstream, and the nervous system-gut-microbiota axis, from a large-scale perspective all right down to the molecular level. We accomplish this in the hopes of demonstrating that it can serve as a source of information and concepts for the investigation and use in this area of study. This review is different from others in that we have concentrated on the toxicological effects, and in more recent times, zebrafish have seen widespread use in the study of vertebrate development, and their significance in the screening of potential toxins has significantly expanded. In addition, this review is unique in that we have emphasised the importance of zebrafish as a model for the screening of potential toxins (Fig 1). The following is a summary of few of the traits that make zebrafish an ideal alternative model for toxicological study. Zebrafish are known for their ability to adapt to a wide variety of environments. The following is a condensed version of these characteristics: To begin, the life cycle of zebrafish is much shorter than that of rats, and they also have a higher fecundity rate. After being fertilised in a controlled environment, the majority of the time it takes them between three and six months to attain reproductive maturity. Adult female fish are the ones responsible for the development of embryos, and this process occurs on a daily basis. In most circumstances, a single adult female may generate anywhere from 100 to 300 embryos at once. 1.
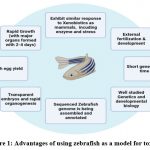 |
Figure 1: Advantages of using zebrafish as a model for toxicity Click here to view figure |
The rapid ability to reproduce exhibited by zebrafish facilitates data processing and enhances the probability significance of research assessments. Embryogenesis and the many stages of development in zebrafish have been the subject of a significant amount of research. As compared to other animal models, zebrafish have a far more rapid developmental rate, which gives it a significant advantage when used for developmental toxicity testing 2. Because of the transparency that exists throughout the embryonic stages, it is simple to see toxic consequences such as teratogenicity, reproductive toxicity, and mortality 3 4 5–8. The sequencing of the zebrafish genome has been performed, revealing the presence of replicas for 71% of human genome and 82% of disease-associated genes.7, suggesting that this research may have significant therapeutic applications. There are developmental parallels, genetic homologies, morphological similarities, and biological resemblances in the main tissues, with the exception of the respiratory organs and the mammary glands, that are not existing in zebrafish 8. Because of its usefulness in experiments and its resemblance to humans, the zebrafish has arisen as a prototype for a range of human illnesses, including haematological disease, heart disease, ophthalmic disease, cancer, muscle disease, and complicated brain disease 9,10.
These fish have developed as a prominent model entity for chemical efficacy evaluation and pharmaceutical research in contemporary times 11 12, and developmental neurotoxicity and nanotoxicology research 1. Although zebrafish are also used for testing organ structure toxicology 13, investigating mechanisms of action 14, and evaluating ecotoxicity and environmental toxicant 11, in latest years, zebrafish have become a mainstay model in chemical toxicity selection 12. In developmental toxicity experiments, the zebrafish model behaves in a manner that is comparable to that of mammalian models. The consonance between zebrafish and mammalian in the evaluation of compounds for developmental toxicity ranges from 55–100% 15–17, as stated in a review that was conducted by Sipes et al. 18. Yet, when screening for known fetotoxic compounds, tests conducted with rats identified 79% of chemicals, while those conducted with rabbits identified 75%, and those conducted with mice found 75%. Unfortunately, the compliance between the mice, rabbits, rats and other rodents is only 56% 19. The fact that the reaction of zebrafish is comparable to that of mammalian models of toxicity lends credence to the fact that the zebrafish model may be useful in toxicology research. The percentage of concordance across all mammalian species was calculated. The use of behavioural endpoints as phenotypic markers of toxicity has expanded as a result of automation, which has led to the growing automation of screening procedures. Moreover, modern molecular tools for modifying the genome make it possible to create specialised models of human diseases and to investigate precise epigenetic pathways in order to investigate their mechanistic underpinnings 1,2. The developmental origins of health and disease (DOHaD) and the determination of xenobiotic- persuade epigenetic modifications over generations are two of the rising areas of study in the area of toxicological exploration, and zebrafish are being used to investigate both of these areas.
The neurotoxicity of chemicals in growing zebrafish may be determined in a few of days using just a little quantity of the substance. Because of developments in automation, it is now possible to swiftly test thousands of substances to determine whether or not they are hazardous to development. Zebrafish will eventually become the go-to model organism for neurodevelopmental biology and toxicology 20, thanks to ongoing efforts to improve and automate relevant lab processes.
In this part, we will discuss how various toxins work on multiple signalling molecules and transcriptional cascades, both of which are known to alter the distinction of embryonic stem cells and/or the initial stages of the growth of heart, hepatic, and dopamine in zebrafish.
Cardiac Cells
The WHO assessments that 17.5 million people deceased from cardiovascular disease in 2017, making it the leading cause of death among non-communicable diseases worldwide. It is estimated that between one and three percent of live births and between ten and twenty percent of stillbirths are affected by congenital heart abnormalities (CHDs) 21,22. Studies that are conducted with zebrafish investigate not only the genetic reasons of CHDs but also analysed that concentrate on the pivotal point that ecological disruptions signify in the process of heart development. This is because it has been shown that CHDs can be caused by both genetic and environmental factors 23. Over the course of the last twenty years, zebrafish have established themselves as a leading prototypic organism for investigating the molecular source of heart ailment and the process of heart development. The heart of zebrafish grows quickly in a predictable pattern that, to a considerable extent, is reminiscent of the initial stages of cardiac growth in mammals 24.
Also, the anatomical structure of cardiac tissue in zebrafish hearts and human hearts are essentially quite similar to one another. An inner endocardial lining makes up the chambers of the heart, which are channels for the circulation of blood. Its lining is encased in a layer of muscular myocardial tissue, which, when it contracts, pumps blood to the many other parts of the body. The myocardium is supplied with oxygenated blood by the coronary artery system, and the epicardium, an exterior protective layer, surrounds the whole of the heart 25,26. Myocardial tissue is also damaged by the coronary artery system.
The cardiac precursors have been traced to the first 4 rows of blastomeres close to the border right before gastrulation (starting at 6 hours post-fertilization, or hpf) in zebra fish27. This mapping was done soon before gastrulation begins. In zebrafish, cardiac precursors are detected in bilateral regions inside the anterior lateral plate mesoderm (ALPM) after the completion of gastrulation. Grinch (grn), a gene that produces a G-coupled Apelin receptor (agtrl1b), is stated in the ALPM and the peripheral zone, while Apelin, the receptor’s ligand, is articulated in the midline. Apelin receptors and Apelin are mandatory to permit relocation of the bilateral pools of myocardial precursor cells towards the mid-line, as the cells obtain the suitable indications to persuade their cardiogenic distinction 28,29. This migration is required for the progress of a fully functional heart. At the age of 16 hours after fertilisation, the cardiac cells migrate medially from their bilateral locations to the midline, where they combine to create a single cardiac ring 30–32. This cardiac ring subsequently goes through a process of cell rearrangement, which results in the development of a primitive heart tube that is bilayer and consists of an internal endocardial stratum that is bordered by an external myocardial coat 33. During 48 hours after fertilisation in zebrafish, a kidney designed ventricular cavity and a tubular atrial cavity are produced. This occurs after the development of the primitive heart tube. Late-differentiating cardiomyocytes make a contribution not only to the formation of chambers but also to the development of outflow and inflow areas, that eventually become the bulbous arteriosus and the sinus venous, correspondingly. In other words, late-differentiating cardiomyocytes are involved in both the formation of chambers and the outflow 24,34,35. When the embryo develops, the heart has significant part in the growth of the body by subsidizing to the development of the embryo’s muscles. At 72 hours post-fibrillation, this enlargement of the muscle is generated by the synthesis of heart cells and their invagination into the lumen of the ventricle. Intussusception of heart cells into the lumen of the ventricle is a process known as trabeculation. This process occurs in all vertebrates and refers to the invagination of myocardial cells 24. Conjunction of endocardial cells from the atrial and ventricular regions of the pumping organ towards the atrioventricular canal border marks the beginning of valve creation at the atrioventricular canal (AVC), which begins at 36 hours after fertilisation 36. Cardiac valves are indispensable part in the efficient operation of the heart in vertebrates, and abnormalities in the valves are at the root of a wide variety of congenital and acquired types of heart disease in humans. The earliest symptoms of endocardial transdifferentiation may be seen in the heart of zebrafish at 36 hours post fertilisation (hpf), and by 48 hours post fertilisation, the endocardial cells that line the AV canal have formed a sole stratum of cuboidal cells that releases in cell adhesion molecule- Dm-grasp. In zebrafish, the growth of heart valves rest on the activation of the Tgf-b signalling pathways, NFAT, ErbB, and Notch 36–39.
During in vitro maturation of human cardiomyocytes, let-7 is the microRNA family that is most substantially up-regulated; however, this is not the case for early differentiation of cardiomyocytes. When it comes to hESC-CMs, upregulated of adherents of the let-7 family leads to an increase in sarcomere length, respiratory capacity, cell size, and force of contraction. It is interesting to note that metabolic flux assays, important expression data, and target analysis, all point to the possibility that the let-7–driven CM progress could be the consequence of the less production in phosphoinositide 3 kinase (PI3K)/AKT protein kinase/insulin pathway and the increased production of fatty acid metabolism. Based on these findings, let-7 seems to be a crucial mediator in the process of increasing the metabolic energetics of developing CMs 40.
Several cytokines belonging to the TGF-b family all show important parts in the processes of embryonic development, cell fate determination, stem cell maintenance, and adult tissue homeostasis and repair 41–43. In man, vascular disease condition and cardiovascular illnesses such as arteriovenous malformations (AVMs), atherosclerosis, aneurysms, cardiac fibrosis, vascular remodelling of the retina (retinopathy), and valvular heart disease are caused by improperly regulated TGF-b signalling 44.
The usage of the zebrafish model as a paradigm for analysing human cardiovascular development and illness has already shown to be very helpful. Studies conducted using zebrafish have revealed information on the hazardous consequences of pollutants such as pesticides, food additives, and nanoparticle exposure, which have been linked to cardiovascular developmental abnormalities (CHDs). But, adult zebrafish have also been utilised lately, which demonstrates the potential of this model organism in adult cardiotoxicity research. Although zebrafish larva has undoubtedly been one of this model organism’s strengths, adult zebrafish have also been used. There are a variety of chemical toxicants, including pesticides (Famoxadone-cymoxanil, Polychlorinated biphenyls (PCBs), Acrolein, Diclofop-methyl (DM), Oxadiazon-butachlor (OB), Iprodione, and Deltamethrin), nanoparticles (Graphene oxide (GO), Selenium, Zinc oxide, silver nanoparticles), and food additive. In order for the adult zebrafish heart to become a more effective model for toxicity, further study into its anatomy and function is required.
Liver cells
Hepatic failure is one of the main causes of death, and the inability of the liver to function may be deadly. Mortality due to liver conditions is significantly greater in parts of the globe where the spread of viral hepatitis virus infection is difficult to control or where access to medical treatment is restricted 45. In 2018, liver cancer ranked seventh on the list of the major causes of cancer and third on the list of the top causes of mortality from cancer 46. It has been shown that work-related and ecological acquaintance to chemicals and toxicants may result in a variety of pathologies, including fatty liver disease, swelling, cell death, and even liver tumour. Research conducted in clinical settings has shown the effects of liver injury, fibrosis, cirrhosis, tumour, and hepatic breakdown. These investigations have also demonstrated the astonishing potential of liver regeneration as well as its limitations. Research conducted on other organisms, such as fish, may thus provide light on the common pathways underlying liver illness.
Even though there is a high level of cell type conservation within the liver, zebrafish have a distinctively different hepatic morphology and cellular architecture in comparison to mammals. The harmfulness of a broad variety of substances, including naturally raised metals and metalloids, manufactured insecticides, and derivatives of user items, industrial processes, and waste firing method, has been investigated using zebrafish. The formation of the liver follows a typical pattern that may be broken down into three stages: the requirement of the progenitor cells, the variation of the cells and tissues, and the outgrowth of the liver. While this process is finished in a matter of days in zebrafish as divergent to a matter of weeks in animals, the DNA and pathways that govern every stage were substantially the same in both species.
The liver of zebrafish is made up of three continuous lobes, two of which are lateral and one of which is ventral. These lobes do not have a pedicle, which is the structure in mammalian livers that divides the various lobes. Hepatocytes in fish livers are not organised in a portal architecture but are instead grouped in tubules 36. The apical membranes are oriented such that they expression the interior of the tubule, and the sinusoids follow the path of the liver cell basal side 45,47,48. The cells in zebrafish livers have a mammalian-like appearance and accomplish many of the same purposes as their mammalian equivalents. These functions include bile excretion 49, glycogen and lipid storage 50, insulin sensitivity, xenobiotic and NH3 metabolism, and the discharge of blood proteins such as complement and clotting elements, transferrin, and albumin51. It is important to note that all liver activities that have been examined up to this point are present in larvae as initial as 5-days after fertilisation (dpf).
Later the development of the foregut endoderm at 24 hours post-fertilization (hpf), which is situated at the midline of embryo and will later cumulated into a liver left to the midline, the hepatic requirement in zebrafish begins initial in progress 52. This occurs shortly after the formation of the foregut endoderm. Hepatoblasts, which are the liver progenitor cells, are determined throughout the process of specification via the appearance of two important transcription factors called hhex and prox153,54. Between 50 and 60 hours after fertilisation, hepatoblasts start their differentiation into mature hepatocytes and biliary epithelial cells. During the hepatocyte differentiation process, which starts between 30 and 48 hours post fertilisation (hpf), the genes gc, fabp10a, and ceruloplasmin are discovered for the first time11,36. Other genes that are involved in important hepatocyte activities include fabp10b.
Preductal epithelial biliary cells, which are also referred to as PDEC, are the cells that are in charge of linking the bile canaliculi of liver cells to the most distal divisions of the bile duct network. These branches, which are related to the Canal of Hering in mammals, subsequently unite to create bigger bile ducts, which are accountable for conveying the bile that is generated by the hepatocytes out of the liver47,55. Although PDECs are too small to be identified, ductal structures may begin to be differentiated as early as 70 hpf by the use of staining48 and transgenic. As early as four days after fertilization, the preductules that are formed by PDECs are able to transmit bile to the gallbladder in an efficient manner 49.
Liver endothelial cells develop from the nearby posterior cardinal vein 56, travel to the first mount by 54 hpf, and then enter the liver, which ultimately results in the creation of hepatic sinusoids and veins by 72 hpf. Hepatocytes in endothelial cells move to establish a branching vascular system by 80 hpf, which coincides with and promotes hepatocyte polarisation 57. This migration of endothelial cells takes place by 80 hpf. The diversity of the hepatic design is essentially finished by 5 days’ post-fertilization (dpf); however, the development of the organ continues until it is fully developed in adulthood. By 5 days after fertilisation, the liver begins to assume responsibility for metabolic processes 49,50, and at this point, liver buildout is basically finished 58.
According to recent findings, between the years 1999 and 2016, there was a 65% rise in the yearly mortality rate caused by cirrhosis 59. Toxic substances such as pesticides (Hexaconazole and Epoxiconazole, Flutolanil, Chlorpyrifos (CH) and beta-cypermethrin (CY), Triazophos, Thifluzamide, Tebuconazole, Naphthalene, 2,4-Dichlorophenoxyacetic acid (2,4-D), Chlorfenapyr, Paclobutrazol (PBZ) and Boscalid), food additive The liver’s protective 60 mechanisms against over-nutrition may be compromised by environmental exposures, which may then promote steatohepatitis as a result of eating diets high in calories. The majority of liver damage is caused by toxic metabolites, which are produced during metabolism. This might possibly lead to continuous exposure of the liver’s intrahepatic cells to chemicals, which can then change gene expression associated to the metabolism of those chemicals. Because of its invertebrate-like advantages as well as its vertebrate biology, the zebrafish has proved to be an important model for the hepatic system. The underlying physiological processes, genetic changes, and symptoms of pathogenic reactions to environmental stressors are quite similar between zebrafish and mammals, despite the fact that the anatomy of the liver in zebrafish is different from that of mammals. Since zebrafish larvae are transparent, they are an excellent model for real-time imaging in liver research because of this trait.
Dopamine neurons
The frequency of neurodegenerative illnesses is rising, and as a result, they have become a public health concern. As a result, research into the disorders’ origins and potential therapies has become more important. In spite of the fact that rodents like rats, mice, and flies may be used in a variety of studies to research neurodegenerative illnesses, the Danio rerio has become more popular due to the fact that it is so similar to humans. Parkinson’s disease is the second most prevalent form of advanced neurodegenerative condition. It is defined by the damage and degradation of dopaminergic neurons (DA) in the substantia nigra pars compacta (SNpc) of the brain as well as by motor dysfunction including bradykinesia, stiffness, and tremor. Dopamine neurons are responsible for controlling movement as one of their many tasks. Their location was primarily identified by the use of immunohistochemistry for tyrosine hydroxylase (TH), which is an enzyme in dopamine production and also interacts in a role in the production of noradrenaline.
Dopamine neurons may be detected in the zebrafish retina, ventral diencephalon, prethalamusdorsal pretectum, preoptic region, subpallium, olfactory bulbs, and rhombencephalon throughout the embryonic and larval stages of development 61–64. Because of its supposed resemblance to the substantia nigra in mammalian brains, the ventral diencephalon’s dopaminergic (DA) neurons have garnered the greatest interest in recent years 61,65. From the posterior tuberculum all the way to the hypothalamus, these diencephalic neurons are arranged in groups that are numbered from DC1 to DC7. During 16 to 19 hours after fertilisation (hpf), the DA neurons of DC2 become post mitotic 64,66,67. This transformation takes place in the DA neurons. Groups DC1 and DC4/5 are also found at these initial stages, although their appearance patterns are not as consistent as those of the other groups. As early as 3 days after fertilisation (dpf), group DC3 may be identified with group DC6 for the first time. In position 4 dpf 68, the Group DC7 emerges. Hence, by the third day after fertilisation, the majority of DA groups located inside the ventral diencephalon had already been produced.
There are a number of genes that contribute to the formation of dopaminergic systems in vertebrates. It is essential to note that Wnt1, Wnt3a, and Wnt5a are necessary for the expansion and differentiation of neuronal axons 69. It has been shown that the growth of dopaminergic neurons in vertebrates is significantly influenced by a number of transcription factors, including Lmx1b, Nurr1 and Pitx3, 70. The Nurr1 zebrafish paralogue known as nr4a2a is released in the preoptic, retinal, and pretectal dopamine neurons, while the Nurr1 zebrafish similarly called as nr4a2b is solely released in the preoptic and retinal DA neurons. The expression of ‘th’ and ‘dat’ in those neurons is dependent upon the presence of nr4a2a. In the regulator of late variation as well as the development of the neurotransmitter phenotype, nr4a2/Nurr1 plays a function that is almost always present. Pitx3 ortholog does not appear to be released in any of the DA clusters, but like the lmx1b genes, it may be released in dopamine neuron precursors 67. However, these genes are not released in mature dopamine neurons.
Neurodevelopmental disorders (NDDs), which are instigated by abnormalities in brain development and development, are incapacitating conditions that may last a person’s whole life and significantly lower their quality of life. Recently, the zebrafish has been used as a model for a wide diversity of neurodegenerative diseases, comprising Parkinson’s disease (PD) and Huntington’s disease (HD). This is possible because the zebrafish’s genes are genetically similar to most human genes, including genes that cause neurodegenerative disorders. Neurodevelopmental disorders are caused by chemical substances like pesticides (Simazine, Boscalid, Clethodim, Rotenone, Triazophos (TRI), Atrazine, Fluazinam (FZN), Strobilurins, Deltamethrin, Penconazole), food additives (Aspartame, Sodium benzoate (SB), butylated hydroxytoluene), and nanoparticles (Graphene To this day, the vast majority of research conducted on zebrafish as a model for neurodevelopmental abnormalities has concentrated mostly on early morphological and straightforward behavioural traits. On the other hand, current developments in functional imaging are anticipated to revolutionise these investigations in the near future, allowing for the evaluation of circuit-level abnormalities that follow from risk gene disruption 71.
The accumulation of information, some of which has been described here, points to zebrafish as a potentially useful model species for the investigation of a broad variety of human neurodegenerative illnesses. In point of fact, zebrafish often similar and summarize rat prototypes of similar illnesses. As a result, they frequently provide a supplementary screening approach that is more throughput-oriented, productive, and less expensive than classic rodent models. The ability to simulate neurodegenerative illnesses in various taxon, such as fish as opposed to humans, is also very essential. This is because it allows researchers to concentrate on common, evolutionarily conserved pathophysiological pathways, which are thus referred to as “core” mechanisms. As we shall see in the following discussion, one of the most important benefits of using models may include those parts of neurodegeneration in which fish give different symptoms or mechanistic insights than rat models. Videlicet, zebrafish have developed into an important prototype organism for neurodegeneration in their own ways, rather than just serving as a metaphor for “little mice.” For instance, as was just said, they possess higher neurodegenerative potential 36, and in contrast to mammals, they have at least 16 brain areas that are capable of adult neurogenesis. Several of these sites are very similar to mammalian areas that are responsible for adult neurogenesis, such as the lateral ventricle and the subgranular zone of the dentate gyrus of the hippocampus 72. On the other hand, zebrafish show evidence of healthy neuronal renewal via the IL-4/STAT6 pathway, which is triggered by microglia-induced stimulation of nSPC 30,73. Therefore, our knowledge of certain neurodegenerative operations and regions in the brain of zebrafish, which do not exist in the brains of mammals 74, may be applied to the development of novel ideas concerning the pathogenesis of neurodegenerative disorders and the treatment of these conditions. In contrast to mammals, zebrafish do not have a neocortex or subcortical areas in their brains; instead, they have pallium and subpallium, respectively 35. Yet, the telencephalon of zebrafish is quite similar to the telencephalon of mammals both neurophysiological and topologically30,75. In addition, many of the fundamental brain structures that are involved in neurodegenerative illnesses include the brainstem, which is a portion of the brain that is rather consistent across species. Hence, zebrafish and other chordates may be employed as a one-of-a-kind model to advance analyse the involvement of non-telencephalic assemblies in neurodegenerative illnesses. This will result in the generation of crucial insights that are difficult to achieve by utilising just rats 71.
Developmental toxicity refers to any abnormalities that appear in an unborn child as a result of exposure during the gestational period. The term “teratogenicity” refers to any deformity or deficiency in structure that is brought on by acquaintance to a lethal substance during the prenatal period. These behaviour of zebrafish is progressively being used into toxicological analysing as a quantifiable phenotype representing abnormalities in normal physiology. This trend is expected to continue in the foreseeable future. Other toxicants include antimony, octocrylene (OC), saxitoxin (STX), and nodularin (NOD), microcystin-LR (MC-LR), fatty alcohol polyoxyethylene ether-7 (AEO-7), fluxapyroxad, maduramicin, flutolanil, ziram, silica nanoparticles (NPs), sodium benzoate (SB), ethoxyquin, citral.
Stem-loop binding protein (SLBP) found in retinal cells found that SLBP attaches to this region and works in conjunction with other components, such as U7 snRNP, to facilitate the processing of histone pre-mRNA and its subsequent conversion into protein. Genetic studies in Caenorhabditis elegans, Drosophila melanogaster, mouse, and human cultured cells The SLBP is necessary for cell cycle that are coupled histone mRNA assembly. These studies were conducted by Despite the fact that cell-cycle evolution is connected with vertebrate retinal neurogenesis, the involvement of SLBP and SLBP-mediated histone metabolism in retinal neurogenesis has not been explored. This is despite the fact that vertebrate eye retina neurogenesis occurs 76. The responsibilities that SLBP1 plays in the formation of the retina, which includes the proliferation of retinal stem cells and retinal progenitor cells, the timing of the production of new neurons in the retina, and lastly the pathfinding of intraocular retinal axons. Throughout development, the duration of the cell cycle is controlled in a variety of tissues in both geographical and temporal ways. Due to the fact that SLBP1 controls the pace of histone formation and degradation in a manner that is reliant upon the cell cycle, SLBP1 serves as an essential midway factor in the pathway that governs the rate at which the cell-cycle advances. The analysis of the zebrafish slbp1 mutant as a whole should, as a result, serve as an excellent model for research into the biological importance of spatial and temporal management of cell multiplying throughout the process of development 77.
Molecular and biological tests based on stem cells for the monitoring of toxicants
Toxicologists have found that the introduction and increase of stemcell biology have rekindled their interest in their field. It is believed that modern stem cell tools, when functional to the examination of possible harmful effects of contaminants on human health, have the potential to revolutionise in vitro toxicology. Both embryonic production and the regeneration of adult tissue may get their starting point from stem cells, which can be further subdivided into embryonic and adult stem cells. The field of environmental application makes extensive use of stem cells, which each possess their own set of distinctive characteristics (Fig 2).
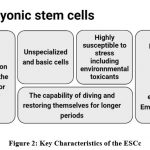 |
Figure 2: Key Characteristics of the ESCc. Click here to view figure |
A stem cell has the potential to self-renew indefinitely without going through the ageing process known as senescence. Stem cells also have pluripotency, which is the capability to develop into one or more specialised cell. Totipotent stemcells are involved in formation of all tissues and organs in the embryo, as well as all extra-embryonic tissues. This is an important function for these cells. Stem cells are very vulnerable to the toxicity of ambient chemicals during embryogenesis, which is a phase of rapid cellular division 78. This might lead to a deleterious influence on the development of both the embryo and the foetus if the toxins are toxic enough. ESCs are pluripotent stemcells that are derived from the internal cell mass of blastocyst stage embryos. ESCs are capable of replicating indefinitely and hold potential as a potential in vitro prototype for the evaluation of progressive toxicity through embryogenesis.
Stem cells may play a number of important functions in cellular assays that are used to test for toxicity. These roles may be performed in either organogenesis or embryotoxicity when analysed for toxicity in adult tissues. Stem cells have the potential to be used in both of these contexts. The second function that stem cells play in toxicological research is one that pertains to the embryotoxicity of substances. ESCs are able to simulate the initial stages of embryonic growth that occur in vivo, and they exhibit tissue-specific expression patterns, which is a feature that is significant for assessing embryotoxicity 79. The research of the interventions in stem cell distinction that are caused by substances can be used to identify probable developmental pollutant and genetic molecular tactics for the subsequent investigation into the mechanisms of action that are responsible for the teratogenic or embryotoxic effects caused by the evaluated chemical. Due to the capability of stem cells to imitate the process of embryo development, in vitro testing of embryotoxicity may be done very well using stem cells 80. Stem cells provide a good platform for this kind of study.
In current years, there has been a growing alarm that exposure to ecological substances in initial life may be related with chronic metabolic ailment in middle age, leading to improved examination interest in this topic 81. This has led to an upsurge in the studies that have been conducted in this area. For example, prenatal contact to lead, methyl mercury, or the pesticide chlorpyrifos hinders with one or more life-threatening phases of human growth, leading to progressive neurotoxicity. Furthermore, even minor contacts during periods of susceptibility can initiate hostile health concerns that can apparent across the life time of persons and generations 82. Widespread contact with potentially harmful environmental chemicals, such as heavy metals (lead, aluminium, and copper), insecticides (organochlorine and organophosphate insecticides, neonicotinoids), industrial chemicals, and air pollutants, poses a risk to healthy human reproduction and permeates all aspects of life across the globe. In these years, there has been important rise in the use of food additives; thus, it is believed that 75% of the modern diet is composed of foods that are produced in industrial settings 83. The average amount of food additives that a person consumes in a year has been estimated to be between 3.6 and 4.5 kg, although these figures might be far higher 84. Additives are often used in all varieties of food, ranging from those with the least amount of processing to those with the greatest amount of processing and modification 85. These are drugs that, despite their widespread usage, have the potential to cause adverse responses, just as any other medicine does. These reactions may include allergic reactions, changes in behaviour, and even the development of cancer 86. Because of an increase in population as well as an increase in the demand for industrial food production, synthetic pesticides have become more widely used. These pesticides alter the body’s metabolism, which in turn leads to conditions such as diabetes, thyroid disease, cancer, and neurodevelopmental disorders 82.
Laschinski et al. (1991) 87 were the first researchers to definitively develop the phrase “stem cell toxicity” in order to identify a new promising trend in in vitro. For the MTT cytotoxicity experiments and the teratogenic assays, Newall et al. 88 employed embryonic stem cells obtained from mice. Their research shows that mouse embryonic stem cells (mESC) have a higher degree of sensitivity than mouse fibroblast. Pratt et al. 89, who examined the effects of environmental teratogens on human embryonic palates that were generated from mesenchymal cells. Scanu et al. 90, who were the first researchers to examine human mesenchymal stem cells for the purpose of determining the toxicity of a variety of substances. It was revealed by Calderon-Gierszal et al. 91. that human embryonic stem cells played a role as the cellular toxicity model system. Under the context of this test, they determined the LD50 value of the poison based on the worldwide agreed method of categorization (GHS). They revealed that human mesenchymal stem cells (hMSCs) offered a more realistic simulation of in vivo settings when compared to approved test techniques such as 3T3 and NHK NRU. The cytotoxic impact of methyl mercury was examined by Tamm et al. 92 using neural stem cell line C17.2 and principal embryonic cortical NSCs as test subjects. Chandler et al. 93 investigated the toxic impact of 309 environmental chemicals (food usage pesticides) on mouse embryonic stem cells. Their results clearly demonstrate that the examined substances affected the cell growth and differentiation capacity. It was revealed that monobutyl phthalate had an influence on the cell differentiation of mouse embryonic stem cells (mESC) by the use of proteome profiling. 94. mESC cardiomyocytes that were exposed to dioxin were studied by Wang et al. 95 and Neri et al. 96, who analysed the signalling pathways and differentiation pathways of the cells. Cardiocytes that were produced from human embryonic stem cells have been employed as a prototype for testing the toxicity of teratogens 97.
The cytotoxic impact of trichloroethylene, which is one of the most frequent industrial toxicants, was also examined using human embryonic stem cell-derived cardiocytes 98. According to the findings of this investigation, the chemical that was put through its paces had a discernible impact on the transition of cardiac progenitor cells into cardiomyocytes. Stem cell toxicology investigations involving neural development of human embryonic stem cells (hESCs) play an essential part in the assessment of a varied variety of constituents, with toxicants 99. It was discovered that silver and gold nanoparticles have a significant influence on the differentiation of brain cells 100. Wilson et al. 101 published the results of neurotoxicity testing using 26 different chemical toxicants on neuronal cell models such as SH-SY5Y and PC12 cells, as well as hN2TM cells produced from human embryonic stem cells. Umbilical cord blood resulting neural stem cell line (HUCB-NSc) was evaluated against a variety of toxicants by Buzanska et al. 102. The researchers found that the studied toxicants alter cell proliferation, neuronal, glial development, and apoptotic cell death.
To investigate the harmful impact of BPA on differentiation, De Long et al. subjected human mesenchymal stem cells (hMSCs) to the DDT (dichlorodiphenyltrichloroethane), an endocrine disruptor (EDC) 81. Significant changes in gene expression, differentiation (both adipogenesis and ostogenesis), self-renewal, proliferation, and differentiation were discovered. These findings may help explain the homeostatic imbalance. The cytotoxic effects of various chemicals, such as insecticides, PAHs, lead, saccharin, mercury, acrylamide, triphenyl phosphate, isphenol, methyl mercuric(II), chloride, saccharin, methyl mercury, berberine chloride, flame retardants, deltamethrin, and D-glycine, were examined in neural cultures made from human pluripotent stem cells (hPSCs) 103. hESC-derived retinal organoids, also known as hERO, have been employed as a remarkable in vitro model system for toxicity. Zeng and colleagues 104 investigated the cytotoxicity of PM2.5 in their study. They found that the chemical under investigation prompted fast apoptosis, which in turn led to the death of the studied cells. Toxicology research is greatly aided by an investigation into the effects of a wide variety of plastics modelled with a variety of stem cell types 105. Recently, using mice and human lung organoids, scholars were able to report on the toxicological impact of nylon microfibers106.
Recent research has focused on employing stem cell toxicology principles to conduct cytotoxicity assessments for the purpose of determining the incidence of harmful pollutants in water. Researchers Masood et al. 107 used grown neural stem cells to perform in vitro stem cell bioassays for the purpose of determining the presence of harmful contaminants in water samples obtained from reservoirs located all across Europe. According to the findings, the neuronal cells that were tested were vulnerable to the various toxic pollutants that were present in the water samples. This, in turn, led to a reduction in the viability and proliferation of stem cells, in addition to a reduction in neuronal and astrocyte differentiation, cell migration, astrocyte growth, and neuronal neurite growth.
Embryonic stem cells derived from zebrafish.
Zebrafish have been presented to be capable of producing ESCs, making them an essential model organism for biological study as well as the discovery and development of novel medications and cell-based treatments. ESCs are pluripotent stemcells that are derived from the inner cell groups of embryos. These cells have the potential to replicate endlessly and may be used as a prospective in vitro model to determine how toxicity changes throughout development. The inner cell mass of embryos at the blastocyst stage is where embryonic stem cells are formed 3.
ES cells originate from certain cell groups inside the blastocyst that are referred to as the inner cell mass (ICM) (ES). During two to two and a half hours of fertilisation, zebrafish embryos have developed to the blastocyst stage. ES cells are pluripotent, meaning they have the prospective to develop into any body cell, and these cells are responsible for giving growth throughout development to all derivatives of the three basic germ layers, which are the ectoderm, endoderm, and mesoderm, respectively. Fish become particularly appealing models for the research and development of embryonic stem cell technologies for two reasons: Piscine species are of significant interest for both fundamental research in cellular, molecular, and developmental biology as well as for business purposes. The zebrafish (Brachidanio rerio) and the medaka (Oryzias latipes) are both viable alternatives to the mouse when it comes to the study of gene functions that are important to humans. Fish have a number of technological advantages over other vertebrates, including high fecundity, big embryos that are transparent, and quick development. These characteristics make manipulation easier and enable phenotypic observations of markers throughout early development, particularly in tiny aquarium fish with relatively quick generation rates, such as zebrafish and medaka, whose generation durations range from two to three months. The direct insertion of transgenes into germ cells, embryos, or fertilised eggs is one of the traditional methods for creating transgenic fish 108.
From the beginning of the 20th century, scientists have been doing research on the origin of ESC in non-mammalian animals, namely fish. It is fascinating that a team of researchers was able to demonstrate that embryonic stem cells (ESCs) produced from zebrafish may be maintained for more than two years without the need that they be grown on a feeder cell layer. The results of this investigation were presented in Zebrafish. This work demonstrated that ZESCs can be maintained for a lengthy period of time deprived of the expenditure of feeder cells. This designation ZES1, has been kept alive for more than 800 days in a medium that has been termed as Dulbecco’s modified Eagle’s medium(DMEM) and that has been accompanied with FBS, human basic fibroblast growth factor, trout serum, and zebrafish embryo extract. ZES1 cells had a morphology that was characteristic of ES cells; they were mostly diploid, spherical or polygonal in form, with a large nucleus and very little cytoplasm. ZES1 cells also had a large nucleus. The cells organised themselves into distinct colonies, each of which was composed of densely packed cells that gave a positive stain for alkaline phosphatase.
Cultivation of embryonic stem cells obtained from zebrafish
With exposure to retinoic acid, this cell underwent the process of differentiating into neuronal cells. By using immunostaining and RT-PCR, they were able to locate the pluripotent marker Sox2 in mammalian embryonic stem (ES) cells. In addition to this, they found that ZESCs had the capability of forming three germ layers when labelled with green fluorescent protein. The results of this group as a whole indicate that zebrafish embryonic stem cells (ZESCs) are pluripotent cells and that they may be maintained for an extended length of time without a feeder coat.
ZESCs cultivation
The embryonic stem cells initially produced from the undifferentiated cells that made up the inner cell mass of germ cells when they were in the blastocyst stage. After being reintroduced into blastocysts, embryonic stem (ES) cells have the likely to participate in embryonic development in vivo. Embryonic stem cells derived from zebrafish are shown in a step-by-step format in Figure 3, which begins with the selection of the blastocyst embryo stage and continues with the isolation of the inner germ layer mass and the cultivation of the cells under optimal conditions. Pluripotent stem cells can be identified by their morphological characteristics (size and shape), cell surface markers (alkaline phosphatase activity), and their expression of transcription factors (klf4, sox2, myc, ronin, sall4, and tcf3 (tcf7l1) Pluripotent stem cells are distinguished from other types of stemcells by their capability to self-renew.
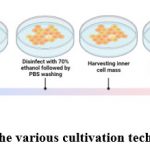 |
Figure 3: shows the various cultivation techniques for ZESCs. Click here to view figure |
In chordates, hematopoiesis happens in 2 waves: the first one is known as the primitive wave and it happens during embryonic development, while the second wave happens throughout adulthood. Out of the whole population of zebrafish, only three of the five antigens on the haemoglobin types present in embryonic zebrafish can be distinguished by gene expression by day one. Hbbe2 is primarily expressed by erythrocytes, and lcp1 by macrophages 109, and neutrophils precisely express mpx 110. With the use of these probes, we were able to identify cells that were morphologically distinct from one another after just 26 hours. The nuclei of macrophages were quite tiny in comparison to their sizes, making them seem to be nearly twice as big as other types of cells. Along with these macrophages and neutrophils showed abnormal cell forms and their nuclei were folded. Erythrocytes all had the same general outline, and their nuclei were spherical. Even though these indicators were preferred for double labelling analysis indicated that each marker brands a different population of blood cells 111, there have been a figures of current reports questioning whether or not lcp1 only marks macrophage cells. This is due to the fact that between 48 and 72 hours, it seems that certain neutrophil cells also express lcp1. We proceeded with caution when utilising gene expression as the only method to distinguish cell types since there is an absence of agreement in the scientific literature about the specificity of gene markers. Because of this, researchers looked at the shape of each of the red blood cells and utilized this information as a foundation for identifying the cells while they were still in the body. At 30 hours, above the sac containing the yolk, before the viteline veins constricted and moved in the front, or in the rostral space of the tail while there was an artery and vein, blood cells were quick to see while they were circulating in the body. This was the case at both locations. Both of these spots date back to a time when there was neither an artery nor a vein. In vivo, we were able to distinguish five unique types of cells, among which three were consistent with the findings of in situ investigation.112 113 114.
Embryonic stem cells, also known as ES cells, have a lot of potential as a tool for the creation of transgenic organisms and as an appropriate investigational model for in vitro research on the development, differentiation, and modification of genes in embryonic cells. We present here the derivation and preliminary characterisation of a pluripotent embryonic stem cell line, which we have labelled as ESSA1 and which was obtained from the blastula stage fertilized egg of Sparus aurata, L. In contrast to other types of embryonic stem cells, ESSA1 cells are cultivated without the use of a feeder layer in Leibovitz’s L-15 mixture that is appended with 5% FBS. They produce dense colonies, might have a spherical or polygonal appearance, and develop at an exponential rate when grown in culture. Strong alkaline phosphatase activity in the cells may also exhibit these features. All-trans retinoic acid treatment induces ESSA1 cells to develop into myocyte, oligodendritic, neuron-like, and melanocyte cells. When sown on bacteriological plates, these cells may also form embryoid bodies, an ability that is often connected to pluripotency. Additionally, it was shown that ESSA1 cells can create a mineralized extracellular matrix in a lab setting. Additionally, ESSA1 cells can be used to create chimaeras, in which they can help zebrafish embryos and fry develop their trunk and gut, among other organs. ESSA1 cells may be used to create chimeras. Therefore, ESSA1 cells offer a useful model for studying fish bone-lineage cell development, while their characteristics also indicate promise for investigation into stem cells featuring fish.
Strategies for reining in genetic instability
Since conditional cassettes have been successfully inserted into the genome using a wide range of techniques, it is clear that any genome editing technique that a specific lab is most familiar with may be used to efficiently generate restricted mutations. The engineering of conditional alleles will undoubtedly become even simpler as a result of ongoing advancements in genome editing, such as the application of modified oligonucleotides, various techniques that boost local HDR template quantity, enhanced CRISPR RNA/trans-activating CRISPR RNA structures in place of sgRNA, crucial editing, and the use additional nucleases like Cas12/Cpf1. 115.
Conclusion:
This review provided valuable insight into the potential use of zebrafish and embryonic stem cells derived from zebrafish to evaluate toxicity during embryonic development. This toxicity during embryonic development may lead to developmental, cardiovascular, and neurodevelopmental toxicity in addition to hepatic disorders and subsequent modifications of specific genes in zebrafish. They provide the opportunity to test many toxicity endpoints by integrating various assays, which makes it possible to create quantitative evaluations of a large number of compounds. Toxic substances have the potential to change the epigenome, which may lead to the development of abnormal behaviours and a variety of illnesses. These modifications may be passed down through the generations and influence subsequent generations even if they have not been exposed to the toxicant directly. It is common practise to use zebrafish to prototypical human syndromes by making genetic modifications, most notably through the use of CRISPR technology. This technology, when combined with the tractability of zebrafish, can be used in toxicology research to examine the effects of genetics on ecological experiences and gain awareness into the aetiologies, mechanisms, and varied consequences of ecological-related disease in humans. This review provides a description of research that is both currently ongoing and that which has been completed in the field of stem cells obtained from a variety of sources and their use in toxicant monitoring assays. In specifically, the study exposes the techniques that may be aimed at the cultivation of embryonic stem cells resulting from zebrafish as well as the methods that can be used for identification. In addition, the majority of toxicity studies are conducted using in vitro cytotoxicity screening assays. These assays are used to monitor various environmental contaminants, all of which do, in fact, play an important part in the monitoring of toxicants.
Acknowledgements: The authors thank Sathyabama Institute of Science and Technology for the various resources provided.
Conflict of interest: NIL
Funding source: No fund received
References
- Bambino K, Morrison J, Chu J. Hepatotoxicity in Zebrafish larvae. Methods Mol Biol. 2019;1965:129-138. doi:10.1007/978-1-4939-9182-2_9
CrossRef - Phillips JB, Westerfield M. Zebrafish models in translational research: Tipping the scales toward advancements in human health. DMM Dis Model Mech. 2014;7(7):739-743. doi:10.1242/dmm.015545
CrossRef - Caballero MV, Candiracci M. Zebrafish as Toxicological model for screening and recapitulate human diseases. J Unexplored Med Data. 2018;3(2):4. doi:10.20517/2572-8180.2017.15
CrossRef - Liu Y, Shen Y. Modelling and optimisation of biomass injection in ironmaking blast furnaces. Prog Energy Combust Sci. 2021;87(July):100952. doi:10.1016/j.pecs.2021.100952
CrossRef - Choi JS, Kim RO, Yoon S, Kim WK. Developmental Toxicity of Zinc Oxide Nanoparticles to Zebrafish (Danio rerio): A transcriptomic analysis. PLoS One. 2016;11(8). doi:10.1371/journal.pone.0160763
CrossRef - Ma YB, Lu CJ, Junaid M, et al. Potential adverse outcome pathway (AOP) of silver nanoparticles mediated reproductive toxicity in zebrafish. Chemosphere. 2018;207:320-328. doi:10.1016/j.chemosphere.2018.05.019
CrossRef - Mesquita B, Lopes I, Silva S, et al. Gold nanorods induce early embryonic developmental delay and lethality in zebrafish (Danio rerio). J Toxicol Environ Heal – Part A Curr Issues. 2017;80(13-15):672-687. doi:10.1080/15287394.2017.1331597
CrossRef - Pecoraro R, Salvaggio A, Marino F, et al. Metallic nano-composite toxicity evaluation by zebrafish embryo toxicity test with identification of specific exposure biomarkers. Curr Protoc Toxicol. 2017;2017(November):1.14.1-1.14.13. doi:10.1002/cptx.34
CrossRef - Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498-503. doi:10.1038/nature12111
CrossRef - Santoriello C, Zon LI. Science in medicine Hooked ! Modeling human disease in zebrafish. Sci Med. 2012;122(7):2337-2343. doi:10.1172/JCI60434.combines
CrossRef - Goessling W, Sadler KC. Zebrafish: An Important Tool for Liver Disease Research. Gastroenterology. 2015;149(6):1361-1377. doi:10.1053/j.gastro.2015.08.034
CrossRef - Peterson RT, MacRae CA. Systematic approaches to toxicology in the zebrafish. Annu Rev Pharmacol Toxicol. 2012;52:433-453. doi:10.1146/annurev-pharmtox-010611-134751
CrossRef - Rennekamp AJ, Peterson RT. 15 Years of Zebrafish Chemical Screening. Curr Opin Chem Biol. 2015;24:58-70. doi:10.1016/j.cbpa.2014.10.025
CrossRef - Kaur J, Khatri M, Puri S. Toxicological evaluation of metal oxide nanoparticles and mixed exposures at low doses using zebra fish and THP1 cell line. Environ Toxicol. 2019;34(4):375-387. doi:10.1002/tox.22692
CrossRef - Brannen KC, Panzica-Kelly JM, Danberry TL, Augustine-Rauch KA. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res Part B – Dev Reprod Toxicol. 2010;89(1):66-77. doi:10.1002/bdrb.20223
CrossRef - Padilla S, Corum D, Padnos B, et al. Zebrafish developmental screening of the ToxCastTM Phase I chemical library. Reprod Toxicol. 2012;33(2):174-187. doi:10.1016/j.reprotox.2011.10.018
CrossRef - Selderslaghs IWT, Van Rompay AR, De Coen W, Witters HE. Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod Toxicol. 2009;28(3):308-320. doi:10.1016/j.reprotox.2009.05.004
CrossRef - Sipes NS, Padilla S, Knudsen TB. Zebrafish-As an integrative model for twenty-first century toxicity testing. Birth Defects Res Part C – Embryo Today Rev. 2011;93(3):256-267. doi:10.1002/bdrc.20214
CrossRef - Hurtt ME, Cappon GD, Browning A. Proposal for a tiered approach to developmental toxicity testing for veterinary pharmaceutical products for food-producing animals. Food Chem Toxicol. 2003;41(5):611-619. doi:10.1016/S0278-6915(02)00326-5
CrossRef - Chen J, Lei L, Tian L, et al. Developmental and behavioral alterations in zebrafish embryonically exposed to valproic acid (VPA): An aquatic model for autism. Neurotoxicol Teratol. 2018;66(October 2017):8-16. doi:10.1016/j.ntt.2018.01.002
CrossRef - Pierpont ME, Brueckner M, Chung WK, et al. Genetic Basis for Congenital Heart Disease: Revisited. Vol 138.; 2018. doi:10.1161/CIR.0000000000000606.The
- Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890-1900. doi:10.1016/S0735-1097(02)01886-7
CrossRef - Jenkins KJ, Correa A, Feinstein JA, et al. Noninherited risk factors and congenital cardiovascular defects: Current knowledge – A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young. Circulation. 2007;115(23):2995-3014. doi:10.1161/CIRCULATIONAHA.106.183216
CrossRef - Brown DR, Samsa LA, Qian L, Liu J. Advances in the study of heart development and disease using zebrafish. J Cardiovasc Dev Dis. 2016;3(2):1-25. doi:10.3390/jcdd3020013
CrossRef - Singleman C, Holtzman NG. Analysis of postembryonic heart development and maturation in the zebrafish, Danio rerio. Dev Dyn. 2012;241(12):1993-2004. doi:10.1002/dvdy.23882
CrossRef - Hu N, Sedmera D, Yost HJ, Clark EB. Developing Zebrafish Heart. Anat Rec. 2000;157(July):148-157.
CrossRef - Shrestha R, Lieberth J, Tillman S, Natalizio J, Bloomekatz J. Using Zebrafish to Analyze the Genetic and Environmental Etiologies of Congenital Heart Defects. Vol 1236.; 2020. doi:10.1007/978-981-15-2389-2_8
CrossRef - Scott K, O’Rourke R, Winkler CC, Kearns CA, Appel B. Temporal single-cell transcriptomes of zebrafish spinal cord pMN progenitors reveal distinct neuronal and glial progenitor populations. Dev Biol. 2021;479(April):37-50. doi:10.1016/j.ydbio.2021.07.010
CrossRef - Zeng XXI, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and Its Receptor Control Heart Field Formation during Zebrafish Gastrulation. Dev Cell. 2007;12(3):391-402. doi:10.1016/j.devcel.2007.01.011
CrossRef - Silva Brito R, Canedo A, Farias D, Rocha TL. Transgenic zebrafish (Danio rerio) as an emerging model system in ecotoxicology and toxicology: Historical review, recent advances, and trends. Sci Total Environ. 2022;848(March):157665. doi:10.1016/j.scitotenv.2022.157665
CrossRef - Kikuchi K. New function of zebrafish regulatory T cells in organ regeneration. Curr Opin Immunol. 2020;63:7-13. doi:10.1016/j.coi.2019.10.001
CrossRef - Zhao Y, Yang Q, Liu D, Liu T, Xing L. Neurotoxicity of nanoparticles: Insight from studies in zebrafish. Ecotoxicol Environ Saf. 2022;242(July):113896. doi:10.1016/j.ecoenv.2022.113896
CrossRef - Rohr S, Otten C, Abdelilah-Seyfried S. Asymmetric involution of the myocardial field drives heart tube formation in zebrafish. Circ Res. 2008;102(2):12-19. doi:10.1161/CIRCRESAHA.107.165241
CrossRef - Hami D, Grimes AC, Tsai HJ, Kirby ML. Zebrafish cardiac development requires a conserved secondary heart field. Development. 2011;138(11):2389-2398. doi:10.1242/dev.061473
CrossRef - Lovett-Barron M. Learning-dependent neuronal activity across the larval zebrafish brain. Curr Opin Neurobiol. 2021;67:42-49. doi:10.1016/j.conb.2020.07.006
CrossRef - Beis D, Bartman T, Jin SW, et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132(18):4193-4204. doi:10.1242/dev.01970
CrossRef - Timmerman LA, Grego-Bessa J, Raya A, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99-115. doi:10.1101/gad.276304
CrossRef - Scherz PJ, Huisken J, Sahai-Hernandez P, Stainier DYR. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development. 2008;135(6):1179-1187. doi:10.1242/dev.010694
CrossRef - Chang CP, Neilson JR, Bayle JH, et al. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118(5):649-663. doi:10.1016/j.cell.2004.08.010
CrossRef - Kuppusamy KT, Jones DC, Sperber H, et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc Natl Acad Sci U S A. 2015;112(21):E2785-E2794. doi:10.1073/pnas.1424042112
CrossRef - Moustakas A, Heldin CH. The regulation of TGFβ signal transduction. Development. 2009;136(22):3699-3714. doi:10.1242/dev.030338
CrossRef - Wu MY, Hill CS. TGF-β Superfamily Signaling in Embryonic Development and Homeostasis. Dev Cell. 2009;16(3):329-343. doi:10.1016/j.devcel.2009.02.012
CrossRef - Itoh F, Watabe T, Miyazono K. Roles of TGF-β family signals in the fate determination of pluripotent stem cells. Semin Cell Dev Biol. 2014;32:98-106. doi:10.1016/j.semcdb.2014.05.017
CrossRef - Goumans MJ, ten Dijke P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb Perspect Biol. 2018;10(2):1-39. doi:10.1101/cshperspect.a022210
CrossRef - Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-2128. doi:10.1016/S0140-6736(12)61728-0
CrossRef - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
CrossRef - Hardman RC, Volz DC, Kullman SW, Hinton DE. An in vivo look at vertebrate liver architecture: Three-dimensional reconstructions from Medaka (Oryzias latipes). Anat Rec. 2007;290(7):770-782. doi:10.1002/ar.20524
CrossRef - Lorent K, John C Moore, Siekmann AF, Lawson N, Michael P. Reiterative Use of the Notch Signal During Zebrafish Intrahepatic Biliary Development. Physiol Behav. 2018;176(1):139-148. doi:10.1002/smll.201502346.Size-dependent
- Farber SA, Pack M, Ho SY, et al. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science (80- ). 2001;292(5520):1385-1388. doi:10.1126/science.1060418
CrossRef - Howarth DL, Yin C, Yeh K, Sadler KC. Defining hepatic dysfunction parameters in two models of fatty liver disease in zebrafish larvae. Zebrafish. 2013;10(2):199-210. doi:10.1089/zeb.2012.0821
CrossRef - Noël ES, Dos Reis M, Arain Z, Ober EA. Analysis of the Albumin/α-Fetoprotein/Afamin/Group specific component gene family in the context of zebrafish liver differentiation. Gene Expr Patterns. 2010;10(6):237-243. doi:10.1016/j.gep.2010.05.002
CrossRef - Field HA, Ober EA, Roeser T, Stainier DYR. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol. 2003;253(2):279-290. doi:10.1016/S0012-1606(02)00017-9
CrossRef - Ober EA, Verkade H, Field HA, Stainier DYR. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442(7103):688-691. doi:10.1038/nature04888
CrossRef - Wallace KN, Yusuff S, Sonntag JM, Chin AJ, Pack M. Zebrafish hhex regulates liver development and digestive organ chirality. Genes (United States). 2001;30(3):141-143. doi:10.1002/gene.1050
CrossRef - Yao Y, Lin J, Yang P, et al. Fine Structure, Enzyme Histochemistry, and Immunohistochemistry of Liver in Zebrafish. Anat Rec. 2012;295(4):567-576. doi:10.1002/ar.22416
CrossRef - Hen G, Nicenboim J, Mayseless O, et al. Venous-derived angioblasts generate organ-specific vessels during zebrafish embryonic development. Dev. 2015;142(24):4266-4278. doi:10.1242/dev.129247
CrossRef - Sakaguchi TF, Sadler KC, Crosnier C, Stainer DY. Endothelial Signals Modulate Hepatocyte Apicobasal Polarization in Zebrafish. Curr Biol. 2008;18(20):1565-1571. doi:10.1016/j.cub.2008.08.065.Endothelial
CrossRef - Wang S, Miller SR, Ober EA, Sadler KC. Making it new again: Insight into liver development, regeneration, and disease from zebrafish research. Curr Top Dev Biol. 2017;124:161-195. doi:10.1016/bs.ctdb.2016.11.012
CrossRef - Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: Observational study. BMJ. 2018;362. doi:10.1136/bmj.k2817
CrossRef - Wahlang B, Jin J, Beier JI, et al. Mechanisms of Environmental Contributions to Fatty Liver Disease. Curr Env Heal Rep. 2019;6(3):80-94. doi:10.1007/s40572-019-00232-w.Mechanisms
CrossRef - Rink E, Wullimann MF. Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res Bull. 2002;57(3-4):385-387. doi:10.1016/S0361-9230(01)00696-7
CrossRef - McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004;480(1):38-56. doi:10.1002/cne.20280
CrossRef - Kastenhuber E, Kratochwil CF, Ryu S, Schweitzer J, Driever W. Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J Comp Neurol. 2010;518(4):439-458. doi:10.1002/cne.22214
CrossRef - Schweitzer J, Löhr H, Filippi A, Driever W. Dopaminergic and noradrenergic circuit development in zebrafish. Dev Neurobiol. 2012;72(3):256-268. doi:10.1002/dneu.20911
CrossRef - Smeets WJAJ, González A. Catecholamine systems in the brain of vertebrates: New perspectives through a comparative approach. Brain Res Rev. 2000;33(2-3):308-379. doi:10.1016/S0165-0173(00)00034-5
CrossRef - Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev. 2001;101(1-2):237-243. doi:10.1016/S0925-4773(01)00287-8
CrossRef - Filippi A, Jainok C, Driever W. Analysis of transcriptional codes for zebrafish dopaminergic neurons reveals essential functions of Arx and Isl1 in prethalamic dopaminergic neuron development. Dev Biol. 2012;369(1):133-149. doi:10.1016/j.ydbio.2012.06.010
CrossRef - Du Z, Hou K, Zhou T, et al. Polyhalogenated carbazoles (PHCZs) induce cardiotoxicity and behavioral changes in zebrafish at early developmental stages. Sci Total Environ. 2022;841(April):156738. doi:10.1016/j.scitotenv.2022.156738
CrossRef - Castelo-Branco G, Wagner J, Rodriguez FJ, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci U S A. 2003;100(22):12747-12752. doi:10.1073/pnas.1534900100
CrossRef - Smidt MP, Burbach JPH. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8(1):21-32. doi:10.1038/nrn2039
CrossRef - Sakai C, Ijaz S, Hoffman EJ. Zebrafish Models of Neurodevelopmental Disorders: Past, Present, and Future. Front Mol Neurosci. 2018;11(August). doi:10.3389/fnmol.2018.00294
CrossRef - Zabegalov KN, Wang D, Yang LE, et al. Decoding the role of zebrafish neuroglia in CNS disease modeling. Brain Res Bull. 2021;166(June 2020):44-53. doi:10.1016/j.brainresbull.2020.09.020
CrossRef - Shimizu Y, Kawasaki T. Histone acetyltransferase EP300 regulates the proliferation and differentiation of neural stem cells during adult neurogenesis and regenerative neurogenesis in the zebrafish optic tectum. Neurosci Lett. 2021;756(March):135978. doi:10.1016/j.neulet.2021.135978
CrossRef - Wang J, Wang D, Hu G, et al. The role of auditory and vibration stimuli in zebrafish neurobehavioral models. Behav Processes. 2021;193(January):104505. doi:10.1016/j.beproc.2021.104505
CrossRef - Zeng CW, Sheu JC, Tsai HJ. A new member of the forkhead box protein family in zebrafish: Domain composition, phylogenetic implication and embryonic expression pattern. Gene Expr Patterns. 2020;35(September 2019):119093. doi:10.1016/j.gep.2019.119093
CrossRef - Yang X, Wang C, Yang L, et al. Neurotoxicity and transcriptome changes in embryonic zebrafish induced by halobenzoquinone exposure. J Environ Sci (China). 2022;117:129-140. doi:10.1016/j.jes.2022.03.042
CrossRef - Imai F, Yoshizawa A, Matsuzaki A, et al. Stem-loop binding protein is required for retinal cell proliferation, neurogenesis, and intraretinal axon pathfinding in zebrafish. Dev Biol. 2014;394(1):94-109. doi:10.1016/j.ydbio.2014.07.020
CrossRef - Estevan C, C. A, Pamies D, Vilanova E, A. M. Embryonic Stem Cells in Toxicological Studies. Embryonic Stem Cells – Basic Biol to Bioeng. Published online 2011. doi:10.5772/24804
CrossRef - de Pater E, Clijsters L, Marques SR, et al. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development. 2009;136(10):1633-1641. doi:10.1242/dev.030924
CrossRef - Wobus AM, Löser P. Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch Toxicol. 2011;85(2):79-117. doi:10.1007/s00204-010-0641-6
CrossRef - De Long NE, Holloway AC. Early-life chemical exposures and risk of metabolic syndrome. Diabetes, Metab Syndr Obes Targets Ther. 2017;10:101-109. doi:10.2147/DMSO.S95296
CrossRef - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care # . Int J Gynecol Obstet. 2015;131:S173-S211. doi:10.1016/s0020-7292(15)30033-3
CrossRef - de Abreu MS, Giacomini ACVV, Genario R, et al. Color as an important biological variable in zebrafish models: Implications for translational neurobehavioral research. Neurosci Biobehav Rev. 2021;124(July 2020):1-15. doi:10.1016/j.neubiorev.2020.12.014
CrossRef - Zengin N, Yüzbaşioĝlu D, Ünal F, Yilmaz S, Aksoy H. The evaluation of the genotoxicity of two food preservatives: Sodium benzoate and potassium benzoate. Food Chem Toxicol. 2011;49(4):763-769. doi:10.1016/j.fct.2010.11.040
CrossRef - Carocho M, Morales P, Ferreira ICFR. Natural Food Additives. Nat Food Addit. Published online 2021. doi:10.5772/intechopen.91548
CrossRef - Cardoso LAC, Karp SG, Vendruscolo F, Kanno KYF, Zoz LIC, Carvalho JC. Biotechnological Production of Carotenoids and Their Applications in Food and Pharmaceutical Products. Carotenoids. Published online 2017. doi:10.5772/67725
CrossRef - Laschinski G, Vogel R, Spielmann H. Cytotoxicity test using blastocyst-derived euploid embryonal stem cells: A new approach to in vitro teratogenesis screening. Reprod Toxicol. 1991;5(1):57-64. doi:10.1016/0890-6238(91)90111-R
CrossRef - Newall DR, Beedles KE. The stem-cell test: An in vitro assay for teratogenic potential. Results of a blind trial with 25 compounds. Toxicol Vitr. 1996;10(2):229-233. doi:10.1016/0887-2333(95)00110-7
CrossRef - Pratt RM, Grove RI, Willis WD. Prescreening for environmental teratogens using cultured mesenchymal cells from the human embryonic palate. Teratog Carcinog Mutagen. 1982;2(3-4):313-318. doi:10.1002/1520-6866(1990)2:3/4<313::AID-TCM1770020312>3.0.CO;2-C
CrossRef - Scanu M, Mancuso L, Cao G. Evaluation of the use of human Mesenchymal Stem Cells for acute toxicity tests. Toxicol Vitr. 2011;25(8):1989-1995. doi:10.1016/j.tiv.2011.07.006
CrossRef - Calderon-Gierszal EL, Prins GS. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol a exposure. PLoS One. 2015;10(7):1-20. doi:10.1371/journal.pone.0133238
CrossRef - Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: Effects on cell survival and neuronal differentiation. J Neurochem. 2006;97(1):69-78. doi:10.1111/j.1471-4159.2006.03718.x
CrossRef - Chandler KJ, Barrier M, Jeffay S, et al. Evaluation of 309 environmental chemicals using a mouse embryonic stem cell adherent cell differentiation and cytotoxicity assay. PLoS One. 2011;6(6). doi:10.1371/journal.pone.0018540
CrossRef - Osman AM, van Dartel DAM, Zwart E, Blokland M, Pennings JLA, Piersma AH. Proteome profiling of mouse embryonic stem cells to define markers for cell differentiation and embryotoxicity. Reprod Toxicol. 2010;30(2):322-332. doi:10.1016/j.reprotox.2010.05.084
CrossRef - Wang W, Wang D, Li X, Ai W, Wang X, Wang H. Toxicity mechanisms regulating bone differentiation and development defects following abnormal expressions of miR-30c targeted by triclosan in zebrafish. Sci Total Environ. 2022;850(August):158040. doi:10.1016/j.scitotenv.2022.158040
CrossRef - Neri T, Merico V, Fiordaliso F, et al. The differentiation of cardiomyocytes from mouse embryonic stem cells is altered by dioxin. Toxicol Lett. 2011;202(3):226-236. doi:10.1016/j.toxlet.2011.02.008
CrossRef - Mayshar Y, Yanuka O, Benvenisty N. Teratogen screening using transcriptome profiling of differentiating human embryonic stem cells. J Cell Mol Med. 2011;15(6):1393-1401. doi:10.1111/j.1582-4934.2010.01105.x
CrossRef - Xu D, Yin X, Zhou S, et al. A review on the remediation of microplastics using constructed wetlands: Bibliometric, co-occurrence, current trends, and future directions. Chemosphere. 2022;303(P1):134990. doi:10.1016/j.chemosphere.2022.134990
CrossRef - Liu D, Liu NY, Chen LT, Shao Y, Shi XM, Zhu DY. Perfluorooctane sulfonate induced toxicity in embryonic stem cell-derived cardiomyocytes via inhibiting autophagy-lysosome pathway. Toxicol Vitr. 2020;69(August):104988. doi:10.1016/j.tiv.2020.104988
CrossRef - Marie-Claude S, Yanhua Z, Fangchao L, Arko S, Douglas RM, Guangzhao M. Size-dependent Toxicity of Gold Nanoparticles on Human Embryonic Stem Cells and Their Neural Derivatives. Physiol Behav. 2018;176(1):139-148. doi:10.1002/smll.201502346.Size-dependent
- Wilson MS, Graham JR, Ball AJ. Multiparametric High Content Analysis for assessment of neurotoxicity in differentiated neuronal cell lines and human embryonic stem cell-derived neurons. Neurotoxicology. 2014;42:33-48. doi:10.1016/j.neuro.2014.03.013
CrossRef - Buzanska L, Sypecka J, Nerini-Molteni S, et al. A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells. 2009;27(10):2591-2601. doi:10.1002/stem.179
CrossRef - Sirenko O, Cromwell EF, Crittenden C, Wignall JA, Wright FA, Rusyn I. Assessment of Beating Parameters in Human Induced Pluripotent Stem Cells Enables Quantitative In vitro Screening for Cardiotoxicity. Toxicol Appl Pharmacol. 2013;273(3):500-501. doi:10.1016/j.taap.2013.09.017. Assessment
CrossRef - Xu Z, Hu Y, Guo Z, Xiao X, Peng C, Zeng P. Optimizing pyrolysis temperature of contaminated rice straw biochar: Heavy metal(loid) deportment, properties evolution, and Pb adsorption/immobilization. J Saudi Chem Soc. 2022;26(2). doi:10.1016/j.jscs.2022.101439
CrossRef - Miloradovic D, Pavlovic D, Jankovic MG, et al. Human Embryos, Induced Pluripotent Stem Cells, and Organoids: Models to Assess the Effects of Environmental Plastic Pollution. Front Cell Dev Biol. 2021;9(September). doi:10.3389/fcell.2021.709183
CrossRef - Dijk F van, Song S, Eck GW. van, et al. Inhalable textile microplastic fibers impair airway epithelial growth (Pre-print). bioRxiv. Published online 2021. https://www.biorxiv.org/content/10.1101/ 2021.01.25.428144v3
- Masood MI, Hauke NT, Nasim MJ, Sarfraz M, Naseem M, Schäfer KH. Neural stem cell-based in vitro bioassay for the assessment of neurotoxic potential of water samples. J Environ Sci (China). 2021;101:72-86. doi:10.1016/j.jes.2020.07.028
CrossRef - Sarkar B, Verma SK, Akhtar J, et al. Molecular aspect of silver nanoparticles regulated embryonic development in Zebrafish (Danio rerio) by Oct-4 expression. Chemosphere. 2018;206:560-567. doi:10.1016/j.chemosphere.2018.05.018
CrossRef - Romano N, Ceci M. The face of epicardial and endocardial derived cells in zebrafish. Exp Cell Res. 2018;369(1):166-175. doi:10.1016/j.yexcr.2018.05.022
CrossRef - Gardiner MR, Gongora MM, Grimmond SM, Perkins AC. A global role for zebrafish klf4 in embryonic erythropoiesis. Mech Dev. 2007;124(9-10):762-774. doi:10.1016/j.mod.2007.06.005
CrossRef - Shin U, Nakhro K, Oh CK, et al. Large-scale generation and phenotypic characterization of zebrafish CRISPR mutants of DNA repair genes. DNA Repair (Amst). 2021;107(June):103173. doi:10.1016/j.dnarep.2021.103173
CrossRef - Dai Y, Zhu L, Huang Z, et al. Cebpα is essential for the embryonic myeloid progenitor and neutrophil maintenance in zebrafish. J Genet Genomics. 2016;43(10):593-600. doi:10.1016/j.jgg.2016.09.001
CrossRef - Xu J, Zhu L, He S, et al. Temporal-Spatial Resolution Fate Mapping Reveals Distinct Origins for Embryonic and Adult Microglia in Zebrafish. Dev Cell. 2015;34(6):632-641. doi:10.1016/j.devcel.2015.08.018
CrossRef - Zhan Y, Huang Y, Chen J, et al. Caudal dorsal artery generates hematopoietic stem and progenitor cells via the endothelial-to-hematopoietic transition in zebrafish. J Genet Genomics. 2018;45(6):315-324. doi:10.1016/j.jgg.2018.02.010
CrossRef - Wogulis M, Wright S, Cunningham D, Chilcote T, Powell K, Rydel RE. Nucleation-dependent polymerization is an essential component of amyloid-mediated neuronal cell death. J Neurosci. 2005;25(5):1071-1080. doi:10.1523/JNEUROSCI.2381-04.2005
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





