How to Cite | Publication History | PlumX Article Matrix
Bioanalytical Studies on Fungal Pathogens Originating from Spoiled Alpan Banana (Musa Paradisiaca)
Amrendra Kumar singh1  and Prakash Chandra Gupta2*
and Prakash Chandra Gupta2*
1Department of Botany, Jai Prakash University Chapra, Bihar, India
2Department of Botany, P.R. College Sonpur (Saran) Bihar, India
Corresponding Author E-mail:pcguptabotany@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3150
ABSTRACT: Background: The entire Alpan banana plant can be utilized medicinally. Since the banana's rapid global expansion, several non-wood plant fibers have been used as substitutes for wood pulp in the manufacture of pulp, paper, and paper board. Currently, modest commercial pulping operations use a range of non-wood fibers, including bamboo, jute, straw, rice, and abaca. The increasing number of banana varieties is a result of the everyday developments that are being achieved in several fields. The Materials and Methods: The purpose of this current study is to check fungal pathogenicity in infected Alpan banana fruit, molecular characterization of the pathogenic species and evaluation of biochemical activities in banana fruit. The studied biochemical activities include protein content, ascorbic acid, total sugar and total phenolic compounds. Ascorbic acid oxidase (AAO) and Phenol Oxidase (PPO) are also studied in the selected banana variety. Result: The fungal pathogenicity assay showed incidence of Aspergillus species on Alpan banana fruit. A very high prevalence of Aspergillus flavus and Aspergillus fumigatus were observed in examined fruits. Species confirmation were based on molecular characterization method and its subsequent bioinformatic analysis. Proteins, total phenolic content and Keto acid were observed to be 223.01, 47.32 and 964.0 mg/g respectively whereas PPO and AAO showed 0.800 and 0.175 U/ml content in the fruit sample. Conclusion:The study concludes the prevalence of fungal infection in ripe bananas by Aspergillus species and changes observed at their biochemical constituents level post fungal infestation
KEYWORDS: Morphology; Pathogenicity; Phenol Oxidase
Download this article as:| Copy the following to cite this article: Singh A. K, Gupta P. C. Bioanalytical Studies on Fungal Pathogens Originating from Spoiled Alpan Banana (Musa Paradisiaca). Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Singh A. K, Gupta P. C. Bioanalytical Studies on Fungal Pathogens Originating from Spoiled Alpan Banana (Musa Paradisiaca). Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/3NOQcGU |
Introduction
Banana is the most extensively consumed fruit in the world, and it is abundant in protein, carbs, and is highly nutritious for individuals of all ages.
It is a tropical fruit that is cultivated by people from over 122 different countries. In terms of output, banana ranked fourth in 2004 behind rice, corn, and milk on 3.8 million hectares and produced 56.4 million metric tonnes1.
The manufacturing of bio-oil, bio sorbents, pulp and paper, cosmetics, energy-related processes, the manufacturing of organic fertilisers, the cleansing of the environment, and biotechnology-related processes are just a few of the businesses that have used bananas2.
With an estimated production value of more than 139 million tons, it is the second largest fruit crop in the world, according to the FAO, due to extensive planting and consumption in the region in recent decades [30].
The countries that produce the most bananas worldwide are India, China, Uganda, Ecuador, the Philippines, and Nigeria. Indigenous people have been using this fruit for food purposes for a long time, but they have recently started experimenting with the advantages of using banana plants in their daily lives. On the other hand, banana plants are a source of pollution because they are cut down after harvest and left in the fields, which results in Sigatoka1.
Alpan banana is cultivated on a large scale in the Vaishali district of Bihar, which covers about 300 acers of land. The Musaceae family has two generations and 42 different species,35species of the genus Musa.Musa Paradisiacaherbaceous plant (up to 9mtr tall). The fruits are oblong, fleshy, 5-7 cm tall in wild varieties and tall on cultivated varieties. The ripe fruit is fresh, spicy and full of seeds and the peel is thicker than any other banana3. The Plantain banana of the ‘‘Terra ’variety (Musaparadisiaca) may be of industrial demandbecause it containshigh starch value. The flour and starch content of that unripe fruit were separated and their morphological, chemical and physicochemical properties were obtained3. With granule sizes of 31.7 and 47.3 mm and temperatures above 65°C, the dry yields of banana flour and starch were 50.6 and 28.5%, respectively. Despite having a much lower swelling capacity than starch, banana flour had a melting point that was greater at all temperatures than that of starch4. Neutrally, carbohydrates can be classified as digestible or digested and as glycemic or nonglycemic. Bananas have been described as another source of indigestible carbohydrates due to their DFs and RS. Starch is a major component of green bananas and undergoes a number of changes during maturation2. Starch, when threatened by the action of amylase, is called digestible starch.A wide range of destructive enzymes on the cell wall produced by the phytopathogenic fungi, are very important biological weapons, which help the pathogen to enter and colonize within the host5.Effective plant infection is highly dependent on the ability of the phytopathogen to reach the internal tissue components5.A key role in this is played by the enzymes that break down cellulase. The breakdown of cellulose into soluble sugars by phytopathogens involves the action of many enzymes. One research paper suggests that cellulose is damaged by the synergistic action of three types of enzymes namely endoglucanase (EC 3.2.1.4), exoglucanase (EC 3.2.1.91) and β-glucosidase (EC 3.2.1.21). According to previous investigation theory endoglucanase (CX) is an accessible intramolecular hydrolyze β-1, a 4-glucosidic bond of cellulose chains randomly to produce the results of a new chain. Exo-glucanase or cello-biohyrolase (C1) further separates cellulose chains from one end to release soluble cellobiose or glucose5.β- glucosidase also lowers cellobiose to release glucose. The purpose of this current study to check their fungal pathogenicity in all the 3 different stages of banana fruit and also find the analytical methods for investigating the protein content, phenol content and ascorbic acid content in all the 3 stages of the fruits and also check the phenol oxidase and ascorbic acid oxidase enzyme activity.
Materials and Methodology
Sample used
The banana fruit for sample was brought from local market, for infected stage we kept the ripe banana for 3 to 4days long for infection and fungus will grow in moisture with their packages.
Chemicals used
Folin Ciocalteu phenol reagent, Sodium Potassium tartrate, ascorbic acid, Copper sulphate, gallic acid, (2,4- Dinitrophenylhydrazine (DNPH)), Coomassie Brilliant Blue, Bovine Serum Albumin etc., were obtained from Sigma Co.
Isolation of Fruit Spoilage Fungi
Separation of mold from diseased fruit is done in the area of Malt Extract Agar (MEA). MEA was prepared by dissolving 20g malt extraction and 20g agar in 1000mL of distilled water. The pH of the mold growth area was modified to 6.5, followed by autoclaving at 121°C and 15lb / inch2 for 15 minutes. In a sterile condition of 40 – 45°C, streptomycin at a dose of 200mg/L was added to prevent bacterial growth6. Approximately, 25mL is poured into each fermented petroleum plate. The center of each petal plate was well rotated and allowed to harden at room temperature. Separation of the different samples was done by direct transfer of visible grains or transfer of a small portion of infected fruit to the growing area. Plates immersed in water in 25 + 2℃ for 3 – 4 days. Emerging colonies are cleaned with a small increase in grain from actively growing colonies to new MEA plates. Pure cultures of all isolated areas were maintained to be identified at 40℃6.
Molecular characterization of fungal isolates
The morphologically identified fungal isolates were confirmed by characterizing their Internal Transcribed Spacer (ITS) regions – the barcode marker for fungal identification. Three isolates were isolated and morphologically identified using Internal Transcribed Spacer (ITS) region.Primer pairs ITS1 and ITS4 was used to amplify ITS regions8. In PTC-100 Peltier Thermal Cycler, PCR amplifications were carried out in a total volume of 25 μL including 0.5 μL of 0.5 μM template DNA, 5 μL of 5 PCR buffer, 4.0 mM MgCl2, 0.8 mM dNTP mix of both forward and reverse primers, and 0.625 U of Taq polymerase (MJ Research Inc., MA, USA).
The initial 34 PCR cycles consisted of a denaturing step at 95 °C for 1 min, an annealing step at 52 °C for 30 sec, an extension step at 72 °C for 1 min, and a final extension step at 72 °C for 10 min. After being purified using a QIA quick Purification kit from Qiagen (Hilden, Germany) in line with the manufacturer’s instructions, the PCR products were transferred to a service provider for sequencing. Sequencing was followed by pairwise alignment of the sequences using the BioEdit program9. BLAST search was used to identify all fungal isolates as it showed the closest match.
Biochemical Parameters of Infected fruit
The protein concentration determination was done by Lowry’s method10. The standard curve was obtained using (BSA) bovine serum albumin (Sigma-Aldrich).The total phenolic content was estimated by the method using standard phenolic compound gallic acid11 with some modification. Briefly, 1ml of the extract was combined with 9ml of distilled water and 1ml of Folin & Ciocalteu’s phenols reagent. A solution of 7% Na2CO3 was then added. Deionized water was used as a blank for the measurement of absorbance at 750 nm, and the amount of phenol was determined from the standard curve plot12.
Ascorbic acid was determined by the method developed by Roe and Kuether in 1943. 0.5g of the sample was weighed, 10ml of 0.4% oxalic acid was added to solubilize it in a test tube for 10 minutes, followed by 5 minutes of centrifugation. 9 ml of 2,6-dichlorophenol indophenol to 1ml of the filtrate that had been deposited into a dry test tube in duplicate and the absorbance at 520 nm was measured after 15 and 30 seconds13.
The Keto acid was determination by Friedemann (1957) method. For standard pyruvic acid was used the sample was crushed with phosphate buffer and supernatant was used for estimation. 1ml of sample containing DNPH Reagent was added and vortexed, and incubated. After 20 min sodium hydroxide was added and content was mixed, absorbance was taken at 540nm.Total sugar content was measured by Titrimetric method14 using Methylene Blue as indicator.
Ascorbic acid oxidase activity, 0.5ml of ascorbic acid solution (1mg/ml in 01M acetate buffer) and 0.5ml of 01M acetate buffer (pH5.6) was poured to each flask. The flasks were incubated at 30°C for 10minutes of slow shaking condition, The content was centrifuged for 20min at 3,000rpm after adding 2.5ml of 10% metaphosphoric acid. Then,1ml of homogenized and extracted sample solution was taken and 1 ml potassium phosphate buffer solution and 0.5 ml ascorbate were added and take the absorbance at 265nm to different time interval13.
In estimation of Phenol oxidase activity, reaction mixture constituted of 2.5 ml phosphate buffer, 0.3ml catechol solution and then 0.2ml of enzyme substrate. The reaction was initiated by the addition of 2.5mM 4-methyl catechol, then absorbance was taken at 495nm15.
Results and Discussion
The single pure fungus was identified according to the key labels in the fungus identification. For the morphological characterization morphology-based diagnosis a broad and minimal observation made using 7-day-old molds grown at 20°C in the SDA growth zone. The pure culture with infected banana sample was done and it was found A. flavus and A. fumigatus.
 |
Figure 1: Infected Banana sample |
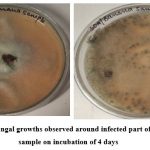 |
Figure 2: Fungal growths observed around infected part of banana sample on incubation of 4 days |
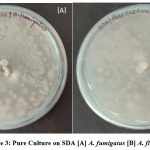 |
Figure 3: Pure Culture on SDA [A] A. fumigatus [B] A. flavus |
Macroscopic characters include colony color, size, texture, texture, genealogical reading and small characters including conidia decoration, conidia size, and color etc. A small test for each type of fungus, small pieces of mycelium fungal were placed in a magnifying microscopic drop. The biochemical test was done and it was found that A. flavus and A.fumigatus. In amylase activity it was found to both fungi are positive while in protease activity was found to both are negative. A complete description of each isolate based on macro and micro morphological letters was prepared16. They have also reported the supportive role in the pathogen’s establishment.
According to reports various fungal pathogens was isolated from the rotten banana fruits17. These pathogens include, Aspergillus fumigatus and Aspergillus flavus. These isolates were identified initially on the basis of their morphological characters and then molecular and genetic analysis was carried out for one of the isolates for DNA -based identifications. The result of molecular characterization is shown in Table 1. Evolutionary history was inferred using maximum parsimony analysis. 1 out of 10 most parsimonious tree is shown in Fig. 3 for an isolate that has 98.19% similarity match to Aspergillus flavus. Likewise, evolutionary history was obtained for all the isolates.In addition, cultures and microphotographs were taken for future reference and comparison in fungal identification12.
Table 1: Species identity of fungi based on ITS sequences
| Isolate | Morphological identification | ITS sequence | % Similarity |
| 1 | Aspergillus flavus | Aspergillus flavus | 98.19 |
 |
Figure 4: Maximum Parsimonyanalysis of Aspergillus flavus Click here to view figure |
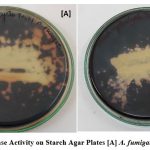 |
Figure 5: Amylase Activity on Starch Agar Plates [A] A. fumigatus [B] A. flavus Click here to view figure |
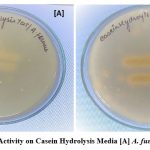 |
Figure 6: Protease Activity on Casein Hydrolysis Media [A] A. fumigatus [B] A. flavus Click here to view figure |
Table 2: In this table shows that the amylase and proteas activity
| S.No | Isolate | Amylase activity | Protease activity |
| 1. | A.flavus | Positive | Negative |
| 2. | A.fumigatus | Positive | Negative |
Banana pulp and peel contain a variety of high-value nutritional compounds that are used in the pharmaceutical industry, including phytosterols and triterpenes like cycloeucalenol and cycloartenol, as well as vitamins (A, B, C, and E), -carotene, and phenolic compounds like gallocatechin, dopamine, lignin, and tannins.
The protein concentration was found in increasing order, in infected banana sample the concentration was 223mg/g. This result infers that protein concentration depend on the growth of banana and its growth while ripening and it is also good protein for human consumption18.Other investigations showed that the total nitrogen content of protein was between 60 and 65 percent. M. acuminata, on the other hand, has a protein value of 1.09 g/100 g. (FW). The protein content increased from 1.3 to 1.8 g/100 g and from 4 to 8 g/100 g as the “Cavendish” and “Dwarf Cavendish” bananas matured, with the concentration rising as ripening progressed9.
Table 3: In this table showing the concentration of protein, total phenolic content, and keto acid
| Estimations | Samples | Concentration
(mg/ml) |
Concentration
(mg/g) |
| Proteins | Infected | 22.30 | 223.01 |
| Total phenolic content | Infected | 4.73 | 47.32 |
| Keto acid | Infected | 96.40 | 964 |
Ascorbic acid, often known as vitamin C, is an organic chemical. Animals may self-synthesize ascorbic acid from glucose; however, humans must get there from food supplements or fruit. Ascorbic acid is essential to all living things. It is measured using a variety of techniques. The ascorbic acid concentration was found to be 10.72mg/100g in infected stage. The ascorbic acid present in the banana is dependent on the growth factor of the banana.
Vitamin C is a potent antioxidant that helps with the production of cortisol, the transport and uptake of non-heme iron at the mucosa, and the decrease of folic acid intermediates. Its flaws include blood capillary fragility, gum degeneration, and scurvy.
According to the reports of18 and19 despite estimates for “Cavendish” bananas’ vitamin C contents ranging from 2.1 to 18.7 mg/100 g, cultivar variations are substantial. The average vitamin C content for “Dwarf Brazilian” was found to be 12.7 mg/100 g and 4.5 mg/100 g for20.These results are in line with a study by21 which 5.1 mg/100 g of vitamin C was determined.
Table 4: In this table shows the %of total sugar and titer value
| S.No | Samples | Titer value | Total sugar (%) |
| 1 | Standard | 18 | 62 |
| 2 | Infected | 6.6 | 57 |
In the total sugar determination titration method was used and sucrose was used for the standard, the result was observed that, in the infected level 57% of total sugar was measured. According to22, Guava has very little phosphorus, but the banana had the highest concentration (32.5mg/100g). Phosphorus is essential for ATP, phospholipids, and acid-base balance. Additionally, it is necessary for the mineralization of bone and teeth. The total sugar content of bananas, ziziphus Mauritania, ziziphus Nammulania, and ziziphus Spina Christi agrees with figures from earlier works of literature23.And in total phenolic content gallic acid was used as a standard, the result showed 47.32mg/g phenolic content in the infected banana sample.
According to 24 in comparison to the 0.9 mg reported by 25, the banana pulp shows antioxidant activity and a total phenolic content of 3.0 mg of gallic acid equivalent/g. Similar to this, it was claimed that the Malaysian banana variety “Pisangmas” contained TPC that varied in concentration from 0.24 to 0.72 mg GAE/g FW, depending on the method of extraction.According to26 the antioxidant activity, total phenolic content, and mineral content of eight varieties of Malaysian bananas differed significantly. According to authors27, catechin and gallic acid are present in the pulp and peel of both ripe and unripe banana varieties. Its pulp included a high percentage of hydroxycinnamic acids (ferulic acid-hexoside with 4.4-85.1 g/g). The keto acid was found in the very high level in the banana and pyruvic acid used as standard, 964mg/g was used in the infected banana sample.
Table 5: In this table showing the phenol oxidase and Ascorbic acid oxidase (U/ml) of the infected banana samples.
| S.No | Test | Result |
| 1. | Phenol oxidase (U/ml) | 0.800 |
| 2. | Ascorbic acid oxidase (U/ml) | 0.175 |
After all this proximate analysis was done, enzyme activity was determined, in ascorbic acid oxidase 0.800U/ml was found in the infected banana sample. The phenol oxidase was observed in the infected banana to be 0.800U/ml
According to 29oxygen intake in mash can result in a number of possible outcomes, including as the possibility of unsaturated fatty acid oxidation, the cross-linking of thiol-rich proteins, and the oxidation of polyphenols, which produces colour. Although this enzyme is extremely thermotolerant, ascorbic acid oxidase is only moderately heat resistant. As a result, it is believed that a mash at 65–70°C has a high level of Ascorbic acid oxidase activity. Furthermore, it is clear that both enzymes can work at a mashed pH.
Increased at the start of tomato fruit ripening, according to several research. The enhanced enzyme activity in samples of infected bananas is documented in the current study. Enzyme activity was found to be minimum in kadali and fruit flesh softened slowly maybe due to the slow activity of cell wall degrading enzymes.
Conclusion
After completion of all the determination and estimation, the conclusions are the concentration of keto acid is highly present in the banana sample while the concentration of ascorbic acid was less while the protein concentration also lowers in the keto acid. The total sugars, glucose, and fructose concentrations increased along with the banana’s developmental phases, which raised their concentration in the banana fritter. The level of nutritional components gets affected by the pathogenicity of various fungal species; therefore, the appropriate conditions must be maintained during the storage and transportation of these fruits to avoid the incidence of such fungal species.
Acknowledgement
This work and the research behind it wouldn’t have been possible without the exceptional assistance of my supervisor, Mr. Prakash Chandra Gupta, Department of Botany, P.R. College Sonpur (Saran) Bihar. His timely guidance, insights, and recommendations kept my work on track. I am also grateful to the CytoGene Research and Development, Lucknow for agreeing me to assist in data collection and analysis.
Conflict of Interest
There are no conflicts of interest to disclose.
Funding Sources
No funding has been provided for the research, authorship and publication of this article.
References
- Sarangi, M., &Instit, S. B. S. P. G. (2014). A review on banana starch. Inventi Rapid: Planta Activa, 2014, 1-8.
- Imam, M. Z., &Akter, S. (2011). Musa paradisiaca L. and Musa sapientum L.: A phytochemical and pharmacological review. Journal of Applied Pharmaceutical Science, (Issue), 14-20.
- Ahmad, I., & Beg, A. Z. (2001). Antimicrobial and phytochemical studies on 45 Indian
medicinal plants against multi-drug resistant human pathogens. Journal of ethnopharmacology, 74(2), 113-123. - Sarkar, S., Girisham, S., & Reddy, S. M. (2011). Cellulase activity in fungal infected banana fruits-Effect of different synthetic media on cellulase production. Recent Advan. Appl. Sci, 26, 12-17.
- Zhang, Y. H. P., & Lynd, L. R. (2004). Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnology and bioengineering, 88(7), 797-824.
CrossRef - Akihisa, T., Kimura, Y., & Tamura, T. (1998). Cycloartane triterpenes from the fruit peel of Musa sapientum. Phytochemistry, 47(6), 1107-1110.
CrossRef - Schoch et al. (2012)
- White et al. 1990
- John P, Marchal J. Ripening and biochemistry of the fruit. In: Gowen S, editor. Bananas and Plantains. London: Chapman and Hall; 1995. pp. 434-467
CrossRef - Lowry, O H, Rosebrough, N J,Farr,A L and Randall, RJ(1951) J Biol Chem 193 265. Mattoo, SRL (1970) Indian J Biochem 7 82.
CrossRef - Agarwal, P. K., Singh, A., Gaurav, K., Goel, S., Khanna, H. D., & Goel, R. K. (2009). Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var. paradisiaca) in rats.
- Sinsabaugh, R. L. 2010. Phenol oxidase, peroxidase and organic matter dynamics of soil. J. Soil Biology & Biochemistry. 42:391-404.
CrossRef - Dadzie, B. K. and J. E. Orchard.1997. Routine Postharvest Screening of Banana /Plantain Hybrids: Criteria and Method, Inihap Technical Guidelines. Haque, M. A. 1988.
- Becalski, A., Lau, B. P. Y., Lewis, D., Seaman, S. W., Hayward, S., Sahagian, M., et al. (2004). Acrylamide in French fries: influence of free amino acids and sugars. Journal of Agricultural and Food Chemistry, 52, 38013807.
CrossRef - Saeidian, S. 2013. Partial purification and characterisation of polyphenol oxidase from tomatoes (solanum lycopersicum). International journal of Advanced Biological and Biomedical Research. 1(6): 637-648.
- Adekunle, O. A., Adejoke, O. O., Adesola, A. A., & Patrick, O. O. (2009). Cellulase activity in tomato fruits infected with Penicillium funiculosum Thom. African Journal of Plant Science, 3(5), 113-116.
- Fatima et al., 2021
- USDA (United States Department of Agriculture). 2012. Nutrient Database.
http://www.nal.usda.gov/fnic/ foodcomp/Data/SR17/wtrank/sr17a306. pdf [Accessed: October 2017] - Amrein, T. M., Bachmann, S., Noti, A., Biedermann, M., Barbosa, M. F., BiedermanneBrem, S., et al. (2003). Potential of acrylamide formation, sugars, and free asparagine in potatoes: a comparison of cultivars and farming systems. Journal of Agricultural and Food Chemistry, 51,5556e5560.
CrossRef - Arumugam, R., and Manikandan, M., 2011. Fermentation of pretreatedhydrolyzates of banana and mango fruit wastes for ethanol production. Asian Journal of Biological Science 2: 246-256.
- Babu, P H., Reddy, C A., Prashanthi, N., and Mahal, J S., 2015. Banana Peel as a Biosorbent in Removal of Nitrate from Water, International Advanced Research Journal in Science, Engineering and Technology. 2 (10), 95-98.
CrossRef - Bhowal, 2012. Utilization of fruit wastes in producing single cell protein, International Journal of Science, Environment and Technology, 1(5), 430-438.
- Birt DF, Boylston T, Hendrich S, Jane J-L, Hollis J, Li L, et al. Resistant starch: promise for improving human health. Advances in Nutrition. 2013; 4(6):587–601. https://doi.org/10.3945/an.113.004325 PMID: 24228189
CrossRef - Chai, M., Ho, Y. W., Liew, K. W. and Asif, J.M., 2004. Biotechnology and in vitro Mutagenesis for Banana Improvement. In: Banana Improvement: Cellular, Molecular Biology and Induce Mutations, Jain, S. and R. Swennen (Eds.). Science Publisher Inc., USA, Pp: 59-77.
- Effect of temperature and time on the formation of acrylamide in starch based and cereal model systems, flat breads and bread. Food Chemistry, 92, 693e700.
CrossRef - Gaithersburg, MD, USA: AOAC. International, Official Method 982.30.
- Iralapati, V., and Kummari, S., 2014. Production of Citric Acid from Different Fruit Peels Using Aspergillus niger, International Journal of Scientific Engineering and Research, 3 (5), 129- 130.
- Itelima, J., Onwuliri, F., Onwuliri, E., Onyimba, I., and Oforji, S., 2013. BioEthanol Production from Banana, Plantain and Pineapple Peels by Simultaneous Saccharification and Fermentation Process, International Journal of Environmental Science and Development, 4,(2),213-216.
CrossRef - Li BW, Schuhmann PJ, Wolf wr. Chromatographic determinations of sugars and starch in a diet composite reference material. Journal of Agricultural and Food Chemistry. 1985; 33(3):531–6.
CrossRef - Outlook, O. F. A. (2010). OECD/FAO 2010.

This work is licensed under a Creative Commons Attribution 4.0 International License.





