How to Cite | Publication History | PlumX Article Matrix
S. Dennis , *R. Ravindhran*
, *R. Ravindhran* P. Charles
P. Charles , S. Leo Arockia Raj
, S. Leo Arockia Raj and V. Kaviarasan
and V. Kaviarasan
T.A.L. Unit for Plant Tissue Culture and Molecular Biology, Department of Plant Biology and Biotechnology, Loyola College (Autonomous), Chennai, Tamil Nadu, India.
Corresponding Author E-mail: raviloyola1998@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3137
ABSTRACT: Buckwheat (Fagopyrum spp.) is a pseudocereal, dicot, economically significant, and nutraceutical crop that belongs to the order Caryophyllales of the family Polygonaceae. The two species Fagopyrum esculentum (common buckwheat) and Fagopyrum tartaricum (tartary buckwheat) are most grown in the Himalayas. A crop that thrives in extremely cold temperatures is Fagopyrum tartaricum. It contains D-chiro inositol, quercetin, vitexin, and the antioxidant polyphenol rutin. This study has devised an effective indirect organogenesis strategy for tartary buckwheat, (Fagopyrum tartaricum). Callus induction medium containing Murashige and Skoog’s (MS) medium with additional 2 mg L-1 of 2,4-dichlorophenoxyacetic Acid (2,4-D) and 0.1 mg L-1 6-benzylaminopurine (BAP) produced the optimum (90.67%) friable yellow callus using leaf explant. Shoot proliferation medium (SPM) containing MS medium supplemented with 3.0 mg L-1 6-benzylaminopurine (BAP) and 0.5 mg/l Naphthalene Acetic Acid (NAA) has produced the most shoots (35.2±1.83) with mean shoot length of 3.41±0.14 in cm. The regenerated shoots were successfully rooted in indole-3-butyric acid-containing full-strength MS medium. A rooting medium with 3 mg L-1 IBA exhibited the most roots with 6.84±0.45 and a mean length of roots being 11.59±0.44 in cm. 100% of the in vitro rooted shoots that were transplanted into the field survived.
KEYWORDS: Cold tolerant; Fagopyrum tartaricum; Multiple Shoot Formation; Nutraceutical crop; Rutin
Download this article as:| Copy the following to cite this article: Dennis S, Ravindhran R, Charles P, Raj S. L. A, Kaviarasan V. Indirect Organogenesis and High Frequency Plant Regeneration in Buckwheat (Fagopyrum tartaricum Gaertn.). Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Dennis S, Ravindhran R, Charles P, Raj S. L. A, Kaviarasan V. Indirect Organogenesis and High Frequency Plant Regeneration in Buckwheat (Fagopyrum tartaricum Gaertn.). Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/468XFIB |
Introduction
Buckwheat (Fagopyrum sp.) is a commercially important cash crop, a member of the Polygonaceae family and order Caryophyllales. It is a pseudocereal1, dicot, nutraceutical plant. It is also a functional food.2 The genus Fagopyrum has 18 species, including the two domesticated species Fagopyrum esculentum and F. tartaricum.3 The major cultivable varieties in India and in the Himalayan, region is Fagopyrum esculentum (sweet buckwheat) and Fagopyrum tartaricum Gaertn. (Bitter buckwheat). It is a common crop in Central and Eastern Europe and in Asia.4
The entire parts of the buckwheat plant have medicinal significance and are used locally in traditional healthcare to treat a variety of alignments (diseases). Its leaf is used as vegetable, soup, medicine, and beverage. Grain hulls are used to stuff pillows, and the blooms, are a good source of nectar for honey. Straw is good fodder for animals. In tartary, rutin levels were found up to five times greater than in normal buckwheat.5 Buckwheat grains typically have a protein concentration of 12–19% and higher lysine content.6 The long-chain fatty acids, which constitute a crucial source of dietary energy is found in buckwheat.5 It has also the highest quantities of Se, Zn, Fe, Co, and Ni.6 Additionally, the phytoremediation of mercury, aluminium toxicity, and other heavy metals is a possible benefit of buckwheat.2 The tartary buckwheat also has unique qualities like self-pollination, frost resistance, and high rutin concentration which is absent in common buckwheat. Tissue culture techniques along with the genetic manipulation techniques could help in integrating the desired features of tartary buckwheat to common buckwheat.
The aim of the current research is to create a highly effective technique for indirect organogenesis and establishment of complete plant from leaves of tartary buckwheat. This plant regeneration system for tartary buckwheat could be useful for potential use in cell selection and genetic modification. The hormone dosage that produces the best results for callus induction, shoot and root induction are also determined.
Material and Methods
The mature seeds of Fagopyrum tartaricum were obtained from Nepal Agricultural Research Council (NARC), Lalitpur, Nepal. These were used for further investigation. The mature seeds of Fagopyrum tartaricum were surface sterilized in the running tap water for 10 minutes and were then washed with Tween 20 solution. Seeds were then washed with double distilled (DD) water. Further, the seeds were washed for 2 minutes each using 0.1% (w/v) HgCl2 and 70% ethanol. After each wash, the seeds were twice rinsed in sterile distilled water to eliminate any remaining traces of HgCl2 and ethanol. The sterilized seeds were allowed to air dry in the laminar airflow chamber for about 20 minutes. The dried seeds were inoculated on the MS medium with pH between 5.7- 5.8 and 30 gm L-1 of Sucrose. The inoculated seeds were allowed to grow under controlled day/night environment. The temperature was kept constant at 25±2 °C with a 16/8 h (light/dark) photoperiod.
For callus induction, the leaves of seedlings that were two weeks old were used as explants. The MS basal medium was added with Plant Growth Regulators (PGR) of different concentrations of dichlorophenoxy acetic acid (0.5 – 4.0 mg L-1 ), and 6-Benzylaminopurine (0.0 – 0.2 mg L-1 ). The pH was maintained between 5.7- 5.8. The explants were inoculated under controlled environment and were kept in a 16/8 h (light/dark) photoperiod at a temperature of 25±2 °C.
The sub cultured friable Calli were placed on the Shoot proliferation medium (SPM) containing MS medium in combinations with Plant Growth Regulators (PGR) like 6-Benzylaminopurine (0.25 – 4.0 mg L-1 ), and 1-Naphthalene Acetic Acid (0.0 – 0.1 mg L-1 ) in various concentrations. The pH was maintained between 5.7- 5.8. The explants were inoculated under controlled day/night environment. The conditions were maintained at 16/8 h (light/dark) photoperiod at 25±2 °C.
The regenerated shoots were for rooting on MS Medium. The MS medium was added with indole-3-butyric acid (0.25 to 4.00 mg L-1 ) and Naphthalene acetic acid (0.25 to 4.00 mg L–1 ) for regeneration of Callus. The plantlets were transferred into perforated plastic cups containing potting mix (having garden soil, coarse sand, and vermiculite in 1: 1: 1 ratio) and were nurtured with reduced MS medium without sucrose. Then the plantlets were transferred to the greenhouse for further hardening. For continued growth, the hardened plantlets were moved to the field area.
The experiments were laid out according to completely randomised block design; Each experiment consisted of three repetitions and each consisted of 25 cultures. The means were compared using Duncan’s new multiple range test (DMRT) at p <0.0531in order to assess the significance of differences between treatment means. Both ANOVA and DMRT analysis were carried out using the SPSS v.20.0 (Statistical Package for the Social Sciences, SPSS)
Results
After two weeks of incubation on media containing growth regulators, leaf explants developed calluses that varied in colour and appearance (Fig1). The majority of them Friable, yellow 90.67% (Table 1) while being compact, not embryonic. Some of them had a more noticeable yellowish tint compact 76.33%. There were others compact and greenish yellow 69.33%. Still others were Greenish, compact 44.00 % displayed spherical golden light structures on their surface (Fig 1). The most effective callus development was seen at 2 mg L-1 of 2,4-D and 0.1 mg L-1 BAP (Table 1) which were friable, yellow (90.67%) callus (Fig 2).
 |
Figure 1: Organogenesis of Fagopyum tartaricum from seeds: a) Seed b) germinated under in-vitro condition c) Callus induction
|
Table 1: The results of various PGRs on the production of callus from leaf of Fagopyrum tataricum, 4 weeks after culture
|
Plant growth regulators (mg/l) (PGR) |
Percentage Response (%) |
Nature of Callus |
|
|
2, 4 D |
BAP |
|
|
|
0.5 |
0 |
52.00 |
C , B |
|
1.0 |
0 |
33.33 |
C , Gw |
|
2.0 |
0 |
30.67 |
F , Cr |
|
4.0 |
0 |
28.00 |
C , B |
|
0.5 |
0.05 |
22.67 |
C , B |
|
1.0 |
0.05 |
38.67 |
C , W |
|
2.0 |
0.05 |
44.00 |
F , Gb |
|
4.0 |
0.05 |
45.33 |
F , Cr |
|
0.5 |
0.1 |
44.00 |
C , Gb |
|
1.0 |
0.1 |
76.33 |
C , Y |
|
2.0 |
0.1 |
90.67 |
F , Y |
|
4.0 |
0.1 |
69.33 |
F , Gy |
|
0.5 |
0.2 |
59.67 |
F , G |
|
1.0 |
0.2 |
56.00 |
C , Cr |
|
2.0 |
0.2 |
61.33 |
F , B |
|
4.0 |
0.2 |
52.00 |
C , Gb |
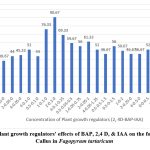 |
Figure 2: Plant growth regulators’ effects of BAP, 2,4 D, & IAA on the formation of Callus in Fagopyrum tartaricum
|
% of response figures show the average response percentage of 25 explants for each treatment of three repeated experiments taken 4 weeks after.
Present data were arcsine transformed (degrees) in advance of statistical analysis
Means within columns that are separated by the same letter are not substantially different at 5% probability level using Duncan’s Multiple Range Test (DMRT)
B brown, F friable, Y yellow, C compact, Cr cream, G green, W white, GW greenish white GB greenish brown, GY greenish yellow,
The morphogenetic response of the callus in full strength of MS medium with different concentrations of BAP and NAA is summarised in table 2. The optimum number of shoots 35.2±1.83 (Fig 3), with mean shoot length 3.41±0.14 (Fig 4) were observed at 3.0 mg L-1 of BAP and NAA 0.5 mg L-1 (Table 2), while there was significant decline on the higher concentration of BAP and NAA thereafter
Table 2: The results of different PGRs on the induction of shots callus and shoot length of Fagopyrum tataricum, after 4 weeks of culture
|
Plant growth regulators (mg/l) |
No. of Shoots / Explant Mean |
Mean Shoot Length (cm) Mean |
|
|
BAP |
NAA |
|
|
|
0.25 |
0 |
3.12±0.17 |
0.45±0.05 |
|
0.5 |
0 |
9.4±0.31 |
0.77±0.11 |
|
1.0 |
0 |
19.48±0.95 |
1.25±0.05 |
|
2.0 |
0 |
12.32±0.4 |
1.29±0.06 |
|
3.0 |
0 |
2.88±0.18 |
0.44±0.05 |
|
4.0 |
0 |
9.64±0.39 |
1.35±0.05 |
|
0.25 |
0.25 |
16.16±0.94 |
1.29±0.05 |
|
0.5 |
0.25 |
12.04±0.36 |
1.33±0.05 |
|
1.0 |
0.25 |
3.8±0.16 |
0.47±0.05 |
|
2.0 |
0.25 |
12.56±0.28 |
1.34±0.05 |
|
3.0 |
0.25 |
22.4±1.31 |
1.15±0.06 |
|
4.0 |
0.25 |
15.64±0.91 |
1.24±0.05 |
|
0.25 |
0.50 |
3.88±0.17 |
0.44±0.05 |
|
0.5 |
0.50 |
12.72±0.3 |
1.25±0.05 |
|
1.0 |
0.50 |
21.04±1.55 |
1.28±0.05 |
|
2.0 |
0.50 |
18.16±0.98 |
1.29±0.05 |
|
3.0 |
0.50 |
35.2±1.83 |
3.41±0.14 |
|
4.0 |
0.50 |
12.28±0.34 |
1.32±0.05 |
|
0.25 |
1.0 |
6.8±0.24 |
0.48±0.04 |
|
0.5 |
1.0 |
20.4±1.2 |
1.35±0.06 |
|
1.0 |
1.0 |
6.72±0.23 |
0.47±0.04 |
|
2.0 |
1.0 |
12.24±0.35 |
1.37±0.06 |
|
3.0 |
1.0 |
12.96±0.32 |
1.43±0.04 |
|
4.0 |
1.0 |
23.2±1.6 |
1.3±0.04 |
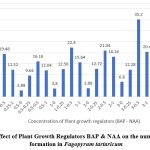 |
Figure 3: Effect of Plant Growth Regulators BAP & NAA on the number of shoot formation in Fagopyrum tartaricum
|
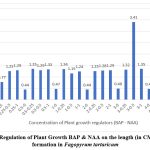 |
Figure 4: Regulation of Plant Growth BAP & NAA on the length (in CM) of shoot formation in Fagopyrum tartaricum
|
Shoots of 5-6 cm were cultured on full strength MS medium supplemented with 0.25 – 4.0 mg L-1 of IBA and 0.25 – 4.0 mg L-1 of NAA (table 3). IBA at 3.0 mg L-1 shows the optimum for rooting (Table-3). The most possible roots mean 6.84±0.45 (Fig 5) with mean root length 11.59±0.44 (Fig 6) was observed (Table 3).
Table 3: Effect of different PGRs on root induction and root length in Fagopyrum tataricum, after 4 weeks of culture
|
Plant growth regulators (mg/l) (PGR) |
No. of roots Mean |
Mean root Length (cm) Mean |
|
|
IBA |
NAA |
|
|
|
0.25 |
0 |
2.72±0.15 |
1.77±0.09 |
|
0.50 |
0 |
2.92±0.17 |
1.74±0.1 |
|
0.75 |
0 |
3.2±0.16 |
1.76±0.09 |
|
1.00 |
0 |
3.04±0.19 |
1.91±0.09 |
|
2.00 |
0 |
4±0.16 |
5.81±0.06 |
|
3.00 |
0 |
6.84±0.45 |
11.59±0.44 |
|
4.00 |
0 |
5.56±0.25 |
6.29±0.24 |
|
0 |
0.25 |
5.2±0.18 |
10.07±0.26 |
|
0 |
0.50 |
5.4±0.22 |
7.84±0.25 |
|
0 |
0.75 |
5.64±0.23 |
7.49±0.28 |
|
0 |
1.00 |
5.48±0.23 |
7.56±0.25 |
|
0 |
2.00 |
5.36±0.24 |
7.19±0.3 |
|
0 |
3.00 |
5.32±0.17 |
7.24±0.28 |
|
0 |
4.00 |
5.08±0.16 |
7.32±0.24 |
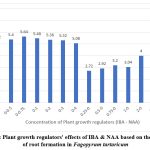 |
Figure 5: Plant growth regulators’ effects of IBA & NAA based on the number of root formation in Fagopyrum tartaricum
|
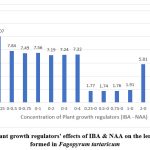 |
Figure 6: Plant growth regulators’ effects of IBA & NAA on the length of roots formed in Fagopyrum tartaricum
|
Discussion
The Organogenic callus was successfully induced on the leaf explant of tartary buckwheat using the combinations of Auxins and Cytokinin in various combinations. Optimal callus development was observed at 2 mg L-1 of 2,4-D and 0.1 mg L-1 BAP (Table 1) which were friable, yellow (90.67%) callus (Fig 2). These outcomes line up with the results observed by Saraswat & Kumar, (2019)7, Woo et al,(2000)8, Jin Hong et al.(2002)9, Mirjana Neskovic, (1987)10 Srejovic Veroslava (1981)11. However, the auxin 2,4-D alone and in various concentrations failed to initiate callus formation. But 2, 4-D showed vital responsibility with Cytokinins for callus induction (Neskovic et al. 1987) 10.
Analysis of variance revealed that frequency of responding explants, mean shoot numbers and mean shoot length were adversely impacted by the auxin concentration. The percentage of explants showing shoot proliferation improved with the increasing concentration of NAA up to 0.5 mg L-1 , beyond which there was a progressive decline.
The concentration of Auxin that supported the maximum shoot proliferation also produced the longest average shoot length for that cytokinin. These outcomes concur with the previous ones with Rashid et al, (2009)12, Lee et al., (2009)13, Woo et al., (2000)8. The quantity of shoots varied between 2.88 to 35.2 per callus explants (Table 2). The average shoot length was 4.38 cm (Fig 3). The length of shoots ranged from 0.44 to 3.41 cm (Fig 4)
The number of roots ranging from 2.92 to a maximum of 6.84 roots per plant observed (Fig 5). The length of the roots varied from 1.77 to 11.59 cm (Fig 6). This is agreement with the results of Saraswat & Kumar (2019)7.
Conclusion
In closing, this paper presents a successful procedure for high frequency plant regeneration from leaf explant. Callus induction was optimum with 2 mg L-1 of 2,4-dichlorophenoxyacetic Acid (2,4-D) and 0.1 mg L-1 6-benzylaminopurine (BAP). 2,4-D alone could not induce callus formation. But in combination with cytokinin produced results. This plant regeneration system of tartary buckwheat could be useful for future application of genetic transformation.
Acknowlegement
I gratefully acknowledge Nepal Agriculture Genetic Resources Centre (NAGRC), Khumaltar, Lalitpur, Kathmandu, Nepal for supplying seeds of Buckwheat (Fagopyrum sp.) for the study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
This study received no funding from any sources.
References
- Wijngaard H. H., E. K. Arendt, Buckwheat, Cereal Chem. 2006;83(4):391–401, 2006 DOI: 10.1094/ CC-83-0391
CrossRef - Kumari Anita & Harinder Kumar Chaudhary, Nutraceutical crop buckwheat: a concealed wealth in the lap of Himalayas, Critical Reviews In Biotechnology, (2020) https://doi.org/10.1080/ 07388551.2020.1747387
CrossRef - Luitel Dol Raj, Mohan Siwakoti, Pramod Kumar Jha, Ajay Kumar Jha, and Nir Krakauer,. An Overview: Distribution, Production, and Diversity of Local Landraces of Buckwheat in Nepal, Hindawi Advances in Agriculture. 2017; Volume 2017, Article ID 2738045, org/10.1155/2017/2738045
CrossRef - Joshi Bal Krishna, Hari Prasad Bimp, Prospects and possibilities of buckwheat development through biotechnology, Proc. National Workshop on Research and Development on Buckwheat, Kathmandu, Nepal. 2001 (HP Bimp & BK Joshi, eds.): 13-1 4 Sept
- Raguindin, Peter Francis, Oche Adam Itodo, Jivko Stoyanov, Gordana M. Dejanovic, Magda Gamba, Eralda Asllanaj, Beatrice Minder, Weston Bussler, Brandon Metzger, Taulant Muka, Marija Glisic, Hua Kern, A systematic review of phytochemicals in oat and buckwheat, Food Chemistry, 2021; 338 2021 127982, https://doi.org/10.1016/j.foodchem.2020.127982
CrossRef - Podolska, G.; Gujska, E.; Klepacka, J.; Aleksandrowicz, E. Bioactive Compounds in Different Buckwheat Species. Plants, 2021; 10, 961. https://doi.org/10.3390/plants10050961
CrossRef - Saraswat Ribha and Mithilesh Kumar, Plant Regeneration in Buckwheat (Fagopyrum esculentum ) via Somatic Embryogenesis and Induction of Meristemoids in Abnormal Embryos, Plant Tissue Cult. & Biotech. 2019,; 29(1): 33-47, 2019 (June) Bangladesh Assoc. for Plant Tissue Culture & Biotechnology, DOI: https://doi.org/10.3329/ptcb.v29i1.41977
CrossRef - Woo Sun Hee, Arun Nair, Taiji Adachi, and Clayton G. Campbell. Plant Regeneration from Cotyledon Tissues of Common Buckwheat (Fagopyrum Esculentum Moench)’ In Vitro Cell. Dev. Biol. -Plant. 2000; 36:358-361, Society for In Vitro Biology1054-5476/00
CrossRef - Jin Hong, Jing-fen Jia, Jian-guo Hao, Efficient plant regeneration in vitro in buckwheat. Plant Cell, Tissue and Organ Culture. 2002; 69: 293–295
CrossRef - Neskovic Mirjana, Radmila Vuji, and Sneana Budimir, 1987 Somatic embryogenesis and bud formation from immature embryos of buckwheat (Fagopyrum esculentum) Plant Cell Reports. 1987; 6:423-426
CrossRef - Srejovic Veroslava, Mirjana Neskovic, Regeneration of Plants from Cotyledon Fragments of Buckwheat (Fagopyrum esculentum )’ Z. Pjlanzenphysiol. Bd. 1981;104. S. 37-42 1981
CrossRef - Rashid Umer, Shaukat Ali, Ghulam Muhammad Ali, Najma Ayub, M. Shahid Masood. Establishment of an efficient callus induction and plant regeneration system in Pakistani wheat (Triticum aestivum) cultivars, Electronic Journal of Biotechnology, 2009; ISSN: 0717-3458, Vol.12 No.3, DOI: 10.2225/vol12-issue3-fulltext-1
CrossRef - Lee SY, Kim YK, Uddin MR, Park NI, Park SU. An efficient protocol for shoot organogenesis and plant regeneration of buckwheat (Fagopyrum esculentum ). Roman Biotech Lett. 2009;14:4524-4529.

This work is licensed under a Creative Commons Attribution 4.0 International License.





