How to Cite | Publication History | PlumX Article Matrix
Sreeja Bopin1* and Kalavati Prajapati2*
and Kalavati Prajapati2*
1Department of Microbiology, HVHP Institute of Post Graduate Studies and Research, KSV Kadi, (North Gujarat), India.
2Department of Microbiology, Shri PHG Muni. Arts and Sci. College, Kalol. (North Gujarat), India.
Corresponding Author E-mail: sreejabopin@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3151
ABSTRACT: The most prevalent nutrient is potassium (K), which makes up around 2.5% of the lithosphere. Approximately 90–98% of soil mineral K is present in the forms of feldspar (orthoclase and microcline) and mica (biotite and muscovite). Particularly in smallholder agriculture, replenishing potassium remains difficult because of its dependence on fertilizer. Potassium shortage in soil can be addressed by the use of soluble mineral potassium fertilizers; however, farmers have been constrained by the high price and restricted availability of these products. The present study aims to identify and select soil Actinomycetes from the soils used in the ceramic industry that may dilute potassium. Since feldspar, an insoluble potassium source, is used by most ceramic manufacturers as a raw ingredient, we gathered samples from these businesses. In the Gujarati cities of Morbi, Meshana, and Kadi, ceramic firms were contacted for a total of fifteen samples. 22 Actinomycetes isolates were chosen for further investigation after primary and secondary screening and inoculation onto Aleksandrov agar supplemented with 0.5 percent potassium aluminium silicate. The 16S rRNA sequence of strain KSA 16 confirmed that it was Streptomyces atacamensis. In a liquid solution, KSA 16 was the most effective in dissolving the insoluble potassium source, feldspar.
KEYWORDS: Actinomycetes; Ceramic soil; Potassium; Streptomyces atacamensis
Download this article as:| Copy the following to cite this article: Bopin S, Prajapati K. Isolation, Screening and Molecular Characterization of Potassium Solubilizing Actinomycete Streptomyces atacamensis (KSA16). Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Bopin S, Prajapati K. Isolation, Screening and Molecular Characterization of Potassium Solubilizing Actinomycete Streptomyces atacamensis (KSA16). Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/44QIzqG |
Introduction
Potassium is a macroelement necessary for all forms of life. Both in terms of its concentration in plant tissues and the biochemical and physiological functions it plays, it is the most important cation in the study of plant physiology.1 The majority of the minerals that include potassium are insoluble. The parent elements from which most soils are derived are typically feldspar and mica. 2. Lack of potassium caused all small grains, potato tubers, and the tips or margins of lower leaves of tobacco, cotton, maize, and legume crops to become black, which in turn decreased photosynthesis and resulted in burning and scorching.3 Banana, cotton, and a few other species have a larger requirement for potassium than nitrogen, despite the fact that most cultivated plants have a far lower absorption amount of potassium by roots than nitrogen do. 4. Research has also indicated that plants have the greatest chance of reaching their potential economic output when potassium is abundant in the soil. It is possible that agricultural soil with low potassium availability will not respond as well to high potassium fertilisation rates, with yield improvements of less than those seen in enriched soil. The enhanced soil stores have ensured that potassium is now widely available in the soil profile, where the vast majority of plant roots are located. As a consequence of crop absorption, potassium stocks in the soil solution are restored. Crops having a shorter growth season stand to gain somewhat more. Because their root systems are limited, these plants need to take up nutrients rapidly in order to flourish. Although potassium fertilizers have been a helpful step forward, the high cost of this input means they are not a viable long-term answer for developing nations like India. 5 The formation of various organic acids, which are then followed by exchange processes known as acidolysis and complexolysis, as well as the mobilisation of insoluble potassium and structurally inaccessible forms of potassium compounds are all significant mechanisms involved in the conversion into a soluble form. Wheat, fodder crop, maize, and Sudan grass crops are only some of the field test crops that have been studied for their potential to lessen the need for chemical or organic fertilisers thanks to potassium solubilizing bugs.6. There has been very little research on the potential uses of Actinomycetes as enzyme makers in the agricultural sector. 7. Recently, actinomycetes have been considered as a kind of rhizobacteria that helps plants develop by encouraging nutrient cycling in the soil. 8 9 10.
Materials and Methods
Sample Collection
Feldspar, an insoluble source of potassium, is used by most ceramic businesses, hence samples from these sectors were gathered. A total of 15 samples were gathered from three different ceramic factories in Gujarat, one each in the towns of Morbi (22.82520 N,70.84910 E), Meshana (23.58800 N,72.3630 E), and Kadi (23.29730 N,72.33020 E). the samples labeled S1 to S22.
Adaptation and Enrichment
Soil samples were mixed with insoluble potassium (Feldspar) and CaCO3 to encourage the development of Actinomycetes (for a week at room temperature). One milliliter of soil was added to each test tube liquid containing broth containing 1% glucose, 0.05% yeast extract, and 0.5% feldspar after a week of acclimatization at 28 ± 2 ◦ c and 120 rpm.
Isolation and Screening of Potassium Solubilizing Actinomycetes
Enriched samples were diluted from 10-1 to 106 before even being plated onto Aleksandrov’s agar medium (1% glucose, 0.05% MgSo4.7H2O, 0.0005% FeCl3, 0.01% CaCO3, 0.2% CaPO4, and 0.5% KAlSi, agar 3% pH6.5 11) and maintained at 28±2 ◦ c. for a week. Colonies with a noticeable solubilization zone for potassium were selected.
The zone diameters of the various isolates grown on Aleksandrov’s agar plates were evaluated using Khandeparkar’s selection ratio for a second round of screening.12
Isolates of Actinobacteria were seeded on Aleksandrov’s medium with a pH indicator dye in order to explore the process by which potassium is absorbed (0.025 percent Bromothymol blue).13
Macroscopic / Morphological Characterization
KSA 16 Actinomycete colony properties were investigated using Glycerol Asparagine Agar (GAA). Cell size, shape, arrangement, and pigmentation were only few of the morphological features that were documented across a wide variety of different isolates. Gram staining was used to confirm the cell morphologies found in the isolates, which were then examined using a compound microscope.14
Scanning Electron Microscopy (SEM) of KSA 16
Three-dimensional SEM images for judging the surface structure of the Actinomycete was studied by preparing a thin smear of matured culture on a glass cover slip. The smear was fixed with 2 % Glutaraldehyde solution and kept for 10-40 min. After fixation, the fixative was removed by tilting on tissue and washing cover slip with sterile distilled water followed by air drying. The cover slip was stuck on SEM holder by using carbon conducting tape and placed in sputter coating unit. The samples were then analyzed by SEM, using 30 kV accurate voltages on a SEM with the model number LEO s- 440i, after being coated with a thin coating of gold ( ~50 or 100 microns). Both 6000x and 9000x magnification images of KSA 16 were obtained. The final image gives a magnified look at the isolated cells’ outer surface.
Potassium Solubilizing ability of KSA 16
Using a medium of modified Aleksandrow agar + bromothymol blue, the degree of potassium solubilization was evaluated subjectively. The modified Aleksandrow medium was used for the Streptomyces atacamensis (KSA16) spot vaccinations, and the plates and liquid broth (GYE) were incubated at 28 ± 2°C for 3–4 days while being stirred at room temperature (RT). The formation of separate halos surrounding the colony was evaluated using Khandeparkar’s selection ratio technique. D/d = Diameter of the hydrolysis zone/Diameter of the growth
Production of Growth Promoting Substances by Potassium Solubilizers
KSA 16 was evaluated for its ability to produce IAA (Indole Acetic Acid) and GA (Gibberellic Acid) on Luria agar treated with SDS (0.06%) and glycerol (1 percent ). All five isolates were judged positive for IAA and GA synthesis based on the growth of red color on the filter paper (Qualitative approach) or green fluorescence under UV light.15
Media Optimization and quantitative estimation of Streptomyces antamenecia (KSA 16)
Effect of pH on Growth of K solubilizers
The studies were carried out in nutritive broth in a medium that had its pH adjusted with 0.1 M HCl and NaOH. 10 ml of N-broth medium were mixed with a loopful of an active bacterial culture, and the combination was then cultured for 3–4 days at 28 ± 2 °C. The development was then visually examined.16
Effect of Temperatures on Growth of K solubilizers
Nutrient broth medium was used to investigate the impact of various temperatures. A bacteriological culture that had already been activated was added to 10 ml of broth medium, and the combination was incubated for 3 to 4 days at varied temperatures. The progress was then inspected visually.
Effect of NaCl concentration on Growth of K solubilizers
To verify their findings, different NaCl concentrations were utilised in the N-broth medium. A loop of the activated bacterial culture was added to 10 ml of the N-broth media after the bacterial culture had been incubated there for 3–4 days at 28 ± 2 °C. After then, the growth was inspected visually.
Detection of Organic acids produced by KSA -16
Analyzing organic acids with a low molecular weight in soil solution is now much simpler due to RPHPLC. A Chrom Sil C 18 HPLC column was used for the research. The best measurement of organic acid was made at room temperature (25°C) at a detection wavelength of 220 nm using a solvent system of 10 mM KH 2 PO 4 -CH 3 OH (955, pH 2.7), flow rate of 0.8 mL/min, and sample size of 10 µL.17
Molecular Characterization and Identification of KSA 16
To discover the additional closely related species members, the 16S rRNA sequence was BLAST searched. Sequence data for related species were retrieved and multiple sequence alignment was performed using the Clustal W program to validate the uniqueness of our sequence. 18The hyper variable nucleic acid region of isolate was compared with 100 closely related sequences. KSA16 from the BLAST program was used for the phylogenetic tree construction, and Streptomyces antamenecia was determined to be the biological parent using 16S rRNA analytical techniques. 19
Result and Discussion
Soil Sample collection and Isolation
The soil samples were collected from 10 different insoluble potassium rich sites in an around Morbi, Meshana, Kadi. The gathered soil samples were analyzed for colony characterization, which led to the isolation of 22 different actinomycetes. Colony morphology of individual colonies on Aleksandrov’s agar dilution plates at 10-4, 10-5, and 10-6 levels. Colonies were chosen based on their capacity to dissolve the potassium aluminum silicate added to the growth medium, as shown by the presence of a distinct zone of solubilization. A total of 22 actinobacterial isolates, designated KSA-1 through KSA-22, were shown to be efficient potassium solubilizers. (Table-3.1).
Table 1: Soil Sampling sites with Isolates code
|
Sr.no |
Industry Name |
Collection Rgion |
Isolates Code |
|
1 |
Golf Ceramics |
Balol,Meshana |
SA1,SA3 |
|
2 |
Aarati Ceramics |
Litarar Road,Morbi |
SA2 |
|
3 |
Aquatech Ceramics |
Thangadh,Morbi |
SA4 |
|
4 |
Kholer Ceramics |
Shobeshwar Road,Morbi |
SA5 |
|
5 |
PSSHDA Ceramic Lab |
S.V Campus,Kadi |
SA6, 7, 8 & 9 |
|
6 |
Bajrang Refacteries Pvt.Ltd |
Kadi,Meshana |
SA10 |
|
7 |
Gobind Glass& Industries Pvt.Ltd |
GIDC,Kadi |
SA11 |
|
8 9 10 |
Alaska Ceramics Italiya Ceramics Cera Sanitaryware |
Dhanali,Kadi Kaiyal,Meshana Kalol,Kadi |
SA12 & 13 SA14, 15, 19,20,21 & 22 SA16, SA17, SA18 |
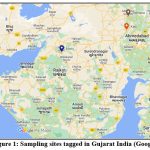 |
Figure 1: Sampling sites tagged in Gujarat India (Google Map)
|
https://goo.gl/maps/fDiGJeCB9Sb87ph97
Isolation and Screening
Aerial mycelium of KSA 16 (Streptomyces atacamensis) was shown to be a dark yellow, smooth, round, reverse bright yellowish pigment generator in modified GAA (Glycerol Asparagine Agar). After 72 hours of incubation on Bromothymol blue-supplemented modified Aleksandrov’s Agar, a yellow hue develops at low pH. The Bromothymol blue-containing zone of potassium solubilization. When grown in Aleksandrov medium supplemented with the pH indicator dye Bromothymol blue, these Actinomycetes strains showed a distinctive zone of potassium solubilization with the creation of a yellow hue owing to acid generation all around growth (Table 2). Table 2 showed morphological characteristics of all isolates.
Secondary Screening
All the bacterial isolates with selection ratio values and solubilization zones of potassium aluminum silicate on Aleksandrov’s agar media over 1 were put through further testing in liquid medium. After incubation for 7, 15, and 20 days in a GYF (Glucose Yeast extract Feldspar) broth containing feldspar, the amounts of K released by the isolates were calculated (DAI). Feldspar with greater potassium release from isolates KSA 9, KSA 10, KSA 12, KSA 16, and KSA 17. Table 3 shows that all strains released a quantity of K from mineral K that peaked 20 days after incubation. This amount increased with the length of incubation. At 20 days post-inoculation (DAI), the isolates’ K release from feldspar varied from 5.8 mg/lit to 45.4 mg/lit.
Table 3: Potassium solubilization values of Actinomycetes isolates by Khandeparkar’s selection ratio (mm)
|
Isolates |
Diameter of Growth and Clearance (D) mm |
Diameter of growth (d) mm |
D/d (ratio) mm |
|
KSA01 |
9 |
9 |
1 |
|
KSA02 |
10 |
10 |
1 |
|
KSA03 |
11 |
10 |
1.1 |
|
KSA04 |
12 |
12 |
1 |
|
KSA05 |
9 |
8 |
1.13 |
|
KSA06 |
10 |
10 |
1 |
|
KSA07 |
11 |
11 |
1 |
|
KSA08 |
9 |
8 |
1.13 |
|
KSA09 |
14 |
9 |
1.56 |
|
KSA10 |
11 |
8 |
1.37 |
|
KSA11 |
10 |
10 |
1 |
|
KSA12 |
10 |
8 |
1.25 |
|
KSA13 |
10 |
10 |
1 |
|
KSA14 |
10 |
10 |
1 |
|
KSA15 |
10 |
9 |
1.11 |
|
KSA16 |
13 |
8 |
1.62 |
|
KSA17 |
10 |
8 |
1.25 |
|
KSA18 |
11 |
11 |
1 |
|
KSA19 |
10 |
10 |
1 |
|
KSA20 |
11 |
10 |
1.1 |
|
KSA21 |
12 |
12 |
1 |
|
KSA 22 |
9 |
8 |
1.13 |
Macroscopic / Morphological Characterization
Colonies of Streptomyces atacamensis (KSA16) are shown in Fig-2. From the were exhibiting smooth, round, flat, entire, leathery, opaque, light orange diffusible pigment.
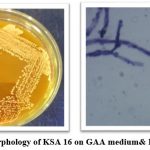 |
Figure 2: Colony morphology of KSA 16 on GAA medium and Its Microscopic view.
|
Molecular Characterization and Identification of KSA 16
Scanning electron microscopy of the Streptomyces atacamensis (KSA16) isolates were carried out and their images are shown in Fig. 3 Isolate KSA-16 cells are long and thin rods (0.5 to 0.7 µm width and 2.0 to 3.7 m long.) that often form chains. Conventional SEM is used to visualize actinomycetes which had been fixed, dehydrated, and dried.
 |
Figure 3: Streptomyces antamenecia strain name in SEM
|
Potassium Solubilizing ability of KSA 16
Seven days of incubation in Aleksandrov medium with bromothymol blue resulted in a potassium solubilization index of 1.162 for Streptomyces atacamensis (KSA16), as reported in Table 3. In bromothymol blue (BTM)-containing Aleksandrov media, Figures 4 and 5 demonstrate that a yellow hue forms around the isolate’s development, indicating that it is creating organic acid to solubilize the potassium from the solid medium. Figure 6 shows an estimate of the solubilization of potassium in liquid medium, as determined using the flame photometry method.
 |
Figure 4: Zone of Potassium solubilization KSA16 on Aleksandrov’s +BTM medium
|
 |
Figure 5: Zone of Potassium solubilization in KSA16 on Aleksandrov’s +BTM medium Liqid medium(GYE broth).
|
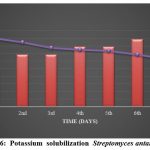 |
Figure 6: Potassium solubilization Streptomyces antamenecia
|
Media Optimization and quantitative estimation of Streptomyces antamenecia (KSA 16)
The pH Effect on K-Solubilizer Development.
In the effect of pH ,total 5 pH variations (2.0, 4.0, 6.0, 8.0 and 10.0) were selected and estimation levels were studied and measured after 72 hrs. as optical density at 600 nm and cell free supernatant was used for estimation of K solubilization. The experiments were conducted in triplicate and mean values are represented KSA 16 showed higher potassium solubilization at pH -6 ( 23.89 mg/l) figure 6. 20
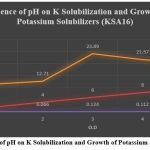 |
Figure 7: Influence of pH on K Solubilization and Growth of Potassium Solubilizers (KSA16).
|
Effect Reconducted of Temperatures on Growth of K solubilizers
In the effect of Temperature, total 5 Temperature variations (5ºC, 10ºC, 25ºC, 37ºC and 55ºC) were selected and estimation levels were studied and measured after 72 hrs. as optical density at 600 nm and cell free supernatant was used for estimation of K solubilization. The experiments were conducted in triplicate and mean values are represented . KSA 16 showed higher potassium solubilization at 25ºC ( 24.66mg/l)( figure 8). 20
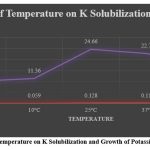 |
Figure 8: Influence of Temperature on K Solubilization and Growth of Potassium Solubilizer (KSA16).
|
Impact of NaCl Concentration on K Solubilizer Growth
In the effect of NaCl concentration, total 6 concentrations variations (2%, 4%, 6% 8% and 10%) were selected and estimation levels were studied and measured after 72 hrs. as optical density at 600 nm and cell free supernatant was used for estimation of K solubilization. The experiments were conducted in triplicate and mean values are represented KSA 16 showed higher potassium solubilization at 6% (26.2 mg/l)( figure 8). 20
 |
Figure 9: Influence of NaCl concentration on K Solubilization and Growth of Potassium Solubilizer (KSA16)
|
Detection of Organic acids produced by KSA -16:
When comparing the KSA16 sample solution to the Oxalic Acid Standard Solution, at a flow rate of 0.8 ml/min and with a 5:95 methanol to phosphate buffer (pH 2.7) ratio, the Oxalic Acid peak at 220 nm (retention time about 4.26) was clearly resolved using HPLC.. (Refer Data report of Test 15.lcd for sample and standard 42.lcd for standard)( figure 9).
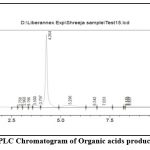 |
Figure 10: HPLC Chromatogram of Organic acids production by KSA -16
|
The isolate was verified to be Streptomyces atacamensis using 16S rRNA gene sequencing and molecular characterization (KSA16). Figure 3.5 shows the related phylogenetic tree.
 |
Figure 11: Streptomyces antamenecia
|
Discussion
Potassium solubilizing actinomycetes are playing an important role in the soil and its fertility.. In addition to bacteria, fungi, actinomycetes, and even algae, a total of 21 different microbial species are crucial to the process of Potassium (P) solubilization. Actinomycetes, a broad class of microorganisms, are entophytes that inhabit the rhizosphere and the roots of plants and are crucial for the growth of plants. The famous ability to convert complex carbohydrates like chitin, cellulose, starch, and lipids into simple sugars is just one illustration of their ability to recycle nutrients. Actinomycetes are essential to agricultural ecosystems because they decompose organic matter near the plant’s root surface, allowing the plant to more easily absorb the nutrients it needs to grow. In addition to potassium solubilization and siderophore formation, hydrogen cyanide, auxin, ammonia, and lytic enzyme production are additional possible functions of these bacteria. 22 Actinomycetes secreted enzymes and low molecular weight organic acids can help dissolve phosphate and potash rock. Several actinomycetes release phytase, an enzyme that breaks down phytate, to make more of the organic phosphorus pool in the soil available to plants. Streptomyces venezuelae, S. alboniger, S. ambofaciens, and S. lienomycini are some of these actinomycetes. Actinomycetes make indole acetic acid often, making them potential candidates for use as biofertilizers. 23 Pseudomonas and Bacillus are two bacterial genera that have been found repeatedly to be efficient potassium solubilizing bacteria. Aspergillus and Penicillium species of fungi are the only ones shown to be able to dissolve potassium in their isolation forms. 24 However, in cases involving barley, chickpea, or soybean, the effectiveness of potassium-solubilizing microorganisms in enhancing plant K uptake was not clearly recognised.25 26 The authors hypothesized that phosphate-solubilizing microorganisms introduced into soil may enhance plant development in ways other than by increasing the soil’s capacity to take up phosphate. Further studies will examine whether or whether this Streptomyces atacamensis strain produces and/or exerts influence on the synthesis of any other growth-promoting substances.
Conclusion
The diversity of potassium solubilizing microbes/actinomycetes at different ceramic industry regions were observed in this study. All actinomycete isolates obtained were able to solubilize the potassium in high concentration. Streptomyces atacamensis, which in the current study had the best potential to increase potassium solubility, is one example of an effective KSM that can be used to improve crop productivity and yields without adversely affecting soil health. Because potassium-solubilizing actinomycetes play a substantial role in plant nutrition by increasing K absorption by plants, their usage as PGPR makes a considerable contribution to biofertilization of agricultural crops.
Acknowledgement
The authors are thankful to the Department of Microbiology, HVHPGR, Kadi and Management of Kadi Sarva Vishwavidyalaya (KSV), Gandhinagar, for providing the facilities for this research.
References
- Zhang C & Kong F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl Soil Ecol.2014; 82:18–25.
CrossRef - Foth HD, & Ellis BG. Soil fertility. CRC Press, Boca Raton, 1997; p 290.
- Ashley DL, Blount B, Singer PC, Depaz E, Wilkes C, & Gordon S. Changes in blood trihalomethane concentrations resulting from differences in water quality and water-use activities. Arch Environ OccupHealth. 2005; 60(1):7–15.
CrossRef - Mora V, Baigorri R, Bacaicoa E, Zamarrenob AM, & Garcı´a-Mina JM . The humic acid-induced changes in the root concentration of nitric oxide, IAA and ethylene do not explain the changes in root architecture caused by humic acid in cucumber. Environ Exp Bot.2012; 76:24–32.
CrossRef - Uroz S, Calvaruso C, Turpault MP & Frey-Klett P .Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol 2009;17:378–387.
CrossRef - Xie JC .Present situation and prospects for the world’s fertilizer use. Plant Nutr Fertil Sci 1998;321–330.
- Kavita Rani, AtulParashar & LeelaWati. Estimation of hydrolyzing potential of chickpea actinomycetes for degradation of complex compounds through enzymes and acid production.The Pharma Innovation Journal 2021; 10(1): 220-223.
- Pathom-aree W, Stach J, Ward A, Horikoshi K, Bull A & Goodfellow M . Diversity of actinomycetes isolated from Challenger Deep sediment (10,898m) from the Mariana Trench. Extremophiles.2006; 10:181-189
CrossRef - Franco-Correa M, Quintana A, Duque C, Suarez C, Rodriguez MX & Barea J-M. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Soil Ecol. 2010; 45:209- 217.
CrossRef - Feng Sun, Qiaojing Ou, Nan Wang,Zi xuan Guo,Yuyi Ou,Na Li & Changlian Peng. Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on micrantha plants. Global Ecology and Conservation.2020 ;23: 1-9.
CrossRef - Sugumaran P & Janartham B. Solubilization of potassium minerals by bacteria and their effect on plant growth. World Journal of Agricultural Sciences 2007 ;3(3): 350-355.
- Gayal, S. & Khandeparkar, V. Production of Amylase by Bacillus subtillus. Indian Journal of Microbiology .1979; 9:226-231
- B.Prajapati & H.A Modi. Isolation and Characterization of Potassium Solubilizing Bacteria from Ceramic Industry Soil, CIBTech Journal of Microbiology. 2012;1(2-3):8-14.
- Gram, HCJ. About the isolated staining of the schizomycetes in cut and Dry Preparations .Advances in Medicine,Berlin, 1884 ;2: 185-189.
- Gordon, S. A. & Paleg, G. Quantitative measurement of indole acetic acid. Plant Physiology, 1957 ;10: 37-48.
CrossRef - Syed G. Dastager, Wen-Jun Li, Ismail Saadoun & Mohammad Miransari, Microbial Diversity-Sustaining Earth and Industry, Applied and Environmental Soil Science, 2011; ArticleID 459195, 2 pages, 2011. https://doi.org/10.1155/2011/459195
CrossRef - Tasev Krste, Violeta Ivanova-Petropulos, & Marina Stefova. Ultra-performance liquid chromatography-triple quadruple mass spectrometry (UPLC-TQ/MS) for evaluation of biogenic amines in wine. Food Analytical Methods2017; 10.12 : 4038-4048.
CrossRef - Higgins D. G., Thompson, J. D & Gibson, T. J. Using CLUSTAL for multiple sequence alignments. Methods in Enzymology.1996 ;266: 383-400.
CrossRef - Saitou, N & Nei, M. The neighbor-joining method for reconstructing phylogenetic trees. Molecular Biologyand Evolution, 1987. 4: 404-425.
- Zakaria A A B. Growth optimization of potassium solubilizing bacteria isolated from biofertilizer.” Dissertation, Faculty of Chemical & Natural Resources Engineering, University of Malaysia Pahang
- Khan M S,Zaidi A & Wani P A . Role of phosphate-solubilizing microorganisms in sustainable agriculture – A review. Agron. Sustain. Dev. 2007; 27:29- 43.
CrossRef - Sousa JAJ, Olivares FL. Plant growth promotion by streptomycetes: ecophysiology, mechanisms and applications. Chem; 2016.
CrossRef - Rani K, Dahiya A, Masih JC, Wati L. Actinobacterial biofertilizers: an alternative strategy for plant growth promotion. Int J Curr Microbiol App Sci. 2018;7(09):607- 614.
CrossRef - Krishnananda P I & Dipika A P. Phosphate solubilizing microbes: an overview. International Journal of Current Microbiology and Applied Sciences. 2017; 6(1): 844- 852.
CrossRef - Oehl F, Oberson A, Probst M, Fliessbach A, Roth H R & Frossard E. Kinetics of microbial phosphorus uptake in cultivated soils. Fertil. Soil. 2001;34: 31–41.
CrossRef - Fernandez L A , Zalba P, Gomez M A & Sagardoy M A . Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Fertil. Soils. 2007; 43: 805–809.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





