How to Cite | Publication History | PlumX Article Matrix
Phytoplankton Diversity of Pandalam Municipal Area, Pathanamthitta District, Kerala
Jithesh Krishnan R1* , Fouzia Hilal2
, Fouzia Hilal2 , Abhilash Sivadasan3
, Abhilash Sivadasan3 , Lekshmi R4
, Lekshmi R4 and Jalaja Vidya4
and Jalaja Vidya4
1Department of Botany, NSS College, Pandalam (Affiliated to Kerala University), Pathanamthitta Dt., Kerala, India.
2Department of Botany, Milad E-Sherif Memorial College, Kayamkulam, Alappuzha Dt., Kerala, India.
3Department. of Botany, Sree Neelakanta Govt. Sanskrit College, Pattambi, Palakkad Dt., Kerala, India.
4Department. of Botany and Biotechnology, Milad E-Sherif Memorial College, Kayamkulam, Alappuzha Dt., Kerala, India.
Corresponding Author E-mail:kjith77@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3158
ABSTRACT: The present investigation deals with the comprehensive and systematic analysis of the unexplored phytoplankton diversity of the unique biodiversity area of Pandalam Municipality of Kerala after the major flood event of the year 2018. The study was carried out between October 2018 and January 2019. Eight study locations were explored in the flood-affected areas of Pandalam Municipality (PN1, PN2, PN3, PN4, PN5, PN6, PN7, and PN8). The study revealed the occurrence of 78 genera belonging to five classes, such as Cyanophyceae, Chlorophyceae, Charophyceae, Euglenophyceae, Bacillariophyceae, and Chrysophyceae. Algae belonging to the Class Bcillariophyceae were more in number (30) compared to all the other classes, followed by-members of class Chlorophyceae (17). The genus Cosmarium and Nitzschia were the most frequently occurring algae throughout the study period (six species each). The genera Pinnularia and Navicula were also abundant (5 and 4 species, respectively).
KEYWORDS: Achenkovil River; Ecosystem Changes, Kerala Floods; Phytoplankton Diversity; Pandalam Municipality
Download this article as:| Copy the following to cite this article: Krishnan R. J, Hilal F, Sivadasan A, Lekshmi R, Vidya J. Phytoplankton Diversity of Pandalam Municipal Area, Pathanamthitta District, Kerala. Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Krishnan R. J, Hilal F, Sivadasan A, Lekshmi R, Vidya J. Phytoplankton Diversity of Pandalam Municipal Area, Pathanamthitta District, Kerala. Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/3rEptWw |
Introduction
The world is facing drastic environmental effects from climate change. Researchers from all over the world are looking into the consequences. Natural disasters not only endanger human lives but also cause irreversible changes and biodiversity loss. The quality of ecosystem services may be negatively affected. Rich biodiversity is an indicator of the safety and pristine nature of the Earth. Climate change, ecosystem degradation due to overuse, pollution, and the appearance of invasive species pose a threat to biodiversity1.
Kerala faced a major flood in August 2018. Many people and animals lost their lives, and it caused irrecoverable biodiversity loss and the mixing up of different water ecosystems. The ecosystems and water bodies of the nearby areas were significantly affected2.
Three southern Districts (Pathanamthitta, Idukki, and Kottayam), three central Districts (Ernakulam, Thrissur, and Palakkad), and two northern Districts (Kannur and Wayanad) were severely affected. Due to the flow of three major rivers—the Pampa, Manimala, and Achencovil—Pathanamthitta and Alappuzha were affected the most. Due to the flooding of the dams, the authorities opened the shutters of the dams to allow the fast movement of excess water. An enormous amount of particulate matter, alongwith dissolved and undissolved solvents, flow into the rivers from adjacent streams. This caused the devastation of micro- and macrohabitats.
The Achencovil River originates in the Western Ghats, flows 128 kilometres, and ends up in the Pampa River. Its surrounding areas were severely affected by ecosystem changes, habitat loss, and species loss3.The Pandalam area is enriched with unique flora. Many of the endemic plants of Kerala exist there4.
A biodiversity study is very important for developing restoration plans. Regular auditing of existing and destroyed species is required in order to create a database. Understanding the existing species and the presence of invasive species, vulnerable species, and harmful species in the changed ecosystem is very important. Therefore, we decided to study the impacts of flooding and the prevailing dynamics of algae in the Pandalam Municipal Area of Pathanamthitta District. It is expected that the enumeration of microalgae will provide an understanding of the community structure. The generated data will help with policymaking and provide guidelines for future conservation studies.
Materials and Methods
The municipality of Pandalam was recently formed (in November 2015)5. It has a total area of 28.72 km2. Pandalam lies between 9.2250N latitude and 76.670E longitude. The total number of wards in Pandalam Municipality is 33. The flood-affected wards were on the banks of the river. The river flows parallel to the Pathanamthitta-Mavelikkara state highway. The northern boundary of the municipality is Kulananda Bridge, and the southern boundary ends at Kurampala. The east boundary of the municipality starts at Kadakkad Bridge, and the west boundary ends at Iranikkuzhi Bridge5.
The entire 18 wards near the river were severely affected. 14 wards were vulnerable. We selected eight severely affected wards for water sampling. A total of 8 sampling stations (one in each ward) were fixed (PN1, PN2, PN3, PN4, PN5, PN6, PN7, and PN8, respectively) (Figure 1).
Place Figure-1
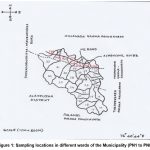 |
Figure 1: Sampling locations in different wards of the Municipality (PN1 to PN8)
|
Sampling
Between October 2018 and January 2019, regular monthly field visits and sample collection were conducted between 10 a.m. and 11 a.m. Water samples were collected from the river Achencovil and other bodies of water in each ward. Planktons were collected in 1L wide-mouthed, pre-sterilized plastic bottles from 2–3 cm of the surface water. Epiphytic algae colonising submerged plants were collected. Mud along the shore was collected using a 50-ml (2-cm-wide) syringe for Benthos. Algae from paddy fields were also collected. All the collected water samples for the phytoplankton study were preserved in Lugol’s iodine in a ratio of 1:10 mL6.
Phytoplanktons were identified using a compound microscope (MX21i Clinical) at 100X magnification. The identification was done using standard keys7, 8, 9. The phytoplankton were separated into classes and arranged in tables. The Round system was followed for the classification of algae10.
Results and Discussion
Phytoplankton Assembly: As per the present study, 78 algal taxa were identified (Table 1). The identified algae belonged to six classes: Cyanophyceae, Chlorophyceae, Charophyceae (Desmids), Bacillariophyceae (Diatoms), Euglenophyceae, and Chrysophyceae. 38.5% of the algae were Diatoms, 21.8% were Chlorophyceae, 16.7% were Charophyceae, 15.4% were Cyanophyceae, 6.4% were Euglenophyceae, and 1.3% were Chrysophyceae (Figure 2).
Table1: Algae identified from Pandalam Municipality
|
Sl.No |
Algal Genera and species |
Class |
Habit |
PN1 |
PN2 |
PN3 |
PN4 |
PN5 |
PN6 |
PN7 |
PN8 |
|
1 |
Anabaena cylindrica |
Cyanophyceae |
P |
– |
+ |
– |
– |
– |
– |
– |
+ |
|
2 |
Anabaenasp.1 |
P |
+ |
+ |
– |
– |
+ |
– |
– |
– |
|
|
3 |
Anabaenasp.2 |
P |
– |
+ |
– |
– |
– |
+ |
– |
– |
|
|
4 |
Chroococcidiopsis sp. |
B |
– |
+ |
– |
– |
+ |
– |
– |
– |
|
|
5 |
Chroococcus sp. |
B |
+ |
– |
– |
– |
– |
+ |
– |
+ |
|
|
6 |
Cylindrospermumsp. |
B |
+ |
– |
– |
– |
– |
+ |
– |
– |
|
|
7 |
Dactylococcopsis rupestris |
P |
– |
+ |
– |
– |
+ |
– |
– |
+ |
|
|
8 |
Merismopedia aeruginea |
E |
– |
+ |
– |
+ |
– |
– |
+ |
– |
|
|
9 |
Nostoc sp. |
P |
– |
– |
+ |
– |
– |
+ |
– |
– |
|
|
10 |
Oscillatoria formosa |
E |
– |
– |
+ |
– |
– |
– |
– |
+ |
|
|
11 |
Oscillatoria limnetica |
B |
– |
+ |
– |
– |
+ |
– |
– |
– |
|
|
12 |
Spirulina meneghiniana Zanardini ex Gomont |
B |
– |
+ |
– |
+ |
– |
– |
– |
+ |
|
|
13 |
Ankistrodesmus falcatus (Corda) Ralfs |
Chlorophyceae |
B |
– |
– |
+ |
– |
+ |
– |
– |
– |
|
14 |
Bulbochaete sp. |
E |
– |
+ |
– |
– |
– |
+ |
– |
– |
|
|
15 |
Chaetophora sp. |
E |
+ |
– |
– |
+ |
– |
– |
– |
– |
|
|
16 |
Closteridium sp. |
P |
– |
– |
– |
+ |
– |
– |
– |
+ |
|
|
17 |
Closteriopsis longissima |
P |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
18 |
Coelastrum sp. |
P |
– |
+ |
– |
– |
– |
+ |
– |
– |
|
|
19 |
Crucigeniella crucifera |
P |
– |
+ |
– |
– |
– |
– |
– |
+ |
|
|
20 |
Crucigeniellasp. |
P |
+ |
– |
– |
– |
– |
+ |
– |
– |
|
|
21 |
Dictyochloropsis |
B |
– |
+ |
+ |
– |
– |
– |
+ |
– |
|
|
22 |
Microspora sp. |
P |
+ |
– |
– |
+ |
– |
– |
+ |
– |
|
|
23 |
Oedogonium sp. |
E |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
24 |
Pediastrum duplex |
B |
– |
+ |
– |
+ |
+ |
– |
– |
– |
|
|
25 |
Pleurotaenium archeri |
P |
+ |
– |
– |
– |
+ |
– |
– |
– |
|
|
26 |
Pleurotaenium ehrenbergii |
P |
– |
– |
+ |
– |
– |
+ |
– |
– |
|
|
27 |
Scenedesmus ellipticus |
P |
– |
– |
– |
– |
– |
– |
– |
+ |
|
|
28 |
Scenedesmus quadricauda |
P |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
29 |
Closterium parvulum |
Charophyceae |
B |
– |
+ |
– |
|
+ |
– |
– |
– |
|
30 |
Cosmarium sp. |
P |
+ |
+ |
– |
– |
– |
– |
– |
– |
|
|
31 |
Cosmarium contractum |
P |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
32 |
Cosmarium hammeri |
E |
– |
– |
+ |
– |
+ |
– |
– |
– |
|
|
33 |
Cosmarium impressulum |
P |
+ |
– |
– |
+ |
– |
– |
– |
– |
|
|
34 |
Cosmarium nymannianum Grunow |
B |
– |
– |
+ |
– |
+ |
– |
– |
– |
|
|
35 |
Cosmarium subtumidum Nordstedt |
P |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
36 |
Euastrum denticulatum |
P |
– |
– |
+ |
– |
+ |
– |
+ |
– |
|
|
37 |
Klebsormidium sp. |
B |
– |
– |
– |
– |
– |
– |
– |
+ |
|
|
38 |
Micrasterias americana |
E |
– |
+ |
– |
– |
– |
+ |
– |
– |
|
|
39 |
Micrasterias laticeps |
E |
+ |
– |
– |
+ |
– |
– |
– |
+ |
|
|
40 |
Mougeotia sp. |
B |
– |
– |
+ |
– |
– |
– |
+ |
– |
|
|
41 |
Pleurotaenium archeri |
E |
– |
– |
– |
+ |
– |
– |
+ |
– |
|
|
42 |
Spirogyra sp. |
P |
– |
+ |
– |
– |
– |
– |
– |
+ |
|
|
43 |
Euglena acus |
Euglenophyceae |
P |
– |
– |
– |
+ |
– |
– |
– |
+ |
|
44 |
Euglena viridis Playfair |
P |
– |
– |
+ |
– |
– |
+ |
– |
+ |
|
|
45 |
Lepocinclis texta (Dujardin) Lemmermann |
P |
– |
– |
+ |
– |
– |
– |
– |
+ |
|
|
46 |
Phacus acuminatus |
B |
+ |
– |
– |
– |
– |
– |
+ |
+ |
|
|
47 |
Phacus sp. |
P |
– |
– |
+ |
– |
+ |
– |
– |
+ |
|
|
48 |
Amphora sp. |
Bacillariophyceae |
B |
– |
– |
+ |
– |
+ |
– |
– |
– |
|
49 |
Amphora inariensis |
B |
+ |
– |
– |
– |
– |
– |
– |
– |
|
|
50 |
Anomoeoneis sphaerophora |
P |
– |
– |
– |
– |
– |
+ |
+ |
|
|
|
51 |
Cyclotella kutzingiana |
P |
– |
+ |
– |
– |
– |
– |
– |
– |
|
|
52 |
Cymbella sp. |
P |
+ |
– |
– |
+ |
– |
– |
– |
+ |
|
|
53 |
Diadesmis confervacea |
P |
+ |
+ |
– |
– |
– |
– |
+ |
– |
|
|
54 |
Gomphonema affine |
P |
+ |
– |
– |
– |
– |
+ |
– |
– |
|
|
55 |
Gomphonema olivaceum |
P |
– |
– |
+ |
– |
– |
– |
+ |
– |
|
|
56 |
Gomphonema venusta Passy, |
P |
– |
– |
– |
– |
+ |
– |
– |
– |
|
|
57 |
Melosira granulata |
P |
+ |
+ |
– |
– |
+ |
+ |
– |
– |
|
|
58 |
Navicula sp. |
E |
– |
– |
+ |
– |
– |
– |
– |
– |
|
|
59 |
Navicula graciloides |
E |
– |
– |
– |
+ |
– |
+ |
– |
– |
|
|
60 |
Navicula acicularis Kutz. |
B |
– |
– |
+ |
– |
– |
+ |
– |
– |
|
|
61 |
Navicula lanceolata |
P |
+ |
+ |
– |
+ |
– |
– |
– |
– |
|
|
62 |
Nitzschia clausii |
P |
– |
– |
– |
– |
– |
– |
+ |
– |
|
|
63 |
Nitzschia desertorum Hustedt |
P |
– |
+ |
– |
– |
– |
– |
– |
+ |
|
|
64 |
Nitzschia filiformis (Krasske) A.Cleve |
E |
– |
– |
– |
+ |
– |
– |
– |
+ |
|
|
65 |
Nitzschia gracilis Hantzsch |
P |
– |
– |
+ |
– |
+ |
– |
– |
– |
|
|
66 |
Nitzschia palea |
B |
+ |
– |
– |
– |
– |
+ |
– |
– |
|
|
67 |
Nitzschia agnita |
P |
+ |
– |
+ |
– |
+ |
– |
– |
– |
|
|
68 |
Pinnularia sp. |
P |
– |
– |
– |
– |
+ |
– |
– |
+ |
|
|
69 |
Pinnularia rectangulata var. |
E |
+ |
– |
+ |
– |
+ |
– |
– |
– |
|
|
70 |
Pinnularia divergentissima |
B |
– |
+ |
– |
– |
– |
+ |
– |
– |
|
|
71 |
Pinnularia gibba |
B |
– |
+ |
– |
+ |
– |
– |
+ |
|
|
|
72 |
Pinnularia viridis (Nitzsch) Ehrenb. |
P |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
73 |
Rhoicosphenia abbreviata (C. Agardh) Lange-Bertalot 1980 |
P |
– |
+ |
– |
+ |
– |
– |
– |
– |
|
|
74 |
Sellaphora pupula (Kutz.) Mereschkow |
P |
– |
– |
+ |
– |
– |
– |
+ |
– |
|
|
75 |
Stauroneis sp. |
B |
– |
– |
– |
+ |
– |
– |
– |
– |
|
|
76 |
Synedra sp.1 |
P |
– |
– |
+ |
– |
– |
+ |
– |
– |
|
|
77 |
Synedra ulna (Kutzing) Hustedt |
P |
+ |
– |
– |
– |
+ |
– |
+ |
– |
|
|
78 |
Dinobryon divergens O. E. Imhof |
Chrysophyceae |
E |
– |
– |
– |
+ |
– |
– |
– |
– |
|
|
27 | 31 | 27 | 25 | 26 | 24 | 20 | 26 |
*P-Plankton, B-Benthos, E-Periphyton
The highest number of genera of algae were observed at PN2 (31) and the lowest at PN7 (20). All the other stations showed a total number of algal taxa between 20 and 37 (PN1 and PN 3-27, PN4-25, PN5 and PN8-26, and PN6-24, respectively). Among the total identified taxa, 45 were plankton, 20 were benthos, and 14 were periphyton (Figure 3). The most abundant Classes were the Basillariophyceae (30 taxa) and Chlorophyceae (17 taxa). The following algae were the most abundant: Cosmarium and Nitzschia (6 species each), Pinnularia (5 species), and Navicula (4 species). Closteriopsis longissima, Scenedesmus quadricauda, Oedogonium sp., Cosmarium contractum, and Pinnularia viridis were present in all the stations.
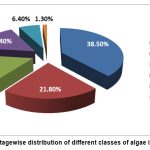 |
Figure 2: Percentagewise distribution of different classes of algae in the Municipality
|
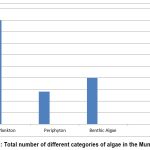 |
Figure 3: Total number of different categories of algae in the Municipality
|
The flood-affected area of Pandalam showed rich algal diversity. PN2 was dense with a larger number of genera (31). This may be due to the presence of large water habitats like the river, Karingali paddy fields (Karingali Puncha), and ponds in the ward. We collected representative samples from all the habitats. PN7 showed the least number of genera of algae (20). The sample collection point was the river at this station. Moreover, the river contained a large number of small rocks and less mud in this area compared to other stations. This station was affected largely due to many small landslides on the shores. Therefore, the possible samples of different categories of algae were smaller at this station.
The Bacillariophyceae were the dominant group of algae in the Municipality. In a pre-monsoon study at Achencovil River Pandalam, Chlorophyceae and Bacillariophyceae were dominant11. Euglenophytes were discovered near PN 8 in our study. The presence of a greater number of Euglenophytes indicates the presence of decaying organic contaminants12. The increased number of Euglenophytes at PN8 may be due to the faecal contamination. This is a station near the pilgrimage area of Pandalam Palace. The presence of the pollution-tolerant alga Scenedesmus in this station indicates enriched water undergoing degradation due to pollution13. The anthropogenic indicator species Nitzschia and Cymbella were present in many stations14.
Different forms of microalgae, plankton, benthos, and epiphytes were abundant in all the stations. Among the six classes of algae identified, the majority were oligotrophic and freshwater forms. Desmids and Diatoms were more numerous. The predominance of Diatoms is an indicator of water quality15. The same Classes of algae were reported in the upstream16. Diatoms and Desmids dominated microalgae from a high-altitude oligotrophic lake17. Bacillariophyceae and Chlorophyceae were dominant in many rivers18.
The most abundant phytoplankton observed were Cosmarium (Charophyceae) and Nitzschia (Bacillariophyceae), with six species each. The greater number of Desmids in a body of water indicates its quality. When eutrophication increases, Desmids decrease and planktonic forms increase19, 20. In the present study, the highest number of algae observed was planktonic forms. Many stations showed anthropogenic influences and a trend towards pollution. Pollution indicator species21, like Nitzschia palea, were observed in PN1 and PN6.
Conclusion
From this investigation, it is understood that the Pandalam Municipal Area is rich in microalgal biodiversity. The flood events caused a disturbance to the community structure, and they paved the way for the mixing up of waters. This may be the reason for the presence of flagellated algae and pollution indicators at some of the stations. In addition, the anthropogenic influence in certain locations of the Achencovil River showed a slight trend towards eutrophication, even though the water is not very contaminated.
Strict measures should be implemented to protect the biodiversity-rich water bodies of the Pandalam Municipality. Especially the Achencovil River. The river is largely used for various purposes during the pilgrimage season. In addition, care should be taken to avoid the loss of habitat and species during flood events since Pandalam is a flood-prone area. A biodiversity register for the micro- and microalgae of Pandalam is the need of the hour. Due to pilgrimage, waste disposal, and other anthropogenic influences, the oligotrophic water resources of Pandalam are degrading. A detailed investigation is needed to understand the lost species, invasive species, and endemic species of the area. This investigation helps to generate baseline data on the algal biodiversity of the precious ecosystems in and around the Pandalam Municipal Area.
Acknowledgements
The authors would like to acknowledge the municipal authorities for providing the map of Pandalam Municipality. The Valiyakoikkal and Mahdevar Temple authorities are highly appreciated for allowing sampling in the river near the temple premise. The first author wishes to acknowledge the Principal of the NSS College, Pandalam, for carrying out the analysis in the College laboratory.
Conflict of Interest
The author(s) declares no conflict of interest.
Funding Sources
The first author wishes to acknowledge the financial assistance received (Grant No. 3371/A8/2018/KSBB) from the Kerala State Biodiversity Board, Thiruvananthapuram, for carrying out a research project, of which this is part.
References
- Smith J., Hitz S. (ed): Estimating Global Impacts from Climate Change, Report of OECD Workshop on the Benefits of Climate Policy: Improving Information for Policy Makers. Organization for economic Co-operation and Development. Cedex 16: France. 2003; pp 390-400.
- Pramanick N., Acharyya R., Mukherjee S., Mukherjee S. SAR based flood risk analysis: a case study of Kerala Flood 2018. in Space Res. 2021; 69 (3) : 01-15.
- Krishnan J. R. (ed) : Impact of Flood on the Micro and Macro Floral Biodiversity of Pandalam and Chengannur with Special Reference to Pandanad: A Post and Pre-flood Analysis, Kerala. Project Report, Kerala State Biodiversity Board. Thiruvananthapuram. 2018; pp 6-7.
- Krishnan J. R., Harikrishnan M. R. Floristic diversity of Kadakkad Sacred Grove in Pandalam Municipality. Tech. and Mangmnt. 2018; 10: 256-266.
- https://pandalammunicipality.lsgkerala.gov.in
- Trivedy R. K, Goel P. K. (ed): Chemical and Biological Methods of Water Pollution Studies. 2nd Karad, India. Environmental Publication. 1986; pp 248.
- Anand N. (ed): Indian Freshwater Micro Algae, 1st Dehradun: Bishen Singh Mahendrapal Singh Ltd. 1998; pp 94.
- Sinha S, Naik M. L. (ed): Phytoplankton and microphytes in the ponds of Raipur city area. 1st Chathizghat. Raipur: Published by Ravishankar ShuklaUniversity. 1997; pp 152.
- Bellinger E. G, Sigee D. C. (ed): Freshwater Algae-identification, enumeration and use as bioindicators. 2nd West Sussex, UK: John Wiley & Sons Publishers Ltd. 2015; pp 275.
- Round F. E. (ed): The Biology of the Algae. 2nd edn. London: Edward Arnold Publishers. 1973; pp 278.
- Krishnan J. R. New report of plankton from Mullaperiyar Lake, Periyar Tiger Reserve, Western Ghats, Kerala. Forstr. 2009; 135 (12): 1750-1751.
- Krishnan M., Dhar P. T., Sreejai R., Thankappan S. Assessment of Phytoplankton Diversity in Midstream of Achenkovil river during Monsoon and Post Monsoon seasons. World. Envt. 2020; 15 (3): 619-623.
- Kumar G. E., Thanzeeha V., Sasikala K., Kumar P. G., Sivadasan K. K., Jaleel A. . V. A Preliminary Study on the Diversity of Planktonic Algae of Kaanam River, Phykos. 2018; 48 (2):13-16.
- Palmer C. M. A composite rating of algae tolerating organic pollution. of Phycol. 1969; 5: 78-82.
- Thomas L. M., Paul T. P. An assessment of phytoplankton and physico-chemical characteristics of Chalakudy river, Kerala. J of Adv. Life Sci. 2015; 8 (2): 197-202.
- Harikrishnan M. R. (ed): Study of the micro flora of three rivers in Pathanamthitta District, Kerala. 1st Kottayam. M.Sc. Project Report-Dept. of Botany, Catholicate College, Pathanamthitta. 2010; pp 35.
- Krishnan J. R. Nutrient (N&P) Enrichment Coupled with Phytoplankton Dynamics of Mullaperiyar reservoir in the Western Ghats of Kerala. Biotec. Res. Asia. 2012; 9 (1): 379-385.
- Paul T. P., Sreekumar R. A report on the pollution algae from the Thrissur Kol wetlands (part of Vembanad-Kol, Ramsar site), Kerala. Envt. Poll. Tech. 2008; 7 (2): 311-314.
- Tas B., Gonulol A. An ecologic and Taxonomic study on phytoplankton of a shallow Lake, Turkey. Biol. 2007; 28 (2): 439-445.
- Coesel P. F. M. Structural Characteristics and Adaptations of Desmid Communities. of Ecol. 1982; 70 (1): 163-177.
- Palmer C. M. (ed): Algae and Water Pollution: The Identification, Significance, and Control of Algae in Water Supplies and in Polluted Water. 1st Los Angeles: Castle House Publications, 1980; pp 110.

This work is licensed under a Creative Commons Attribution 4.0 International License.





