How to Cite | Publication History | PlumX Article Matrix
Silver Nanoparticles and Its Invitro Cytotoxic Behaviour- A Fungi Aided Synthesis
Vardhana Janakiraman1* , Kathiravan Govindarajan2
, Kathiravan Govindarajan2 and Thenmozhi1
and Thenmozhi1
1Department of Biotechnology, Vels Institute of Science Technology and Advanced Studies, Pallavaram, Chennai-600117. India.
2Department of Botany, RKM Vivekananda College, University of Madras, Chennai-600004.India.
Corresponding Author E-mail: vardhana88@ymail.com
DOI : http://dx.doi.org/10.13005/bbra/3145
ABSTRACT: Nanotechnology is concerned with the creation and stabilisation of nanoparticles. The biological method necessitates the creation of nanoparticles that are eaten by microorganisms capable of digesting nanoparticles in various forms. The fungus Pestaloptiopsis breviseta is used in this study to demonstrate the extracellular production of stable silver nanoparticles. The fungal culture was isolated from a stable Catharanthus roseus (L) G.don leaf sample, a common therapeutic plant. They were produced after the AgNO3 solution was employed to treat the cell filtrate and the fungal mat at room temperature and in the dark. (1 mM). The cell filtrate made silver nanoparticles that were between 171-378 nm in size, whereas the fungal biomass was between 140-280 nm in size. The cell lines MCF-7 and A549 were likewise treated with the silver nanoparticles made by the fungi. GraphPad Prism 5 software was used to track the percentage of living cells for 24 and 48 hours at different concentrations of the MCF-7 and A549 cell lines based on the IC50 value.
KEYWORDS: AgNP’s; A549; Cytotoxicity; Endophytic Fungi; MCF-7; Pestaloptiopsis Breviseta
Download this article as:| Copy the following to cite this article: Janakiraman V, Govindarajan K, Thenmozhi T. Silver Nanoparticles and Its Invitro Cytotoxic Behaviour- A Fungi Aided Synthesis. Biosci Biotech Res Asia 2023;20(3). |
| Copy the following to cite this URL: Janakiraman V, Govindarajan K, Thenmozhi T. Silver Nanoparticles and Its Invitro Cytotoxic Behaviour- A Fungi Aided Synthesis. Biosci Biotech Res Asia 2023;20(3). Available from: https://bit.ly/3ZI6B5S |
Introduction
Nanotechnology is the study of nanoparticle creation and stability. Currently, there is a continuing need to develop environmentally safe nanoparticle production technologies. This synthesis changed its focus from physical and chemical processes to “natural” chemistry and biological processes. 1. Nanoparticles can be made in a number of ways, including by itching, pyrolysis, and hydrothermal product synthesis. It can also be made with the help of chemical rays and biological processes. The biological method entails synthesising nanoparticles with microorganisms that are capable of processing nanoparticles of various shapes and sizes. Several inorganic nanoparticles with well-defined chemical composition, scale, and form have been manufactured utilising various microorganisms, and their applications have been investigated in a wide range of cutting-edge technical fields.
Microorganisms biosynthesize nanoparticles by capturing target ions in their environment and then converting the metal ions into the metal product using enzymes produced by cell activity. Based on where the nanoparticles are created, it may be divided into intracellular synthesis and extracellular synthesis. 2,3.
AgNPs are a very important type of nanoparticle in both Nanotechnology and Nanomedicine. The silver nanoparticles serve as an excellent weapon against a wide range of dangerous germs, and scientists are looking at both chemical and biological ways to make them. 4. Specific microorganisms have been identified as reducing Ag+ ions to generate silver nanoparticles, the bulk of which are spherical 5, 6, 7.
Biogenic synthesis of nanoparticles can be performed using organisms such as bacteria, fungi, and plants, or the byproducts of their metabolism, which act as reducing and stabilizing agents8 . These nanoparticles are capped with biomolecules derived from the organism used in the synthesis, which can improve stability and may present biological activity 9 . Biogenic synthesis is relatively simple, clean, sustainable, and economical, and provides greater biocompatibility in the uses of nanoparticles10.
During the production of metal nanoparticles, a champignon exposes the fungal mycelium to a metal salt solution, prompting the fungus to create enzymes and metabolites in order to live. During the process of making metal nanoparticles, a champignon exposes the mycelium of fungi to a metal salt solution. This makes the fungus create proteins and compounds in order to stay alive. Through the action of an extracellular enzyme and fungal metabolites, harmful metal ions are changed into stable metal nanoparticles that are not poisonous. When fungi were utilized, AgNPs were made as a film, made in solution, or built up on the cell surface. 1, 11, 12, 13. Microbes like bacteria and fungus are used to get rid of toxic metals. Recently, these microbes were found to be nanofactories that are safe for the environment. 14 Several studies have shown that nanoparticles of silver 15–20 and gold 21 may be made from microorganisms.
Pestaloptiopsis breviseta, a fungus, is used in this study to demonstrate the surface manufacture of constant silver nanoparticles. Researchers previously employed this fungus to make Taxol, an anti-cancer chemical 22. The fact that silver nanoparticles could be generated with endophytic fungus biomass and aqueous extract adds to the relevance of this study. To the best of our knowledge, the biomass of this species has never been employed in the creation of nanoparticles.
Materials and Methods
Preparation of the extract
A strong leaf sample of Catharanthus roseus (L) G.don-a traditional medicinal plant–was used to isolate the fungal culture. Under running tap water the leaf Samples were cleaned and then air-dried. Surface sterilization was conducted with minor modifications according to the Suryanarayan and Thennarasan (2004) and Schulz et al. (1993) procedures 23, 24. Endophytic fungal colonies from leaf segments were monitored daily in Petri plates. Hyphae emerging from the plant material were moved to other plates after several days, incubated again for 10 days, and regularly tested for culture purity. The isolated endophytic fungi (front and reverse side of the fungal colony) were classified according to their macroscopy and microscopic characteristics such as the morphology of fruiting structures and spore morphology.
The fungus was inoculated (roughly 3 mm in diameter) for 48 hours at 25 °C in an orbital shaker at 120 rpm in 250 ml Erlenmeyer flasks containing 100 cc potato dextrose broth. Mycelial biomass was filtered after incubation, washed with sterile distilled water in order to remove medium components, resuspended in 100 ml distilled water, and incubated at 25 °C. Whatman paper was used to filter the suspension after 24 hours. The cell filtrate was treated with 1 mM AgNO3 in the dark at room temperature. In a 100 rpm shaker, wet fungal biomass was combined with 100 cc of 1mM AgNO3 for 120 hours.
Characterization of nanoparticles
The biosynthesis of P. breviseta nanoparticles was subjected visual characterization. Absorption tests for the extract were performed on the Perkin Elmer spectrophotometer. The instrument has been set to scan mode and the absorption spectrum in the 400-800 nm wavelength range. Perkin Elmer Spectrum One FT-IR used globar and mercury vapor lamp sources, KBr and Mylar beam splitters, a sample chamber, and detectors. This instrument covers the entire area from 400- 4000 cm-1. Used for characterizing peaks and their functional groups. The peak values were recorded. The silver nanoparticle synthesized with Fungi was allowed to dry completely for SEM, and well-grounded into a powder. Normally the specimen needs to be fully dry. As the specimen is at high vacuum, the survival and stabilization of living cells, tissues, and entire soft-species typically involve chemical fixation. Fixing is normally achieved by incubating a buffered chemical fixative, such as glutaraldehyde, in a solution. This then dehydrates the set tissue 25.
To test the cytotoxicity using MTT assay, the synthesized silver nanoparticle extract was treated against human breast and lung cancer cell lines [26]. The cells were placed separately at a concentration of 1 to 105 cells / well in 96 well plates. Using 100 μl of serum-, cells were washed twice after 24 h and starved at 37 ˚C for an hour. Cells were treated for 24 hours with a particular test compound after starvation. Following the treatment cycle, serum-free media containing MTT (0.5 mg/ml) was aspired and incubated in a CO2 incubator for 4 hours at 37 °C.
Following the removal of MTT-containing medium, 200 μl PBS washed the cells. Pipetting mixed 100 μl of DMSO to dissolve the crystals. A microplate reader detected purple-blue formazan dye absorption at 570 nm (Biorad 680). GraphPadPrism5 computed cytotoxicity.
Percentage of cell viability = A570 of treated cells / A570 of control cells × 100%.
Result
The study focused on the synthesis of silver nanoparticles using the fungus P. breviseta. The researchers compared water-based extracts as positive controls and unfiltered pure silver nitrate as negative references. Both the fungal mat and the cell filtrate were exposed to 1 mM AgNO3 in the dark at room temperature. The resulting color change to yellow or dark brown in the fungal mats and cell filtrates indicated the formation of silver nanoparticles through plasmon surface vibrations 27. The control group showed no color change under similar conditions (Fig. 1).
 |
Figure 1: (a) AgNO3: Representation of the silver nanoparticle synthesis process using silver nitrate (AgNO3) as the precursor.
|
Using a UV-Vis spectrophotometer, the optical properties of the fungal cell filtrate were examined. After adding 1 mM AgNO3, a peak absorption at 420 nm was observed, suggesting the presence of silver nanoparticles (Figure 2).
 |
Figure 2: Cell filtrate UV spectra – Pestaloptiopsis breviseta
|
The researchers analyzed the fungal cell filtrate using FT-IR spectroscopy to identify potential bioactive compounds that could be interacting with silver and contributing to nanoparticle formation and stability. This was an attempt to identify the “capping substance.” Bioactive compounds often exhibit distinct infrared absorption patterns, such as the amide interactions found in protein amino acid residues, which produce characteristic fingerprints in the infrared spectrum (Fig. 3). Peaks associated with unbound OH and NH groups were identified at 3380, 3300, and 3467, while the peak at 2140 indicated an increase in aromatic CH bonds. Peaks at 1965 suggested unsaturation, 1643 indicated C-C stretching, 710 implied C-Cl stretching, and 646 signified -C C-H: C-H bending.
 |
Figure 3: Oxidized biomolecule FTIR band peaks during AgNPs production.
|
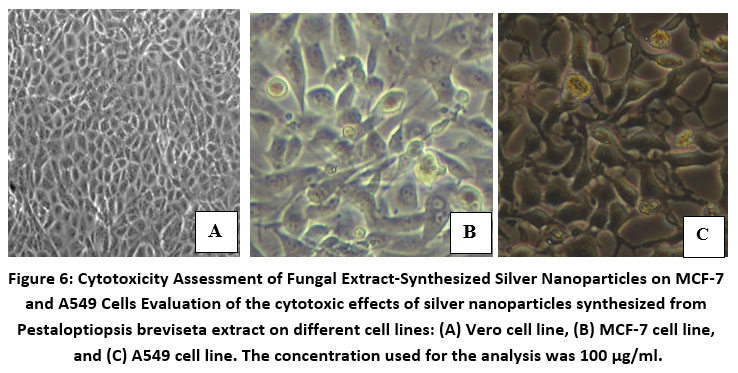
The size of the silver nanoparticles was investigated using scanning electron microscopy. The nanoparticles produced by the cell filtrate ranged from 171 to 378 nm, while those from the fungal biomass were smaller, ranging from 117 to 125 nm (Fig. 4). Despite their relatively large size, the cell filtrate-generated nanoparticles exhibited significant cytotoxicity when tested on human cancer cell lines. Both MCF-7 and A549 cell lines were exposed to silver nanoparticles produced by the fungus cells. Cell viability analysis using Graph Pad Prism 5 revealed that higher nanoparticle concentrations led to greater cytotoxicity. This analysis was conducted over 24 and 48 hours (Fig. 6), and the results demonstrated a clear concentration-dependent cytotoxic effect (Fig. 7).
 |
Figure 4: Fungal Mat-Derived Silver Nanoparticles Characterization. Scanning electron micrographs depicting silver nanoparticles produced by the fungal mat of Pestaloptiopsis breviseta.
|
 |
Figure 5: Cell Filtrate-Derived Silver Nanoparticles Visualization Scanning electron micrographs showcasing silver nanoparticles synthesized from Pestaloptiopsis breviseta cell filtrate.
|
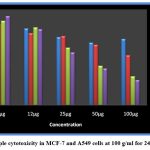 |
Figure 7: Sample cytotoxicity in MCF-7 and A549 cells at 100 g/ml for 24 and 48 hours.
|
Discussion
The use of fungi for the synthesis of silver nanoparticles has gained considerable attention in recent years due to the eco-friendly and cost-effective nature of the process. In this study, the fungus P. breviseta was used for the synthesis of silver nanoparticles, and the results showed the formation of nanoparticles with sizes ranging from 117 to 378 nm. The nanoparticles exhibited high cytotoxicity when tested on human cancer cell lines, indicating their potential as a therapeutic agent against cancer.
The high cytotoxicity of silver nanoparticles has been demonstrated in several previous studies. For instance, a study showed that silver nanoparticles synthesized by the fungus Verticillium sp. were highly cytotoxic against human lung cancer cells 28. Another study by demonstrated the cytotoxicity of silver nanoparticles synthesized by the fungus Aspergillus flavus against human breast cancer cells 29.
The exact mechanism by which silver nanoparticles exert their cytotoxic effects is not fully understood, but it has been suggested that they induce oxidative stress and disrupt cellular processes such as DNA replication and protein synthesis 30. Additionally, the large size of the nanoparticles synthesized in this study may contribute to their cytotoxicity, as larger particles have been shown to have greater toxicity than smaller particles 31.
Despite the high cytotoxicity of silver nanoparticles, their potential as a therapeutic agent against cancer cannot be ignored. Several studies have shown that silver nanoparticles have selective cytotoxicity against cancer cells while sparing normal cells. This selectivity may be due to the higher metabolic activity and greater susceptibility of cancer cells to oxidative stress compared to normal cells 32
Conclusion
Instead of using complex chemical or physical methods, biologically produced nanoparticles can be used instead. SEM analysis confirmed that P. breviseta in this work formed nanoparticles with sizes ranging from 171-378 nm, and that the silver nanoparticles in the fungal biomass had sizes between 140 and 280 nm. Mycelial biomass nanoparticles and taxol-fungus cell filtrate have not been reported on in the literature before. More research at the molecular level is needed to use these nanoparticles to develop a broad-spectrum antibiotic.
Conflict of Interest
The writers claim no conflict of interest.
Funding of Sources
Department of Science and Technology (INSPIRE), India, Grant Recipient: Vardhana Janakiraman
References
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Materials Letters 2007;61:1413–8.
CrossRef - Mann S (2001). Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, Oxford University Press, Oxford, UK.
- Simkiss K and Wilbur KM (1989) Biomineralization, Academic, New York, NY, USA.
- Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO. Biomimetic synthesis and patterning of silver nanoparticles. Nature Materials 2002;1:169–72.
CrossRef - Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar PV, Alam M, Kumar R, and Sastry M (2001). Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Letters. 1: 515-519.
CrossRef - Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids and Surfaces B Biointerfaces 2003;28:313–8.
CrossRef - Fayaz AM, Balaji K, Girilal M, Yadav R, Kalichelvan PT, and Venkatesan R (2010). Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine: Nanotechnology, Biology, and Medicine. 6(1): 103-109.
CrossRef - Durán, N., Marcato, P. D., Durán, M., Yadav, A., Gade, A., and Rai, M. (2011). Mechanistic aspects in the biogenic synthesis of extracelular metal nanoparticles by peptides, bacteria, fungi and plants. Appl. Microbiol. Biotechnol. 90, 1609–1624. doi: 10.1007/s00253-011-3249-8
CrossRef - Ballotin, D., Fulaz, S., Souza, M. L., Corio, P., Rodrigues, A. G., Souza, A. O., et al. (2016). Elucidating protein involvement in the stabilization of the biogenic silver nanoparticles. Nanoscale Res. Lett. 11:313. doi: 10.1186/s11671-016-1538-y
CrossRef - Gholami-Shabani, M., Akbarzadeh, A., Norouzian, D., Amini, A., Gholami-Shabani, Z., Imani, A., et al. (2014). Antimicrobial activity and physical characterization of silver nanoparticles green synthesized using nitrate reductase from Fusarium oxysporum. Appl. Biochem. Biotechnol. 172, 4084–4098. doi: 10.1007/s12010-014-0809-2
CrossRef - Jain N, Bhargava A, Majumdar S, Tarafdar JC, and Panwar J (2011) Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale. 3(2) 635–641.
CrossRef - Bhainsa KC and D’Souza SF (2006). Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus, Colloids and Surfaces B. 47(2) 160–164.
CrossRef - Senapati S, Mandal D, Ahmad A (2004). Fungus mediated synthesis of silver nanoparticles: a novel biological approach, Indian Journal of Physics A. 78(1) 101–105.
- Southam G, Beveridge TJ. The in vitro formation of placer gold by bacteria. Geochimica et Cosmochimica Acta 1994;58:4527–30.
CrossRef - Balaji DS, Basavaraja S, Deshpande R, Mahesh DB, Prabhakar BK, Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides Colloids and Surfaces B Biointerfaces 2009;68(1):88–92.
- Ingle A, Gade A, Pierrat S, So˝ Erichsen C, Rai M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Current Nanoscience 2008; 4(2):141–4.
CrossRef - Kumar SA, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmad A, et al. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnology Letters 2007;29(3):439–45.
CrossRef - Lengke MF, Fleet ME, Southam G. Biosynthesis of silver nanoparticles by filamentous Cyanobacteria from a silver (I) nitrate complex. Langmuir 2007;23(5):2694–9.
CrossRef - Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P. The use of microorganisms for the formation of metal nanoparticles and their application. Applied Microbiology and Biotechnology 2006;69(5):485–92.
CrossRef - Parikh RY, Singh S, Prasad BLV, Patole MS, Sastry M, Schouche YS. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella : towards understanding biochemical synthesis mechanism. ChemBioChem 2009;9(9):1415–22.
CrossRef - Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Colloids and Surfaces B Biointerfaces 2007;57 (1):97–101.
CrossRef - Gowri K, Kathiravan G, and Sukumaran N (2012). Production of taxol by Pestalotiopsis breviseta cr01 isolated from the Catharanthus roseus and its growth studies. International Journal of Pharma and Bioscience 2(3): 1046 – 1053.
- Suryanarayanan TS and Thennarasan S (2004). Temporal variation in endophyte assemblages of Plumeria rubra Fungal Diversity. 15: 197-204.
- Schulz B, Wanke U, Draeger S, and Aust HJ (1993). Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycological Research. 97: 1447-1450.
CrossRef - Bharathidasan R and Panneerselvam A (2012). Biosynthesis and Characterization of Silver Nanoparticles Using Endophytic Fungi Aspergillus concius, Penicillium janthinellum, And Phomosis International Journal of Pharmaceutical Science and Research. 3(9): 3163-3169.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 65: 55-63.
CrossRef - Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles.Langmuir 1996;12:788–800.
CrossRef - Asharani PV, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009; 3: 279-290.
CrossRef - Wu T, et al. Molecular responses of human lung epithelial cells to the toxicity of copper oxide nanoparticles inferred from whole genome expression analysis. ACS Nano. 2010; 4: 7411-7423.
- Prasad RY, et al. Cellular uptake and cytotoxic potential of silver nanoparticles in human bladder cancer cells. J Appl Toxicol. 2016; 36: 954-963.
- Khan I, et al. Biogenic synthesis of silver nanoparticles: Its potential in biomedical applications. J Nanopart Res. 2019; 21: 139.
- Choi JE, et al. Size-dependent and reactive oxygen species-related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol. 2008; 42: 4583-4588
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.